How Can Nanoplastics Affect the Survival, Reproduction, and Behaviour of the Soil Model Enchytraeus crypticus?
Abstract
1. Introduction
2. Material and Methods
2.1. Test Organism
2.2. Test Materials and Characterization
2.3. Exposure via Soil
2.3.1. Test Soil and Spiking Procedures
2.3.2. Enchytraeid Reproduction Test (ERT) Procedures
2.3.3. Avoidance Test Procedures
2.4. Exposure via Water
2.5. Data Analysis
3. Results
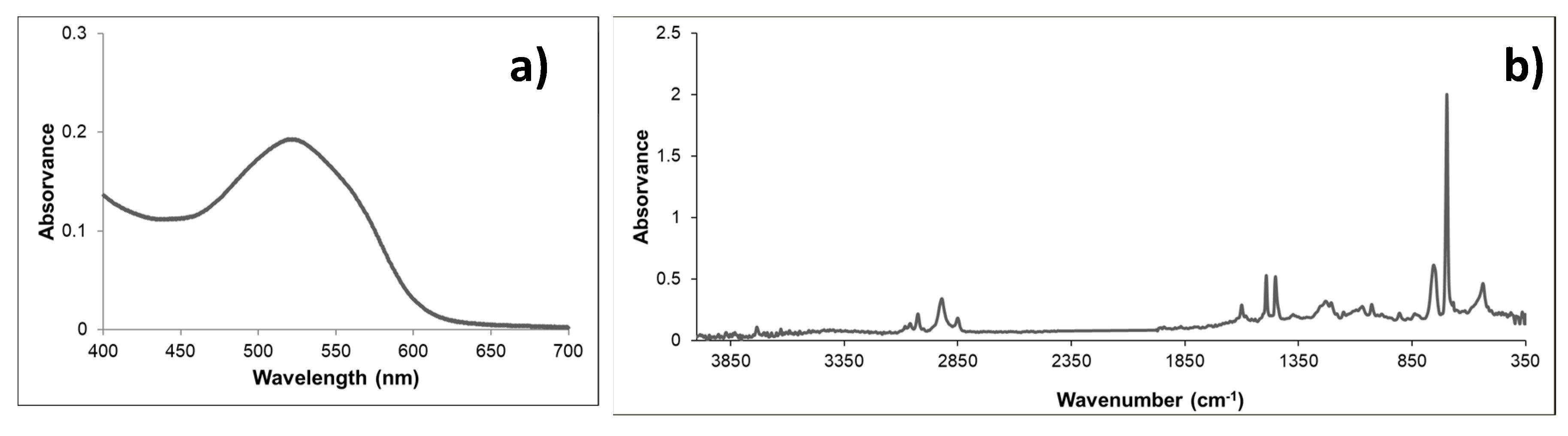
3.1. Characterization of Nanoplastics (NPls)
3.2. Exposure via Soil
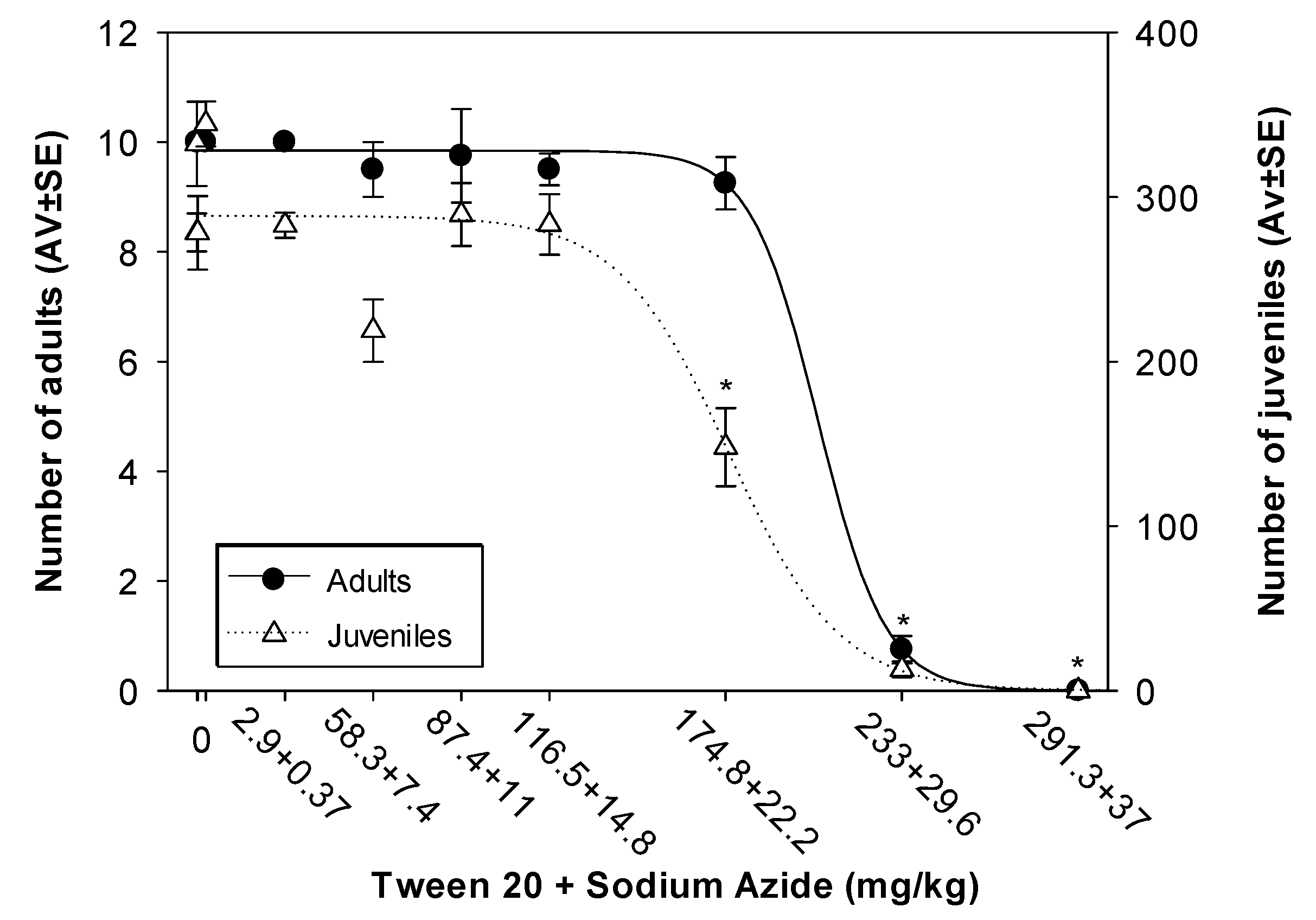
3.2.1. Enchytraeid Reproduction Test (ERT)
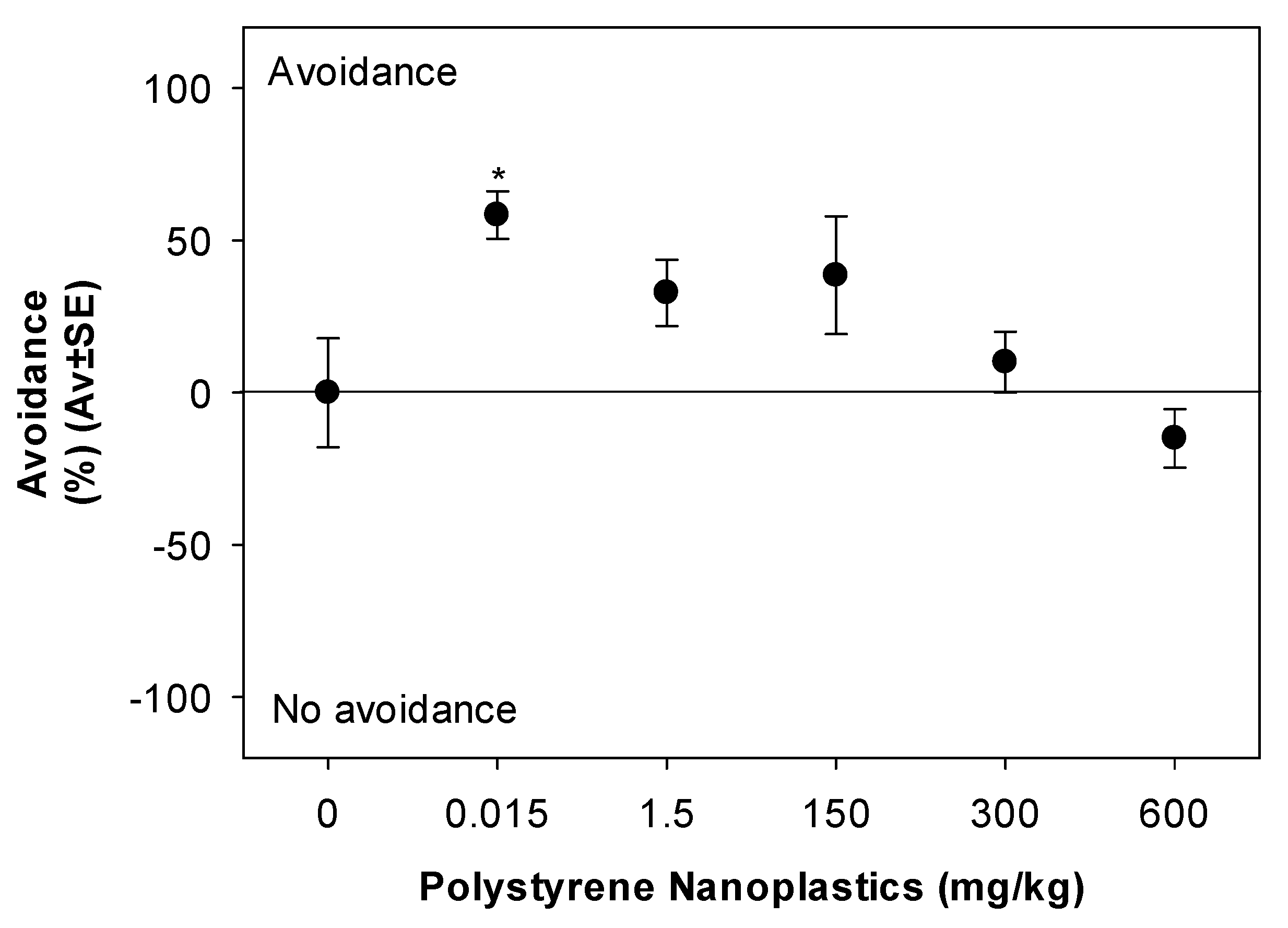
3.2.2. Avoidance Test
3.3. Exposure via Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwach, B.; Shikuku, V. Microplastics as Emerging Contaminants: Occurrence, Toxicology, and Analysis. In Effects of Emerging Chemical Contaminants on Water Resources and Environmental Health; IGI Global: Hershey, PA, USA, 2020. [Google Scholar]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Machado, A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2017, 24, 1405–1416. [Google Scholar] [CrossRef]
- Zhu, B.-K.; Fang, Y.-M.; Zhu, D.; Christie, P.; Ke, X.; Zhu, Y.-G. Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus. Environ. Pollut. 2018, 239, 408–415. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten maximus at Environmentally Realistic Concentrations. Environ. Sci. Technol. 2018, 52, 14480–14486. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Hansson, L.-A.; Cedervall, T. Nano-plastics in the aquatic environment. Environ. Sci. Process. Impacts 2015, 17, 1712–1721. [Google Scholar] [CrossRef]
- Sendra, M.; Saco, A.; Yeste, M.P.; Romero, A.; Novoa, B.; Figueras, A. Nanoplastics: From tissue accumulation to cell translocation into Mytilus galloprovincialis hemocytes. resilience of immune cells exposed to nanoplastics and nanoplastics plus Vibrio splendidus combination. J. Hazard. Mater. 2020, 388, 121788. