Effect of Adjuvant Use of NSAID in Reducing Probing Pocket Depth in the Context of Conventional Periodontal Therapy: A Systematic Review of Randomized Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria Screening and Selection
2.3. Outcome Measures
2.4. Data Extraction
2.5. Data Analysis and Synthesis
2.6. Assessment of Quality of Studies
3. Results
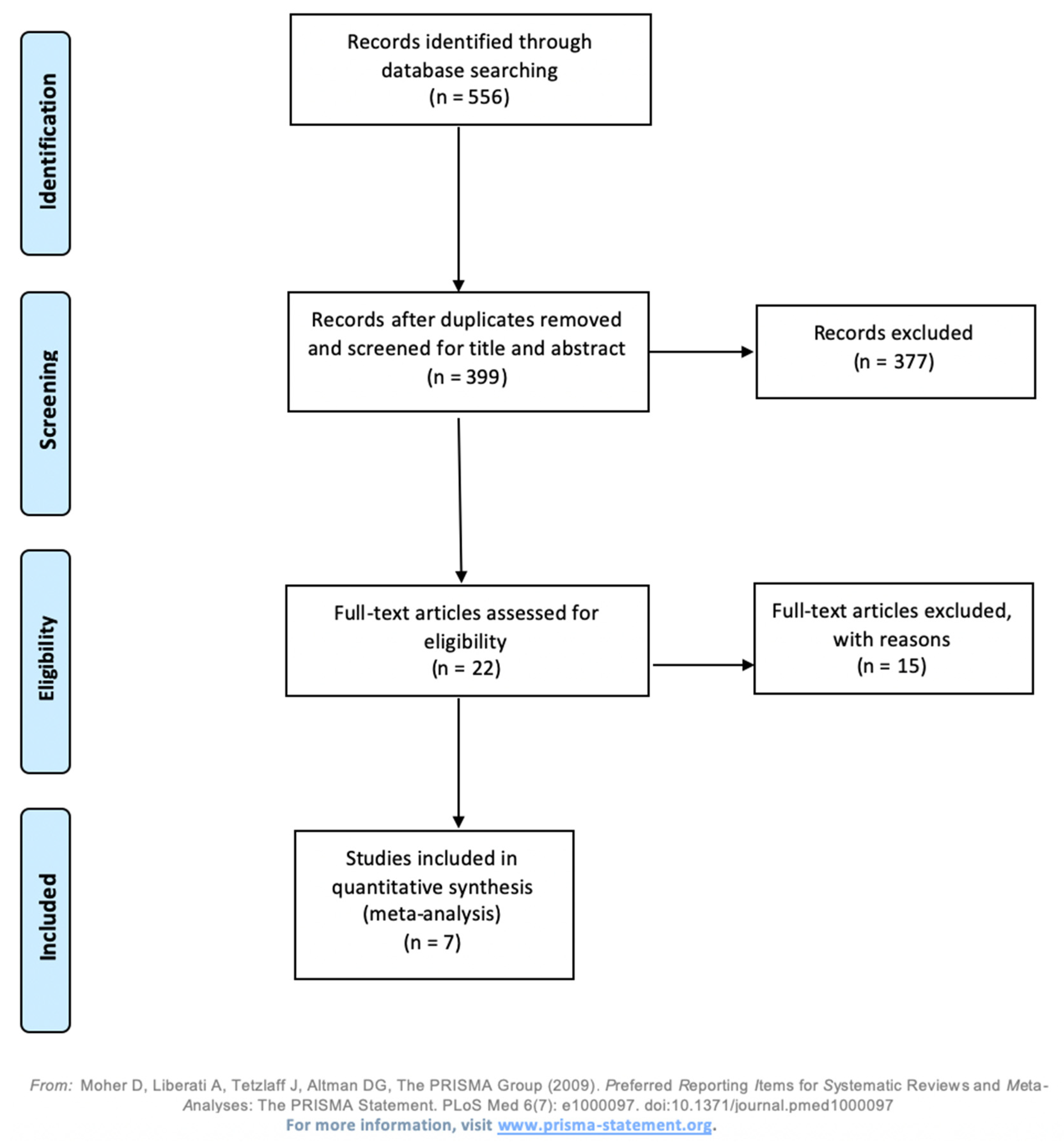
3.1. Study Selection
3.2. Description of Characteristics
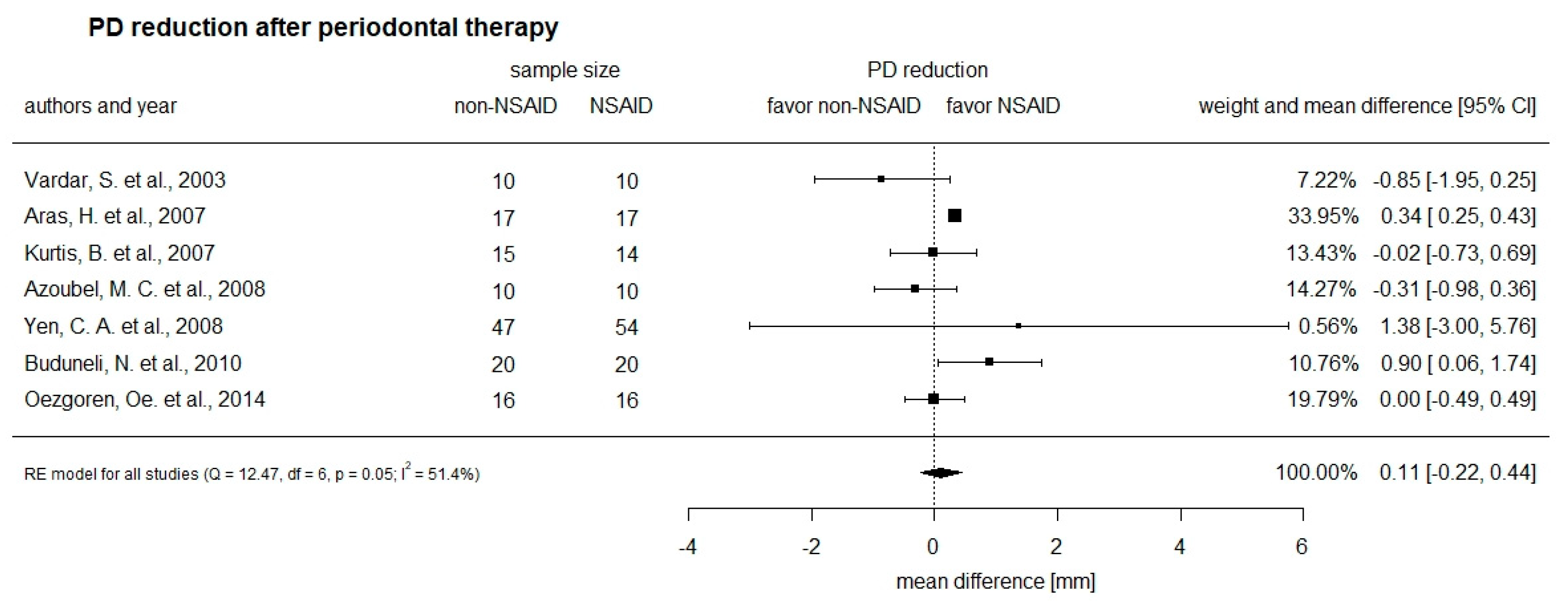
3.3. Primary Outcome of the Intervention on Probing Pocket Depth (PD) Reduction
3.4. Secondary Outcome of the Intervention on Bleeding on Probing (BOP)
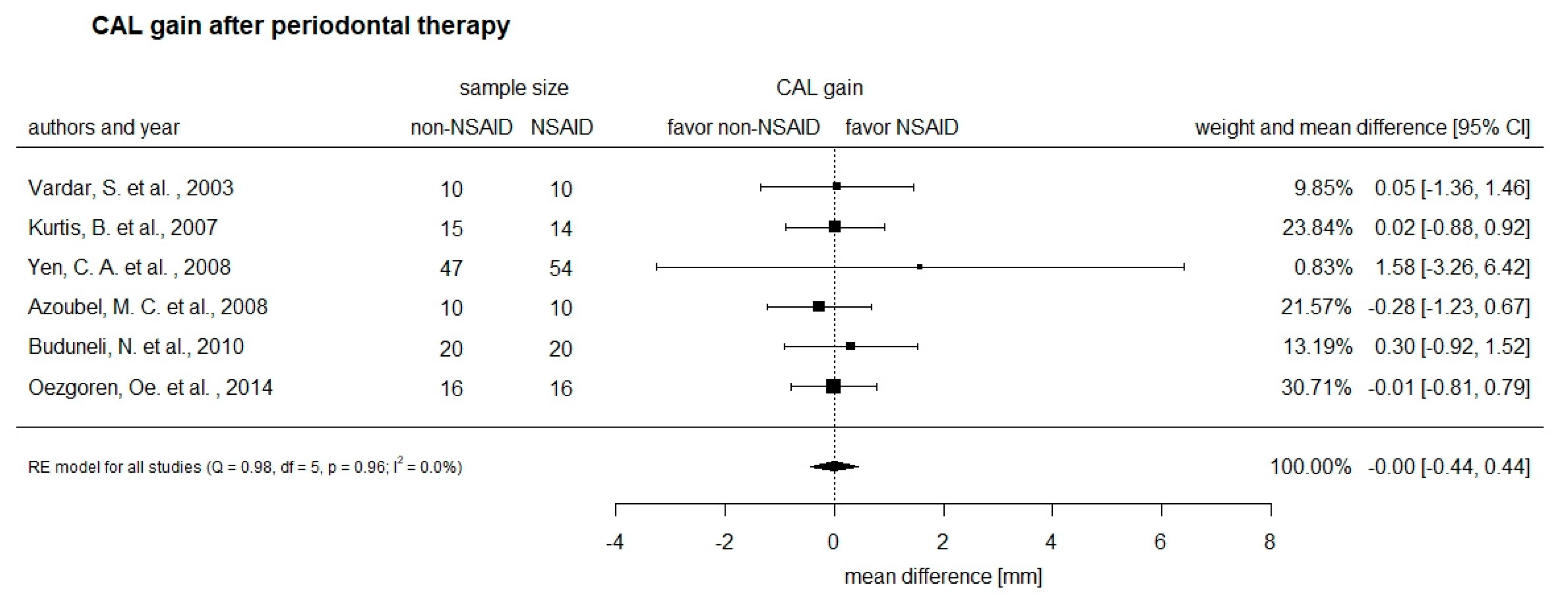
3.5. Secondary Outcome of the Intervention on Clinical Attachment Gain (CAL)
3.6. Secondary Outcome of the Intervention on Plaque Index (PI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Step | Query | Hits |
|---|---|---|
| 1 | (periodontal diseases/or exp periodontitis/or ((periodont* or parodont* or paradont*) adj3 (disease* or loss or pocket* or abscess*)).ti,ab. or (pericementitides or pericementitis or periodontitides or periodontitis or periodontoses or periodontosis or paradontitis or parodontitis).ti,ab.) not (animals not humans).sh. | 58,306 |
| 2 | Dental Scaling/or “Root Planing”/or periodontal diseases/th, dt or exp periodontitis/th, dt or ((dental or root or subgingival or supragingival) adj3 scaling*).ti,ab. or (root adj3 (planing* or planning*)).ti,ab. or ((periodont* or parodont* or paradont* or pericement*) adj5 (treat* or therap*)).ti,ab. | 23,330 |
| 3 | exp anti-inflammatory agents, non-steroidal/or exp cyclooxygenase 2 inhibitors/or ((anti-inflammatory or antiinflammatory) adj3 (agents* or drug* or therapy)).ti,ab. or ((COX-2 or cyclooxygenase-2) adj3 inhibitor*).ti,ab. or “systemic chemotherapeutic*”.ti,ab. or (NSAID* or aspirin or “acetylsalicylic acid” or acetysal or acylpyrin or aloxiprimum or colfarit or dispril or easprin or ecotrin or endosprin or magnecyl or micristin or polopirin or polopiryna or solprin or solupsan or zorprin or ibuprofen or brufen or ip-82 or ip82 or ibumetin or motrin or nuprin or rufen or salprofen or dolgit or traumadolgit or flubiprofen or ansaid or cebutid or dobrofen or flugalin or flurbiprofen or fluriproben or froben or neo-artrol or novo-flurprofen or nu-flurbiprofen or ocufen or ocuflur or strefen or ratio-flurbiprofen or meloxicam or acticam or aflamid or artrilox or contacera or dormelox or ecax or emdocam or exel or flexicam or flodin or hexaphlogin or inflacam or loxibest or loxicam or loxicom or masflex or mecox or mel-od or melcox or melicam or melocam or melocox or melosolute or melosteral or melosus or melovem or melox or meloxidolor or meloxidyl or meloxin or meloxivet or meloxoral or merapiran or mesoxicam or metacam or mevamox or mexpharm or miloxam or mobec or mobic or mobicox or mopik or movalis or movatec or