Abstract
Hypersaline tidal flats (HTF) are ecotones associated with mangrove ecosystems in arid and semiarid coasts. They are predominantly vegetated by halophytes which are related to environmental stabilization and fauna protection. Some plants thrive in HTF by modifying soil biogeochemical conditions at their rhizospheres, expanding across barren soils. Thus, we aimed to study rhizospheric and the adjacent bulk soils of the three most abundant plant species in HTF under a seasonal semiarid climate of northeastern Brazil. We analyzed both rhizospheric and bulk soils of vegetation patches in wet and dry seasons. We found that HTF soils are a heterogeneous system highly influenced by water availability and plant activity. The soils were mostly sandy textured, containing low C and N contents, and hypersaline conditions. Comparing bulk and rhizospheric soils, we concluded that plants changed their own rhizosphere by creating nutrient pools to improve survival under saline conditions. Seasonal changes also affected soil biogeochemical processes in HTF, mainly the bulk soils by changing water availability. Understanding rhizospheric changes by halophytes, their expansion over barren soils, and the amelioration of soil physicochemical conditions, are fundamental to provide support for preservation and management of coastal ecosystems, including HTF.
1. Introduction
Halophyte plants are able to thrive in extreme environmental conditions of coastal areas, such as hypersaline tidal flats (HTF) [1,2]. HTF are evaporative environments (referred as Apicun in Brazil) found in semiarid or seasonal-dry coasts around the globe, and at the confluence of terrestrial/estuarine/marine systems, that contain soils with elevated salinity and pH usually greater than 7.0 [3,4,5]. In these ecosystems, plant growth is strongly limited by fluctuations in temperature, availability of fresh- and tidal-water, salinity, and nutrients [6]. Consequently, species that colonize HTF, mostly grass and herbaceous halophytes, occupy a patchy pattern [7], and have some physiological, morphological, and anatomical adaptations to face the harsh environmental conditions [8,9,10].
To colonize these saline environments, plants may create some adaptation at the “plant-rhizosphere-soil” system, by altering the soil properties through root’s activity [11], in order to increase nutrients availability at the rhizosphere compared to nonrhizospheric (bulk) soils [12]. Besides promoting conditions for the colonization, changes in the rhizosphere may have ecological implications in controlling ecological successions [11]. In fact, the first species to colonize the ecosystem (i.e., the “foundation species”) are critical species that defines much of the structure of a community [13]. For example, marsh plants on saline soils have strong ameliorating effects by reducing soil evaporation and accumulation of soil salts, which creates positive forces on natural community [14]. Thus, investigations regarding rhizospheric soils may not only increase the knowledge about the plants effects on natural occurring soil processes, but also shed light on their ability to change the soil environment on their benefit, and the mechanisms for ecological succession [15].
Since HTF are characterized by a short and sparce vegetation cover, they are strongly vulnerable to human impacts and degradation [7,16]. Indeed, these ecosystems are subjected to increasing rates of anthropogenic disturbances, such as aquaculture (e.g., shrimp farming), domestic sewage disposal, and salt producing ponds [6,17]. The HTF adjacent to mangrove forests are also threatened by those human activities and land use expansion posing serious concerning for these intertwined coastal ecosystems [18,19,20,21]. Contrasting with the adjacent mangrove forests [7,22,23], the HTF soils endure in extreme physicochemical (high pH and salinities) and geochemical conditions [2,24]. Recent work suggests that HTF may be formed in response to the dieback of mangroves, which is accompanied by substantial changes in the soil environment [1]. Contrastingly, mangrove forests and salt marshes can replace the HTF, if hydrological conditions change in response to input of freshwater and the rainfall increases [23].
In fact, rain and drought events regulated by seasonal changes strongly influence the edaphic components of coastal ecosystems soils and, as a consequence, several ecological processes and ecosystem services [25,26,27]. Semiarid seasonal precipitation induces changes in pH, organic matter content, and nutrient bioavailability for plants in hypersaline environments [1,28]. In HTF, the bare soils are usually more influenced by seasonality than the more vegetated soils, which may influence nutrient dynamics [29].
Our study aims to characterize soil changes induced by plant activity on the soils of adverse environments for plant development, such as the hypersaline tidal flats (HTF). We hypothesize that soil biogeochemical changes are more pronounced in the rhizospheric soils, compared to nonrhizospheric (bulk) soils, driven by plants changings the soil characteristics at the rhizospheric zone in order to reduce edaphoclimatic stresses. To accomplish these objectives, physical and chemical parameters (such as: grain size composition, Eh, pH, exchangeable and soluble cations, total C, N, S, salinity, exchangeable NH4+, NO3−, and partitioning of solid-phase Fe) were analyzed in rhizospheric and nonrhizospheric (bulk) soils under three dominant plant species in hypersaline tidal flats of the semiarid Brazilian Coast (Ceará State) in two seasons (dry and wet), providing important information on plant-soil relationships through biogeochemical processes. To our knowledge, there are no studies that have examined both the physicochemical and nutrient changes induced by rhizosphere and seasonal fluctuations in HTF soils, demonstrating the novelty of the present research.