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna-Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Hu, S.; Xiao, X.; Wu, J.; Wei, S.; Xiong, Y.; Ouyang, G. Investigating the toxicities of different functionalized polystyrene nanoplastics on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 180, 509–516. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.-J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.A.P.; da Costa, J.; Duarte, A.C.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, Q.; An, X.; Yang, X.; Christie, P.; Ke, X.; Wu, L.-H. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar]
- Römbke, J.; Jänsch, S.; Didden, W. The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 249–265. [Google Scholar] [CrossRef]
- Peijnenburg, W.; Capri, E.; Kula, C.; Liess, M.; Luttik, R.; Montforts, M.; Nienstedt, K.; Römbke, J.; Sousa, J.P.; Jensen, J. Evaluation of Exposure Metrics for Effect Assessment of Soil Invertebrates. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1862–1893. [Google Scholar] [CrossRef]
- Castro-Ferreira, M.P.; Roelofs, D.; van Gestel, C.A.M.; Verweij, R.A.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeus crypticus as model species in soil ecotoxicology. Chemosphere 2012, 87, 1222–1227. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Maria, V.L.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Fate and Effect of Nano Tungsten Carbide Cobalt (WCCo) in the Soil Environment: Observing a Nanoparticle Specific Toxicity in Enchytraeus crypticus. Environ. Sci. Technol. 2018, 52, 11394–11401. [Google Scholar] [CrossRef]
- Santos, J.; Barreto, Â.; Nogueira, J.; Daniel-da-Silva, L.A.; Trindade, T.; Amorim, J.B.M.; Maria, L.V. Effects of Amorphous Silica Nanopowders on the Avoidance Behavior of Five Soil Species—A Screening Study. Nanomaterials 2020, 10, 402. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Publishing: Paris, France, 2004; 12p. [Google Scholar] [CrossRef]
- OECD. Test No. 220: Enchytraeid Reproduction Test; OECD Publishing: Paris, France, 2016; 22p. [Google Scholar] [CrossRef]
- ISO. Soil quality. Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour—Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei); ISO Publishing: Geneva, Switzerland, 2008; ISO 17512-1; 25p. [Google Scholar]