movi-cox or movicox or mowin or muvera or novem or ostelox or parocin or rafree or recocam or revitacam or rheumocam or rumonal or vivlodex or tenoxicam or alganex or arthrinal or aspagin or doxican or hobaticam or indo bros or indobros or istotosal or liman or mobiflex or neo endusix or neoendusix or octiveran or oxytel or palitenox or reutenox or “ro 12 0068” or soral or tenalgin or tilatil or tilcitin or tilconox or tilcotil or toscacalm or indomethacin or amuno or indocid or indocin or indomet-140 or indometacin or metindol or osmosin or alrheumat or alrheumum or benzoylhydratropic-acid or ketoprofen or orudis or profenid or ketorolac or rs-37619 or rs37619 or naproxen or aleve or anaprox or methoxypropiocin or naprosin or naprosyn or naproxenate or proxen or synflex or etoricoxib or acoxxel or algix or arcoxia or auxib or bericox or caretor or coxerit or coxeta or coxient or coxiloc or coxitor or coxolin or doloxib or ecoxyton or etori or etoriax or etorican or etoricox or “etoricoxib hydrochloride” or etorikoksib or etorilin or etoxib or evetore or exinef or exxiv or halliztolva or imesol or itoroxx or kostarox or l-791456 or l791456 or linzavo or mk-0663 or mk-663 or mk0663 or mk663 or narox or nucoxia or oxidraxib or roticox or tauxib or turox or celecoxib or celebrex or sc-58635 or sc58635).ti,ab. | 250,911 |
| 4 | 1 and 2 and 3 | 361 |
| 5 | Periodontal Index/or ((periodontal or parodont* or paradont* or pericement* or gingival) adj3 (index or indices or indexes or status)).ti,ab. or (bleeding* or pocket*).ti,ab. | 236,334 |
| 6 | 4 and 5 | 101 |
| 7 | (“18771374” or “22092475” or “23594239” or “18166099” or “19180322”).ui. | 5 |
| 8 | 6 and 7 | 5 |
| 9 | 4 not 6 | 260 |
Appendix B
| Excluded Studies | Reason for Exclusion |
|---|---|
| O. Aboul-Dahab, A clinical evaluation of non-steroidal anti-inflammatory drugs (NSAIDS) as adjuncts in the management of periodontal disease, Egypt Dent J 39(3) (1993) 511-8. | No full text available |
| N.C. Deshpande, K.M. Bhat, G.S. Bhat, A.N. Deshpande, Randomized, controlled clinical study to evaluate efficacy of novel indigenously designed controlled release flurbiprofen gel system for management of periodontal diseases, Contemp Clin Dent 4(1) (2013) 32–6. | Primary outcome (PD) not investigated |
| H. El-Sharkawy, N. Aboelsaad, M. Eliwa, M. Darweesh, M. Alshahat, A. Kantarci, H. Hasturk, T.E. Van Dyke, Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin, Journal of periodontology 81(11) (2010) 1635–43. | Not addressing research question |
| N.M. Elwakeel, H.H. Hazaa, Effect of omega 3 fatty acids plus low-dose aspirin on both clinical and biochemical profiles of patients with chronic periodontitis and type 2 diabetes: a randomized double-blind placebo-controlled study, Journal of periodontal research 50(6) (2015) 721–9. | Not addressing research question |