2. Materials and Methods
2.1. Study Site Description
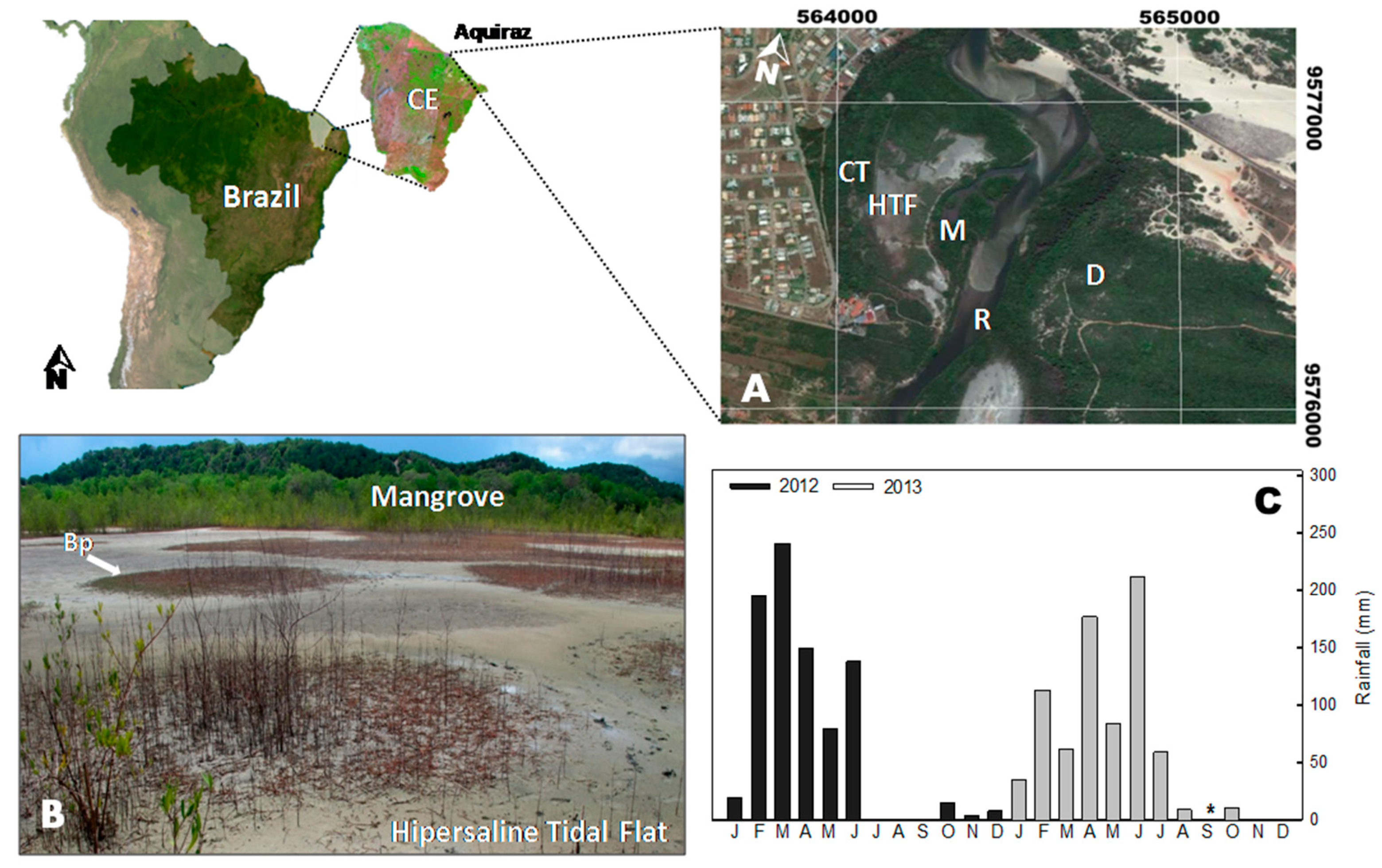
The study area is located in an Environmental Protection Area at the Pacoti River estuary, Ceará state, NE-Brazil (Figure 1A). The Pacoti River extends for 150 km of stream and contains two large reservoirs built to supply freshwater for human consumption. These reservoirs decrease the freshwater flow resulting in an increase of soil salinity at the estuary [30].
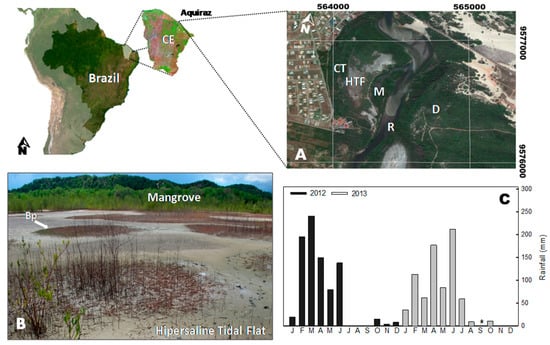
Figure 1.