- Bicho, R.C.; Gomes, S.I.L.; Soares, A.M.V.M.; Amorim, M.J.B. Non-avoidance behaviour in enchytraeids to boric acid is related to the GABAergic mechanism. Environ. Sci. Pollut. Res. 2015, 22, 6898–6903. [Google Scholar] [CrossRef]
- Roembke, J.; Knacker, T. Aquatic toxicity test for enchytraeids. Hydrobiologia 1989, 180, 235–242. [Google Scholar] [CrossRef]
- Bicho, R.C.; Soares, A.M.V.M.; Nogueira, H.I.S.; Amorim, M.J.B. Effects of europium polyoxometalate encapsulated in silica nanoparticles (nanocarriers) in soil invertebrates. J. Nanopart. Res. 2016, 18, 360. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Mechanisms of response to silver nanoparticles on Enchytraeus albidus (Oligochaeta): Survival, reproduction and gene expression profile. J. Hazard. Mater. 2013, 254, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.L.; Caputo, G.; Pinna, N.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effect of 10 different TiO2 and ZrO2 (nano)materials on the soil invertebrate Enchytraeus crypticus. Environ. Toxicol. Chem. 2015, 34, 2409–2416. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Pikuda, O.; Xu, E.G.; Berk, D.; Tufenkji, N. Toxicity Assessments of Micro- and Nanoplastics Can Be Confounded by Preservatives in Commercial Formulations. Environ. Sci. Technol. Lett. 2019, 6, 21–25. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The behaviors of microplastics in the marine environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Cho, H.-J.; Kim, E.; Huh, Y.H.; Kim, H.-J.; Kim, B.; Kang, T.; Lee, J.-S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef]
- Barreto, Â.; Luis, L.G.; Girão, A.V.; Trindade, T.; Soares, A.M.V.M.; Oliveira, M. Behavior of colloidal gold nanoparticles in different ionic strength media. J. Nanopart. Res. 2015, 17, 493. [Google Scholar] [CrossRef]