| T.F. Flemmig, B. Epp, Z. Funkenhauser, M.G. Newman, K.S. Kornman, I. Haubitz, B. Klaiber, Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy, Journal of clinical periodontology 22(6) (1995) 427–33. | Not addressing research question |
| E. Funosas, G. Feser, L. Escovich, L. Maestri, Alteration of hemostasis in patients treated with subgingival NSAIDs during periodontal therapy, Acta Odontol Latinoam 25(1) (2012) 103-8. | Not addressing research question |
| E.R. Funosas, L. Escovich, L. Maestri, The use of topical subgingival gels of non-steroidal anti-inflammatory drugs (NSAIDs) as an adjunct to non-surgical management of chronic periodontitis, Acta Odontol Latinoam 22(3) (2009) 215–9. | No full text available |
| P.A. Heasman, D.K. Benn, P.J. Kelly, R.A. Seymour, D. Aitken, The use of topical flurbiprofen as an adjunct to non-surgical management of periodontal disease, Journal of clinical periodontology 20(6) (1993) 457–64. | Not addressing research question |
| NCT02149758, Effect of selective COX-2 Inhibitor (Etoricoxib) along with SRP on clinical parameters and salivary level of superoxide dismutase in chronic generalized periodontitis a double-blind, placebo-controlled, double-masked randomized controlled trial (2014) | No full text available |
| NCT02538237, The Effect of Sub-gingival Irrigation With Ibuprofen 2% Mouthwash in Treatment of Periodontal Diseases (2015) | No full text available |
| Flemmig TF, Rumetsch M, Klaiber B. Efficacy of systemically administered acetylsalicylic acid plus scaling on periodontal health and elastase-alpha 1-proteinase inhibitor in gingival crevicular fluid. J Clin Periodontol. 1996; 23(3 Pt 1):153–159. doi:10.1111/j.1600-051x.1996.tb02070.x | Not addressing research question |
| Ng VW, Bissada NF. Clinical evaluation of systemic doxycycline and ibuprofen administration as an adjunctive treatment for adult periodontitis. J Periodontol. 1998; 69(7):772–776. doi:10.1902/jop.1998.69.7.772 | Not addressing research question |
| Pinho Mde N, Pereira LB, de Souza SL, et al. Short-term effect of COX-2 selective inhibitor as an adjunct for the treatment of periodontal disease: a clinical double-blind study in humans. Braz Dent J. 2008; 19(4):323–328. doi:10.1590/s0103-64402008000400007 | Not addressing research question; study design |
| Taiyeb Ali TB, Waite IM. The effect of systemic ibuprofen on gingival inflammation in humans. J Clin Periodontol. 1993; 20(10):723–728. doi:10.1111/j.1600-051x.1993.tb00697.x | Not addressing research question |
| Shiloah J, Bland PS, Scarbecz M, Patters MR, Stein SH, Tipton DA. The effect of long-term aspirin intake on the outcome of non-surgical periodontal therapy in smokers: a double-blind, randomized pilot study. J Periodontal Res. 2014; 49(1):102–109. doi:10.1111/jre.12085 | No original data; Pilot study |
Appendix C