(A) Map of the study site (Pacoti River estuary, Aquiraz, Ceará state, Brazil), distinguishing the coastal tableland (CT), the studied hypersaline tidal flat (HTF), the associated mangrove (M), the Pacoti river (R), and the costal dunes (D). (B) Landscape of the studied site, showing the patchy distribution of Blutaparon portulacoides (Bp) and mangrove forest at the background. (C) Average monthly precipitation of the Pacoti estuary for 2012 and 2013 (studied years). Data from FUNCEME database of historical precipitation records. The * represents missing data.
The studied HTF is influenced by a tropical semi-arid climate, with mean annual temperatures ranging from 26 to 28 °C [18,31]. The rainfall is distributed in two seasons, as follows: a rainy summer (from February to June) and a dry season (from July to January) with mean annual rainfall of up to 1200 mm [30]. The tides on the estuary are semidiurnal, with maximum tidal amplitudes of about 3.1 m and a minimum of 0.9 m [32]. In a previous study, the soils of the studied HTF were classified as Tidalic Gleyic Sodic Protic Arenosol (Protocalcic), according to the WRB-FAO soil classification system, and as GLEISSOLO SáLICO sódico típico according to the Brazilian soil classification system [33].
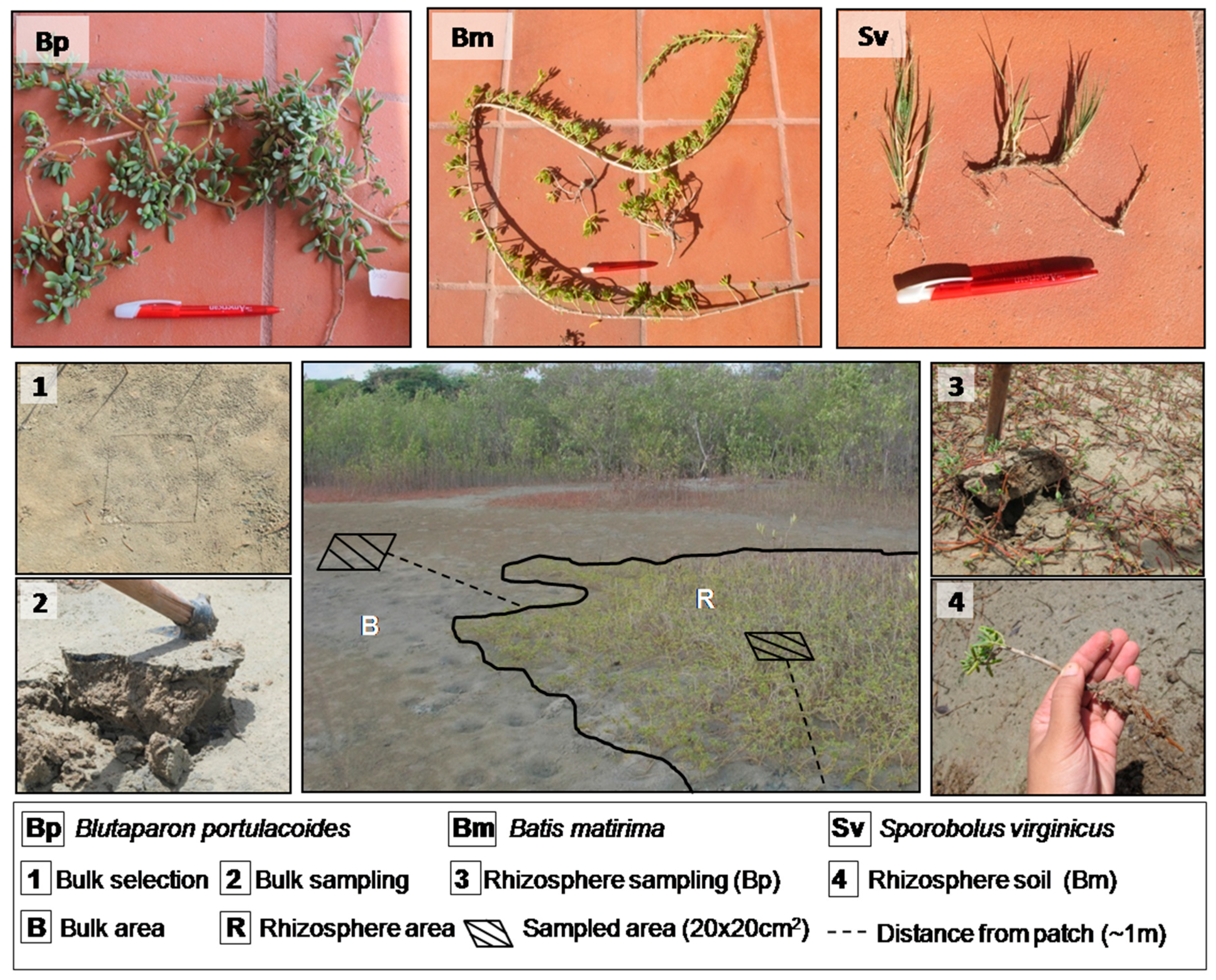
Due to the hypersaline conditions, the HTF present a patchy-distributed vegetation, which allowed the distinction of vegetated and bare soil (Figure 1B). We studied the three most dominant plant species in HTF of Ceará coastal region, as follows: Batis maritma (C. Linnaeus), Blutaparon portulacoides (St. Hil.), and Sporobolus virginicus (C. Linnaeus) K. Kunth (Figure 2). For each of these plants, rhizospheric and nonrhizospheric (bulk) soils were sampled. The studied plant species are clonal plants typical from these saline environments and normally considered as pioneer species able to colonize and stabilize flats and dunes [34,35,36].