- Bicho, R.C.; Ribeiro, T.; Rodrigues, N.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of Ag nanomaterials (NM300K) and Ag salt (AgNO3) can be discriminated in a full life cycle long term test with Enchytraeus crypticus. J. Hazard. Mater. 2016, 318, 608–614. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Novel understanding of toxicity in a life cycle perspective-The mechanisms that lead to population effect-The case of Ag (nano)materials. Environ. Pollut. 2020, 262, 114277. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; He, D. Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environ. Sci. Nano 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.H.; Liang, S.T.; Chen, J.R.; Chen, K.H.C.; Hsiao, C. Der Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: Throwing up alarms of wide spread health risk of exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, M.; Wu, D.; Yu, P.; Jiao, Y.; Jiang, Q.; Zhao, Y. Effects of nanoplastics at predicted environmental concentration on Daphnia pulex after exposure through multiple generations. Environ. Pollut. 2020, 256, 113506. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.B.; Fernández-Cruz, M.L.; Hund-Rinke, K.; Scott-Fordsmand, J.J. Environmental hazard testing of nanobiomaterials. Environ. Sci. Eur. 2020, 32, 101. [Google Scholar] [CrossRef]

| Characteristics of the Particles | Species | Characteristics of the Exposure | Assessed Endpoints | Main Findings | Rf |
|---|---|---|---|---|---|
| Nanoplastics (NPls) | |||||
| Polystyrene, 0.05 to 0.1 μm | E. crypticus | Via food 0.025, 0.5 and 10% 7 days | Reproduction Growth Gut microbiome NPls detection | NPls reduced the weight, had a hermetic-like effect on reproduction and changed the gut microbiome. | [5] |
| Microplastics (MPls) | |||||
| High-density polyethylene, 1.32 ± 0.72 mm | L. terrestris | Via soil spiked 236, 1261 and 4505 mg/kg with 0.35 wt % zinc 28 days | Survival Growth MPls adsorption /desorption capacity and accumulation | MPls had the potential to act as vector for increasing uptake of zinc. | [14] |
| Polyethylene, 250 to 1000 μm | E. andrei | Via soil spiked 62.5, 125, 250, 500 and 1000 mg/kg 28 days | Reproduction Survival Growth Histopathology and molecular analysis | Severe histological damages in the gut. Molecular changes related to immune system. | [15] |