| Aras, H. et al., 2007 | Azoubel, M.C. et al., 2008 | Buduneli, N. et al., 2010 | Flemmig, T.F. et al., 1996 | Kurtis, B. et al., 2007 | Ng, V.W. et al., 1998 | Özgören, O. et al., 2014 | Pinho Mde, N. et al., 2008 | Shiloah, J. et al., 2014 | Taiyeb Ali T.B. et al., 1993 | Vardar, S. et al., 2003 | Yen, C.A. et al., 2008 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Described as randomized * | 1 | 1 | 1 | 1/split mouth | 0 | 1/split mouth | 1 | 1/split mouth | 1 | 1/split mouth | 1 | 1 |
| Described as double-blind * | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| Randomization method described and appropriate ** | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Double-blinding method described and appropriate ** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Description of dropouts and withdrawals * | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| SCORE | 3 | 4 | 4 | 3 | 0 | 2 | 3 | 4 | 4 | 1 | 2 | 5 |
References
- Liu, R.; Li, N.; Liu, N.; Zhou, X.; Dong, Z.M.; Wen, X.J.; Liu, L.C. Effects of systemic ornidazole, systemic and local compound ornidazole and pefloxacin mesylate on experimental periodontitis in rats. Med. Sci. Monit. 2012, 18, BR95–BR102. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Lamster, I.B.; Lalla, E.; Borgnakke, W.S.; Taylor, G.W. The relationship between oral health and diabetes mellitus. J. Am. Dent. Assoc. 2008, 139, 19S–24S. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Gotsman, I.; Lotan, C.; Soskolne, W.A.; Rassovsky, S.; Pugatsch, T.; Lapidus, L.; Novikov, Y.; Masrawa, S.; Stabholz, A. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J. Periodontol. 2007, 78, 849–858. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Pink, C.; Kocher, T.; Meisel, P.; Dörr, M.; Markus, M.R.; Jablonowski, L.; Grotevendt, A.; Nauck, M.; Holtfreter, B. Longitudinal effects of systemic inflammation markers on periodontitis. J. Clin. Periodontol. 2015, 42, 988–997. [Google Scholar] [CrossRef]
- Suvan, J.E.; Petrie, A.; Nibali, L.; Darbar, U.; Rakmanee, T.; Donos, N.; D’Aiuto, F. Association between overweight/obesity and increased risk of periodontitis. J. Clin. Periodontol. 2015, 42, 733–739. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Del Fabbro, M.; Francetti, L.; Weinstein, R.; Ferrazzi, E. Adverse pregnancy outcomes and periodontitis: A systematic review and meta-analysis exploring potential association. Quintessence Int. 2016, 47, 193–204. [Google Scholar] [CrossRef]
- Michalowicz, B.S.; Hodges, J.S.; DiAngelis, A.J.; Lupo, V.R.; Novak, M.J.; Ferguson, J.E.; Buchanan, W.; Bofill, J.; Papapanou, P.N.; Mitchell, D.A.; et al. Treatment of periodontal disease and the risk of preterm birth. N. Engl. J. Med. 2006, 355, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. The management of inflammation in periodontal disease. J. Periodontol. 2008, 79, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Tegner, W.S. The Treatment of the Rheumatic Diseases in the United States and the Continent of Europe. Ann. Rheum. Dis. 1939, 1, 249–303. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.J.; Fudin, J. Nonsteroidal Antiinflammatory Drugs for Acute and Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef]