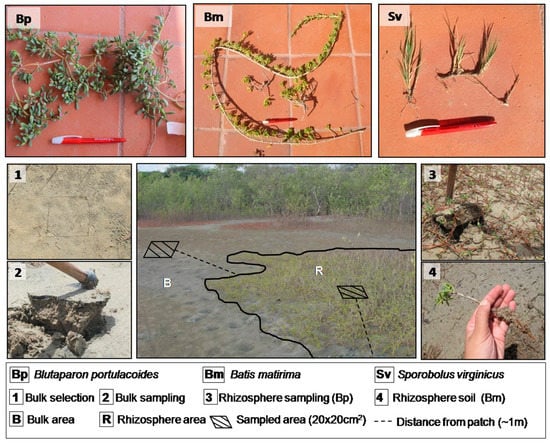
Figure 2.
Studied plant species and soil sampling in the hypersaline tidal flat (HTF) of the Pacoti estuary.
In order to facilitate comparisons and interpretations, the samples were named according the species (i.e., Batis maritima—Bm; Blutaparon portulacoides—Bp; and Sporobolus virginicus—Sv), with the indication of rhizospheric (R) or bulk (B) soil sample (e.g., BmR refers to rhizospheric samples from Batis maritima whereas SvB refers to bulk soil of Sporobolus virginicus).
2.2. Soil Sampling and Field Measurements
The rhizospheric and bulk soil samples were collected in November (dry season) and April (wet season) (Figure 1). The rhizospheric soil (composed of particles directly attached to the roots) were collected in triplicates and were carefully separated from plant roots (B. maritima, S. virginicus and B. portulacoides). The bulk soil samples were separately collected in triplicate for each species outside the patches, within one meter from the board of the vegetation patch (Figure 2). The samples were collected during both seasons (dry and wet), accounting for a total of 36 samples. Bulk soil samples were collected from 0–20 cm soil depth, stored in plastic bags, and transported to the laboratory under refrigeration (approximately 4 °C). Each sampling site was georeferenced using a portable GPS and the coordinates of each site were used for the measurement of the distance to the nearby river using geoprocessing techniques. At the laboratory, the soil adhered to the roots were removed for analysis by gently shaking and brushing the soil-rhizosphere system [37,38,39,40]. Subsequently, the samples of bulk and rhizospheric soil were air-dried, crushed, and 2-mm sieved, whereas subsamples were frozen for posterior determination of iron forms by a sequential extraction procedure.
Redox potential (Eh) was measured in situ by using a portable Eh-pH electrode (Hanna, model HI98121). The Eh values (mV) were obtained after equilibrating the electrodes for 2 min and the final readings were corrected by adding the potential (+244 mV) of a calomel reference electrode.
2.3. Analytical Procedures
The air-dried soil subsamples were used for the determination of grain size composition; pH; total contents of Organic Carbon (TOC), Nitrogen (TN), and Sulfur (TS); NH4+; NO3− available P; cations exchange capacity (CEC); electrical conductivity (EC); exchangeable and soluble cations. The pH (in water; 1:2.5 solid:liquid ratio) was measured with a glass electrode calibrated using pH 4.0 and 7.0 standards. EC was measured with a conductivity meter (Crison Basic 30), whereas the soluble cations (Na+, Ca2+, Mg2+, K+) were determined by atomic absorption spectrophotometry (AAS; Perkin Elmer 1100B) in soil solution obtained by centrifugation (3.500 RPM during 15 min) immediately after soil sampling. TOC, TN, and TS were determined with a LECO CNS-2000 auto-analyzer (LECO Corp., St. Joseph, MI, USA).
The grain size distribution was determined by using a combination of physical (overnight shaking) and chemical (0.015 M (NaPO3)6 + 1.0 M NaOH) dispersal methods [41]. To determinate exchangeable cations, the air-dried samples were washed with 60% (v/v) aqueous ethanol to remove soluble salts until the silver nitrate test (AgNO3 0.05N) indicated the absence of chloride before chemical and physical analyses [42]. Exchangeable K+ and Na+ were extracted with HCl 0.05 M and determined by flame photometry, whereas exchangeable Ca2+ and Mg2+ were extracted with KCl 1 M (1:5 soil/solution) and determined by AAS [43]. Cation exchange capacity (CEC) was calculated as the sum of exchangeable cations (K+, Na+, Ca2+, and Mg2+) [42]. The equivalent sodium percentage (ESP) was calculated as the percentage of exchangeable sodium divided by the cation exchange capacity. The calcium carbonate equivalent (CCE) was obtained by the AOAC method [44]. Available P was extracted with Mehlich-3 [45], whereas NH4+ and NO3− were extracted with KCl 2 M and determined by colorimetry [46].