| Polyethylene, < 150 μm | L. terrestris | Via soil spiked 7, 28, 45 and 60% 60 days | Reproduction Survival Growth MPls ingestion | Mortality increased and growth rate was reduced. MPls were concentrated in cast. | [6] |
| Polyvinyl chloride, 80 and 250 μm | F. candida | Via soil spiked 1 g/kg 48 h | Reproduction Growth Gut microbiome Isotopic turnover | Growth and reproduction were inhibited. MPls changed the gut microbiome. Enhanced δ15N and δ13C values. | [16] |
| NPls Nominal Concentration (mg/kg) | Number of Nanospheres/kg |
|---|---|
| 0.015 | 0.000036825 × 1016 |
| 1.5 | 0.0036825 × 1016 |
| 15 | 0.036825 × 1016 |
| 150 | 0.36825 × 1016 |
| 300 | 0.7365 × 1016 |
| 450 | 1.10475 × 1016 |
| 600 | 1.475 × 1016 |
| 900 | 2.2095 × 1016 |
| 1200 | 2.946 × 1016 |
| 1500 | 3.6825 × 1016 |
| NPls Nominal Concentration (mg/kg) | Tween 20 + NaN3 Nominal Concentrations (mg/kg) |
|---|---|
| 0.015 | 0.0029 + 0.00037 |
| 1.5 | 0.29 + 0.037 |
| 15 | 2.9 + 0.37 |
| 150 | 29.1 + 3.7 |
| 300 | 58.3 + 7.4 |
| 450 | 87.4 + 11.1 |
| 600 | 116.5 + 14.8 |
| 900 | 174.8 + 22.2 |
| 1200 | 233 + 29.6 |
| 1500 | 291.3 + 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. How Can Nanoplastics Affect the Survival, Reproduction, and Behaviour of the Soil Model Enchytraeus crypticus? Appl. Sci. 2020, 10, 7674. https://doi.org/10.3390/app10217674
Barreto A, Santos J, Amorim MJB, Maria VL. How Can Nanoplastics Affect the Survival, Reproduction, and Behaviour of the Soil Model Enchytraeus crypticus? Applied Sciences. 2020; 10(21):7674. https://doi.org/10.3390/app10217674
Chicago/Turabian StyleBarreto, Angela, Joana Santos, Mónica J. B. Amorim, and Vera L. Maria. 2020. "How Can Nanoplastics Affect the Survival, Reproduction, and Behaviour of the Soil Model Enchytraeus crypticus?" Applied Sciences 10, no. 21: 7674. https://doi.org/10.3390/app10217674
APA StyleBarreto, A., Santos, J., Amorim, M. J. B., & Maria, V. L. (2020). How Can Nanoplastics Affect the Survival, Reproduction, and Behaviour of the Soil Model Enchytraeus crypticus? Applied Sciences, 10(21), 7674. https://doi.org/10.3390/app10217674