- Cascorbi, I. Drug interactions—Principles, examples and clinical consequences. Dtsch. Arztebl. Int. 2012, 109, 546–555. [Google Scholar] [CrossRef]
- Mutschler/Geisslinger/Menzel/Ruth/Schmidtko. Pharmakologie Kompakt Allgemeine und Klinische Pharmakologie, Toxikologie; Wiss. Verl: Stuttgart, Germany, 2016. [Google Scholar]
- Offenbacher, S.; Odle, B.M.; Gray, R.C.; Van Dyke, T.E. Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J. Periodontal Res. 1984, 19, 1–13. [Google Scholar] [CrossRef]
- Offenbacher, S.; Odle, B.M.; Van Dyke, T.E. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J. Periodontal Res. 1986, 21, 101–112. [Google Scholar] [CrossRef]
- Feldman, R.S.; Szeto, B.; Chauncey, H.H.; Goldhaber, P. Non-steroidal anti-inflammatory drugs in the reduction of human alveolar bone loss. J. Clin. Periodontol. 1983, 10, 131–136. [Google Scholar] [CrossRef]
- Vogel, R.I.; Copper, S.A.; Schneider, L.G.; Goteiner, D. The effects of topical steroidal and systemic nonsteroidal anti-inflammatory drugs on experimental gingivitis in man. J. Periodontol. 1984, 55, 247–251. [Google Scholar] [CrossRef]
- Alani, A.; Seymour, R. Systemic medication and the inflammatory cascade. Periodontology 2000 2014, 64, 198–210. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid. Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 16 July 2019).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Aras, H.; Caglayan, F.; Guncu, G.N.; Berberoglu, A.; Kilinc, K. Effect of systemically administered naproxen sodium on clinical parameters and myeloperoxidase and elastase-like activity levels in gingival crevicular fluid. J. Periodontol. 2007, 78, 868–873. [Google Scholar] [CrossRef]
- Buduneli, N.; Buduneli, E.; Cetin, E.O.; Kirilmaz, L.; Kutukculer, N. Clinical findings and gingival crevicular fluid prostaglandin E2 and interleukin-1-beta levels following initial periodontal treatment and short-term meloxicam administration. Expert Opin. Pharm. 2010, 11, 1805–1812. [Google Scholar] [CrossRef]
- Kurtis, B.; Tuter, G.; Serdar, M.; Pinar, S.; Demirel, I.; Toyman, U. GCF MMP-8 levels in smokers and non-smokers with chronic periodontitis following scaling and root planing accompanied by systemic use of flurbiprofen. J. Periodontol. 2007, 78, 1954–1961. [Google Scholar] [CrossRef]
- Ozgoren, O.; Develioglu, H.; Guncu, G.; Akman, A.; Berker, E. The adjunctive effect of tenoxicam during non-surgical periodontal treatment on clinical parameters and gingival crevicular fluid levels of MMP-8 and TNF-alpha in patients with chronic periodontitis - randomized, double-blind clinical trial. Adv. Clin. Exp. Med. 2014, 23, 559–565. [Google Scholar] [CrossRef]