Additionally, a partitioning of the solid-phase Fe was performed using fresh samples based on a combination of methods [47,48], which enables the identification of six operationally distinct fractions, defined as follow:
- (F1)
- Exchangeable and soluble Fe, extracted with MgCl2 1M (pH 7; 30 min shaking);
- (F2)
- Iron associated with carbonate extracted with NaOAc 1M (pH 5; 5 h shaking);
- (F3)
- Iron associated with poorly crystalline Fe (ferrihydrite) extracted with hydroxylamine 0.04 M + acetic acid 25% (6 h shaking at 30 °C);
- (F4)
- Iron associated with poorly crystalline Fe (lepidocrocite) extracted with hydroxylamine 0.04 M + acetic acid 25% (6 h shaking at 96 °C);
- (F5)
- Iron associated with crystalline oxyhydroxides extracted with sodium citrate 0.25 M + 0.25 M + sodium bicarbonate 0.11M + Sodium dithionite (30 min shaking at 75 °C);
- (F6)
- Iron associated with pyrite extracted with HNO3 (2 h shaking). This fraction was extracted following two pre-treatments (10 M HF and concentrated H2SO4), for the removal of Fe associated with silicate and organic matter.
The degree of iron pyritization (DOP), calculated as DOP (%) = (F6 × 100)/[(∑F1→F6)]. The DOP determines the percentage of Fe which is combined into the pyritic fraction, allowing the comparison of pyrite content in soils with different reactive iron contents [49].
2.4. Statistical Data Analysis
Analyses were performed with the software SPSS version 21 (SPSS Inc. Chicago, IL, USA). Nonnormal data were log-transformed to meet conditions of normality and homogeneity of variance. To test the effects of rhizosphere on soils and to compare with bulk soils, we performed a one-way analysis of variance (one-way ANOVA). A two-way ANOVA was used to assess the main effects of seasons, three plant species and their interaction on soil parameters. Two-way ANOVA tests were followed by Bonferroni’s post hoc comparison tests (at 5% probability for statistical significances). Furthermore, a discriminant analysis (DA) was performed to identify an optimal differentiation (maximize the variance) among the plant species, among rhizosphere and bulk soil, and the seasons, identifying the most important variables for the group distinction.
3. Results
3.1. Differences between Rhizospheric and Bulk Soils
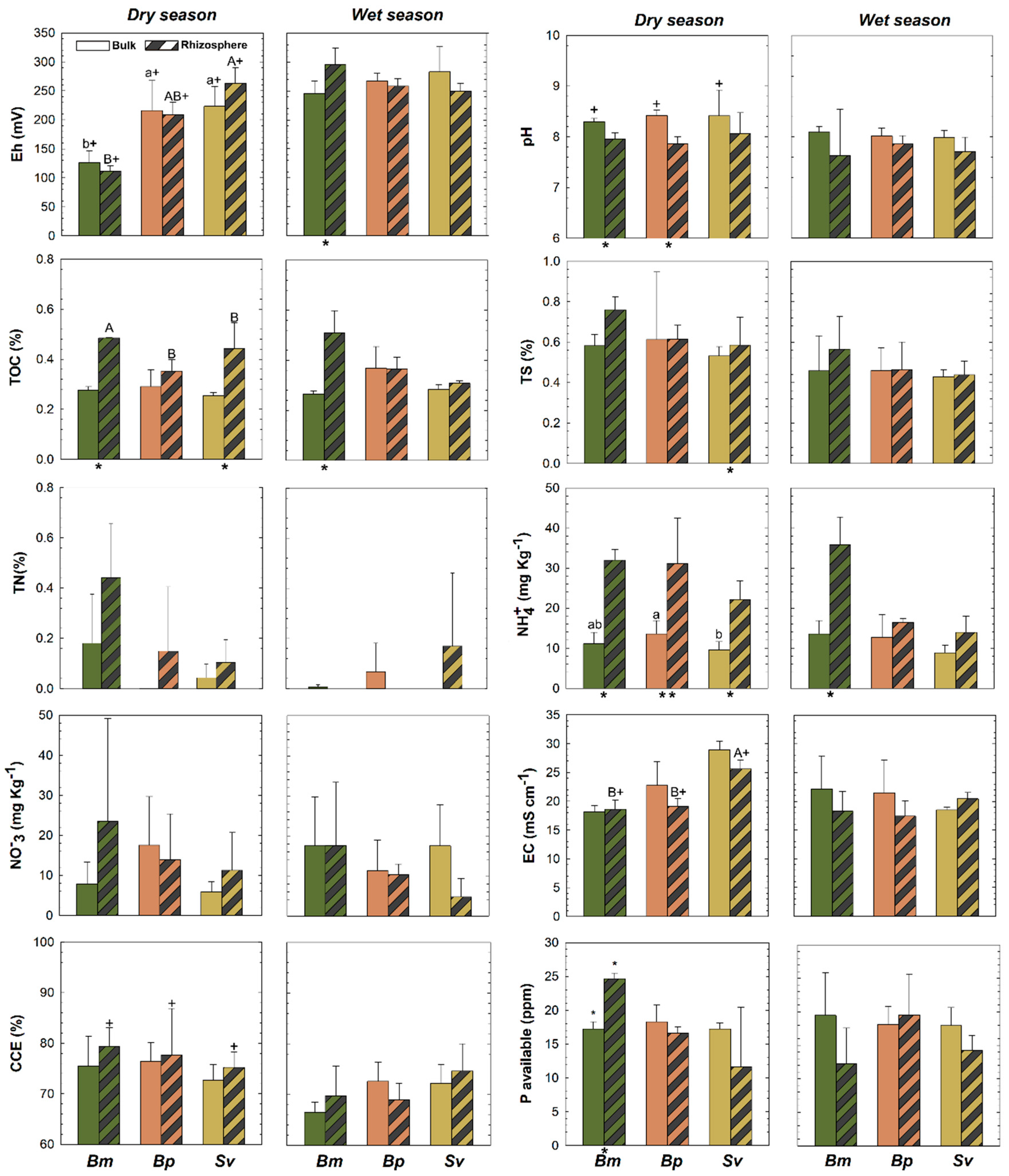
Rhizospheric (R) and bulk (B) soils showed statistical differences (p < 0.05) in some soil physicochemical properties in both dry and wet seasons (Figure 3). The pH was lower in rhizospheric soils (7.7 ± 0.6 and 7.8 ± 0.3, for BmR and BpR, respectively), when compared to the bulk soils (8.2 ± 0.1 and 8.2 ± 0.1, for BmB and BpB, respectively), in the dry season (except for Sv; p > 0.05). Significant differences were recorded for redox potentials (Eh) between BmR and BmB, in the wet season, but no changes in Eh between R and B for the three plants in the dry season (Figure 3)
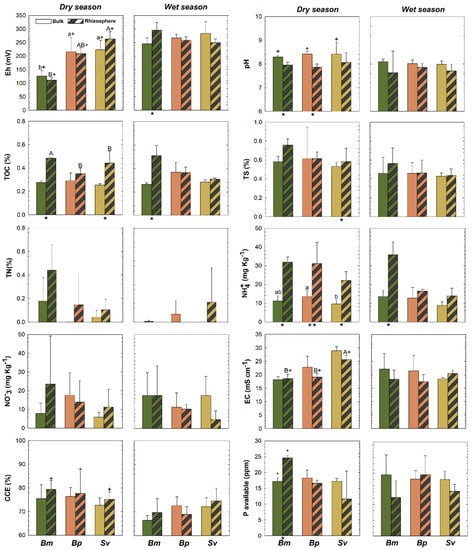
Figure 3.
Soil properties for rhizosphere and bulk soils for Batis maritima (Bm), Blutaparon portulacoides (Bp), and Sporobolus virginicus (Sv) contrasted between dry and wet seasons in the hypersaline tidal flat. TOC: Total Organic Carbon, TN: Total Nitrogen, TS: Total Sulfur, EC: Electric Conductivity, and ESP: Exchangeable Sodium Potential. Different lowercase letters indicate significant differences at the 5% probability among different plants in the bulk soils. Different uppercase letters indicate significant differences at the 5% probability among different plants in the rhizospheric soils. The symbol (*) represents significant difference (p < 0.05) between bulk and rhizospheric soil for each plant and season. The symbol (+) represents significant seasonal variation (p < 0.05) comparing bulk and rhizospheric soils for each plant species. The absence of the symbols (lowercase letters, uppercase letters, + and *) represents no statistically significant difference between the studied parameters.