- Vardar, S.; Baylas, H.; Huseyinov, A. Effects of selective cyclooxygenase-2 inhibition on gingival tissue levels of prostaglandin E2 and prostaglandin F2alpha and clinical parameters of chronic periodontitis. J. Periodontol. 2003, 74, 57–63. [Google Scholar] [CrossRef]
- Azoubel, M.C.; Sarmento, V.A.; Cangussu, V.; Azoubel, E.; Bittencourt, S.; Cunha, F.Q.; Ribeiro, R.A.; Brito, G.A. Adjunctive benefits of systemic etoricoxib in non-surgical treatment of aggressive periodontitis: Short-term evaluation. J. Periodontol. 2008, 79, 1719–1725. [Google Scholar] [CrossRef]
- Yen, C.A.; Damoulis, P.D.; Stark, P.C.; Hibberd, P.L.; Singh, M.; Papas, A.S. The effect of a selective cyclooxygenase-2 inhibitor (celecoxib) on chronic periodontitis. J. Periodontol. 2008, 79, 104–113. [Google Scholar] [CrossRef]
- Marsh, P.D.; Moter, A.; Devine, D.A. Dental plaque biofilms: Communities, conflict and control. Periodontology 2000 2011, 55, 16–35. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef]
- Waerhaug, J. Healing of the dento-epithelial junction following subgingival plaque control: II: As observed on extracted teeth. J. Periodontol. 1978, 49, 119–134. [Google Scholar] [CrossRef]
- Waerhaug, J. Healing of the dento-epithelial junction following subgingival plaque control: I: As observed in human biopsy material. J. Periodontol. 1978, 49, 1–8. [Google Scholar] [CrossRef]
- Donos, N.; Calciolari, E.; Brusselaers, N.; Goldoni, M.; Bostanci, N.; Belibasakis, G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2019. [Google Scholar] [CrossRef]
| Study (Year) | Population Characteristics (Ethnicity, Age, Inclusion Criteria) | No. of Patients (m/f), Smoking Status | Study Design | Intervention (Test vs. Control) | Dose | Duration | Study Period | Analyzed Parameters |
|---|---|---|---|---|---|---|---|---|
| Aras, H. et al., 2007 |
| 34 (17/17); NS; CP | RCT |
| 275 mg | 1x/d for 6 weeks | 6 weeks |
|
| Azoubel, M.C. et al., 2008 |
| 21 (2/19); NS; AP | RCT randomized, placebo-controlled, parallel-design, double-masked clinical trial |
| 120 mg | 1x/d for 7 days | 30 days |
|
| Buduneli, N. et al., 2010 |
| 50 randomized; 40 analyzed; (20/20); NS; CP | RCT randomized, double-blind, placebo-controlled and parallel-design |
| 7.5 mg | 1x/d for 10 days | 4 weeks |
|
| Kurtis, B. et al., 2007 |
| 29 NS/29 smoker Group 1 (NSAID): 14 NS; CP (12/2) Group 2 (Placebo): 15 NS; CP (8/7) Group 3 (NSAID): 14 smoker; CP (10/4) Group 4 (Placebo): 15 smoker; CP (9/6) Smokers had smoked ≥10 cigarettes daily for >5 years. NS had never smoked. | NR |
| 100 mg | 2x/d for 10 days | 10 days |
|
| Özgoren, Ö. et al., 2014 |
| 32 Group 1: 16; NS; CP (9/7) Group 2: 16; NS; CP (9/7) | RCT randomized, double-blind, placebo-controlled |
| 20 mg | 1x/d for 10 days | 30 days |
|
| Vardar, S. et al., 2003 |
| 30 (17/13);
| RCT double-blind placebo controlled |
| nimesulide 100 mg naproxen; 275 mg | 2x/d for 10 days | 3 months |
|
| Yen, C.A. et al., 2008 |