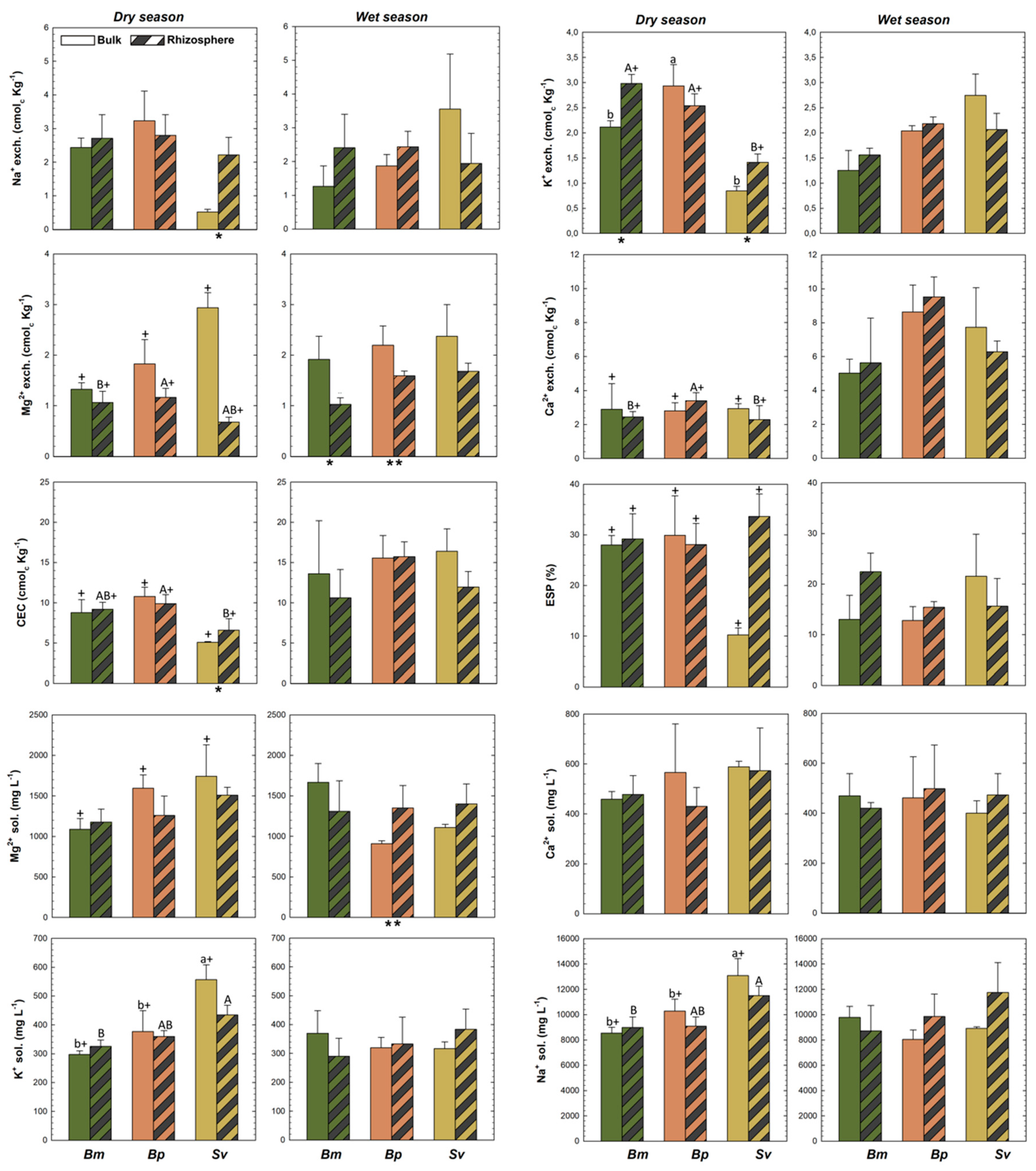
Higher TOC contents were recorded for BmR than BmB in both wet and dry seasons, and higher in SvR than SvB at the dry; and no differences in TN across plants, but a higher TS in SvR compared to SvB at dry (Figure 3). Additionally, a significant increase in NH4+ contents for Bm and Bp were recorded for the rhizospheric soils compared to bulk soils, but no differences in NO3−, available-P, CCE, EC, ESP, and exchangeable-Ca2+ in both seasons (Figure 3 and Figure 4). Soil nutrients also presented differences between rhizosphere and bulk soils due to seasonal variation. We observed greater content of exchangeable K+ and Na+ and CEC in SvR compared to SvB, and greater exchangeable K+ and Mg2+ for BmR than BmB, all for the dry season (Figure 4). Contrarily, exchangeable-Mg2+ was greater in bulk than rhizospheric soil for Bm and Bp in the wet season, and no significant changes were observed in exchangeable-Ca2+ and ESP (Figure 4).
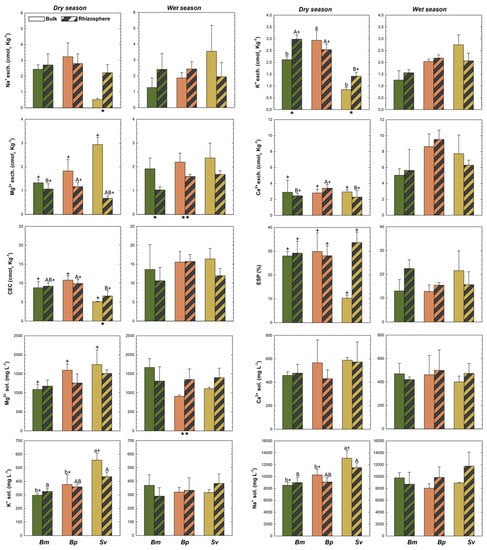
Figure 4.
Soil properties for rhizosphere and bulk soils for Batis maritima (Bm), Blutaparon portulacoides (Bp), and Sporobolus virginicus (Sv) contrasted between dry and wet seasons in the hypersaline tidal flat. CEC: Cation exchangeable capacity; exch.: exchangeable; sol.: soluble. Different lowercase letters indicate significant differences at the 5% probability among different plants in the bulk soils. Different uppercase letters indicate significant differences at the 5% probability among different plants in the rhizospheric soils. The symbol (*) represents significant difference (p < 0.05) between bulk and rhizospheric soil for each plant and season. The symbol (+) represents significant seasonal variation (p < 0.05) comparing bulk and rhizospheric soils for each plant species. The absence of the symbols (lowercase letters, uppercase letters, + and *) represents no statistically significant difference between the studied parameters.