| 131 (54 male); CP; smoker/NS/Ex-smoker (40/35/24) 101 (54 celecoxib/ 47 placebo) returned for 3-month follow-up; 85 (45 celecoxib/ 40 placebo) returned for 6-month visit; 74 (40 celecoxib/ 34 placebo) returned for 9-month visit; 65 (35 celecoxib/ 30 placebo) completed 1-year study | RCT double-masked, randomized, placebo-controlled clinical trial |
| 200 mg | 1x/d for 6 months | 12 months |
|
| Study | NSAID | Dose | Duration | Max. Dose/d |
|---|---|---|---|---|
| Early follow-up: 7 d–4 wks | ||||
| Azoubel et al., 2008 | Etoricoxib | 120 mg | 1x/d, 7 d | 120 mg (max 8 d) |
| Buduneli et al., 2010 | Meloxicam | 7.5 mg | 1x/d, 10 d | 15 mg |
| Kurtis et al., 2007 | Flurbiprofen | 100 mg | 2x/d, 10 d | 300 mg |
| Özgoren et al., 2014 | Tenoxicam | 20 mg | 1x/d, 10 d | 40 mg |
| Late follow-up: 6–12 wks | ||||
| Aras et al., 2007 | Naproxen Sodium | 275 mg | 1x/d, 6 wks | 600 mg 1250 mg (prescription) |
| Vardar et al., 2003 | Nimesulide | 100 mg | 2x/d, 10 d | 200 mg (max 15 d) |
| Naproxen | 275 mg | 2x/d, 10 d | s.o. | |
| Yen et al., 2008 | Celecoxib | 200 mg | 1x/d, 6 mths | 400 mg |
| Study and Groups | Non-NSAID (n) | NSAID (n) | ΔPD in mm Non-NSAID (SD) | ΔPD in mm NSAID (SD) | Dose (%) |
|---|---|---|---|---|---|
| 10 day-follow-up | |||||
| Buduneli et al., 2010 | 20 | 20 | −1.30 (1.42) | −1.60 (1.13) | 50 |
| Kurtis et al., 2007 | / | / | / | / | 33.3 |
| smokers | 15 | 14 | −0.01 (0.50) | 0.01 (1.51) | |
| non-smokers | 15 | 14 | −0.01 (0.62) | 0.01 (1.21) | |
| 4 week/30 days-follow-up | |||||
| Buduneli et al., 2010 | 20 | 20 | −1.90 (1.48) | −2.80 (1.20) | 50 |
| Azoubel et al., 2008 | 10 | 10 | −2.48 (0.99) | −2.17 (0.44) | 100 |
| Özgoren et al., 2014 | 16 | 16 | −0.72 (0.77) | −0.72 (0.64) | 50 |
| 12 week-follow-up | |||||
| Vardar et al., 2003 | 10 | −4.1 (1.39) | |||
| Nimesulide | 10 | −3.25 (1.09) | 50 | ||
| Naproxen | 10 | −4.45 (1.33) | 137.5 | ||
| Yen et al., 2008 | 47 | 54 | 50 | ||
| moderate pockets | −0.98 (6.17) | −1.14 (6.76) | |||
| deep pockets | −1.89 (10.97) | −3.27 (11.46) | |||
| Study and Groups | Non-NSAID (n) | NSAID (n) | ΔBOP in mm Non-NSAID (SD) | ΔBOP in mm NSAID (SD) | Dose (%) |
|---|---|---|---|---|---|
| 10 day-follow-up | |||||
| Buduneli et al., 2010 | 20 | 20 | −1.60 (0.99) | −1.50 (1.13) | 50 |
| Kurtis et al. 2007 | 33.3 | ||||
| smokers | 15 | 14 | −1.50 (0.41) | −1.32 (0.40) | |
| non-smokers | 15 | 14 | −1.60 (0.16) | −1.28 (0.69) | |
| 4 week/30 days-follow-up | |||||
| Buduneli et al., 2010 | 20 | 20 | −1.8 (0.76) | −2.1 (0.89) | 50 |
| Azoubel et al., 2008 | 10 | 10 | −47.58% (22.01) | −57.44% (15.43) | 100 |
| Özgoren et al., 2014 | 16 | 16 | −0.53 (0.37) | −0.59 (0.29) | 50 |
| 12 week-follow-up | |||||
| Vardar et al., 2003 | 10 | −1.85 (1.28) | |||
| Nimesulide | 10 | −2.10 (1.00) | 50 | ||
| Naproxen | 10 | −1.85 (1.13) | 137.5 | ||
| Study and Groups | Non-NSAID (n) | NSAID (n) | ΔCAL in mm Non-NSAID (SD) | ΔCAL in mm NSAID (SD) | Dose (%) |
|---|---|---|---|---|---|
| 10 day-follow-up | |||||
| Buduneli et al., 2010 | 20 | 20 | −0.50 (1.98) | −1.20 (2.13) | 50 |
| Kurtis et al.,2007 | 33.3 | ||||
| smokers | 15 | 14 | 0.29 (0.51) | 0.01 (1.50) | |
| non-smokers | 15 | 14 | 0.01 (1.16) | 0.01 (1.31) | |
| 4 week/30 days-follow-up | |||||
| Buduneli et al., 2010 | 20 | 20 | −1.20 (1.91) | −1.50 (2.00) | 50 |
| Azoubel et al., 2008 | 10 | 10 | −2.23 (1.36) | −1.95 (0.71) | 100 |
| Özgoren et al., 2014 | 16 | 16 | −0.009 (1.02) | 0.00 (1.27) | 50 |
| 12 week-follow-up | |||||
| Vardar et al., 2003 | 10 | −1.3 (1.62) | |||
| Nimesulide | 10 | −1.35 (1.59) | 50 | ||
| Naproxen | 10 | −1.15 (2.02) | 137.5 | ||
| Yen et al., 2008 | 47 | 54 | 50 | ||
| moderate pockets | −0.83 (7.54) | −1.06 (7.42) | |||
| deep pockets | −1.46 (12.82) | −3.04 (11.83) | |||
| Study and Groups | Non-NSAID (n) | NSAID (n) | ΔPI Non-NSAID (SD) | ΔPI NSAID (SD) | Follow-up | Max. Dose/Day (%) |
|---|---|---|---|---|---|---|
| Aras et al., 2007 | 17 | 17 | −0.7 (0.23) | −1.41 (0.18) | 6 weeks | 46 |
| Azoubel et al., 2008 | 10 | 10 | −65.82 (12.9) | −57.83 (20.2) | 30 days | 100 |
| Buduneli et al., 2010 | 20 | 20 | −74 (12.2) | −74 (34.9) | 10 days | 50 |
| −71 (11.2) | −64 (35.8) | 4 weeks | ||||
| Kurtis et al., 2007 | 15 | 14 | −1.48 (0.29) | −1.3 (0.53) | 10 days | 33.3 |
| Özgoren et al., 2014 | 16 | 16 | −0.79 (0.57) | −0.82 (0.56) | 4 weeks | 50 |
| Vardar et al., 2003 | 10 | 10 | −0.75 (0.12) | −0.7 (0.35) | 12 weeks | 50 |
| Yen et al., 2008 | 47 | 54 | −18.6 (25.0) | −20.9 (27.4) | 12 weeks | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartenmann, S.; Maier, N.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P. Effect of Adjuvant Use of NSAID in Reducing Probing Pocket Depth in the Context of Conventional Periodontal Therapy: A Systematic Review of Randomized Trials. Appl. Sci. 2020, 10, 7657. https://doi.org/10.3390/app10217657
Gartenmann S, Maier N, Wiedemeier DB, Attin T, Schmidlin P. Effect of Adjuvant Use of NSAID in Reducing Probing Pocket Depth in the Context of Conventional Periodontal Therapy: A Systematic Review of Randomized Trials. Applied Sciences. 2020; 10(21):7657. https://doi.org/10.3390/app10217657
Chicago/Turabian StyleGartenmann, Stefanie, Nicole Maier, Daniel B. Wiedemeier, Thomas Attin, and Patrick Schmidlin. 2020. "Effect of Adjuvant Use of NSAID in Reducing Probing Pocket Depth in the Context of Conventional Periodontal Therapy: A Systematic Review of Randomized Trials" Applied Sciences 10, no. 21: 7657. https://doi.org/10.3390/app10217657
APA StyleGartenmann, S., Maier, N., Wiedemeier, D. B., Attin, T., & Schmidlin, P. (2020). Effect of Adjuvant Use of NSAID in Reducing Probing Pocket Depth in the Context of Conventional Periodontal Therapy: A Systematic Review of Randomized Trials. Applied Sciences, 10(21), 7657. https://doi.org/10.3390/app10217657