Soils across the three plant species, and for both rhizospheric (R) and bulk (B) soils, were predominantly sandy, and we found no statistical differences in texture between R and B for the three plant species for sand, silt, and clay particle sizes (Table S1). The overall average content for all soils was 871 g kg−1 of sand, 92 g kg−1 of silt, and 37 g kg−1 of clay.
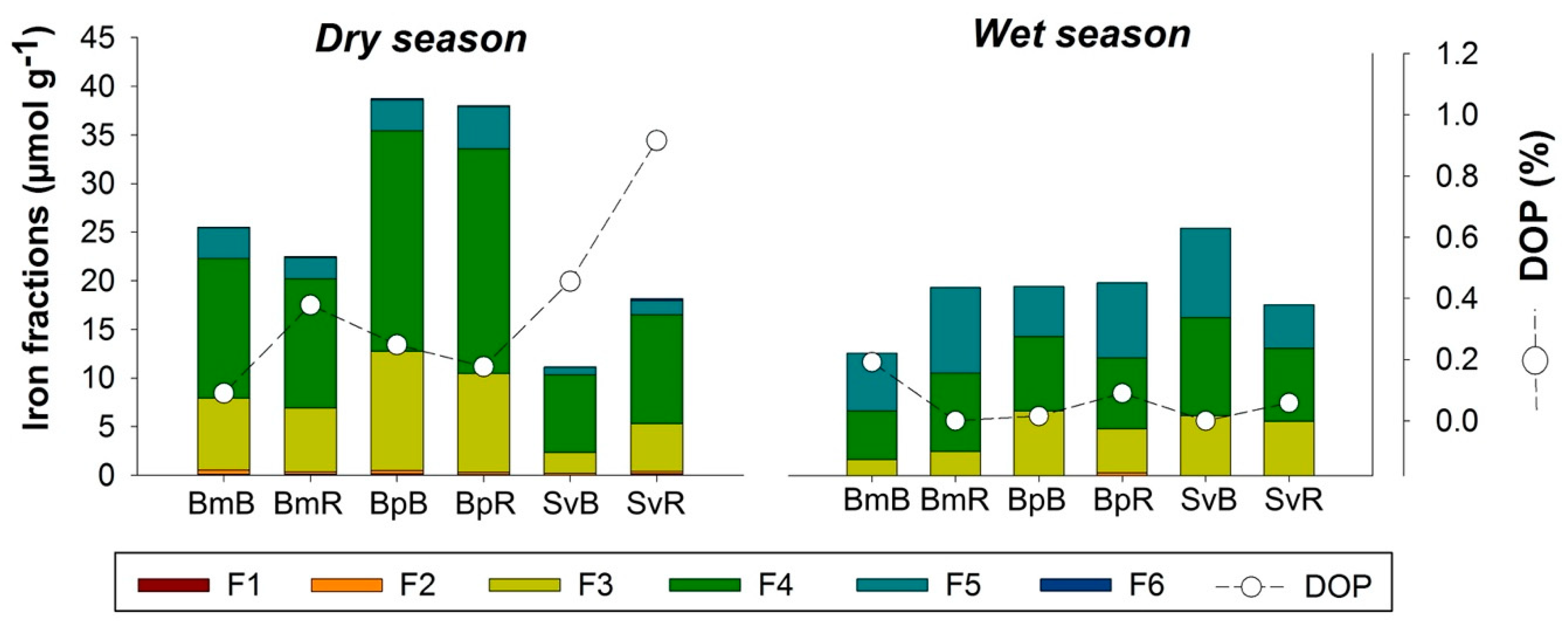
The Fe partitioning and the Degree of Pyritization (DOP) in rhizospheric soils showed contrasting differences in relation to bulk soil (Figure 5). In the dry season, the DOP was higher in the rhizospheric soil (compared to bulk soil) for the plants Bm and Sv, but did not change for Bp, which contained the greatest amount of total Fe (all fractions) (Figure 5).
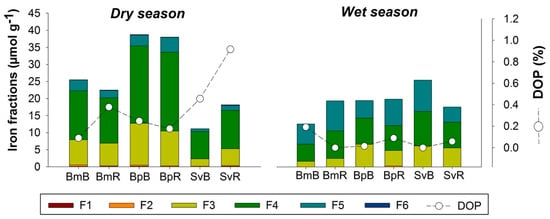
Figure 5.
Iron (Fe) fractions obtained in sequential extraction for bulk and rhizospheric soils in three plant species and two seasons in the hypersaline tidal flat. (F1) Exchangeable and soluble Fe; (F2) Iron associated with carbonate; (F3) Iron associated with poorly crystalline Fe (Ferrihydrite); (F4) Iron associated with poorly crystalline Fe (Lepidocrocite); (F5) Iron associated with crystaline oxyhydroxide; (F6) Iron associated with Pyrite; (DOP) Degree of Pyritization. Plant species are represented by Bm: Batis maritima; Bp: Blutaparon portulacoides; Sv: Sporobolus virginicus. Seasonality represented by d: dry season; w: wet season.
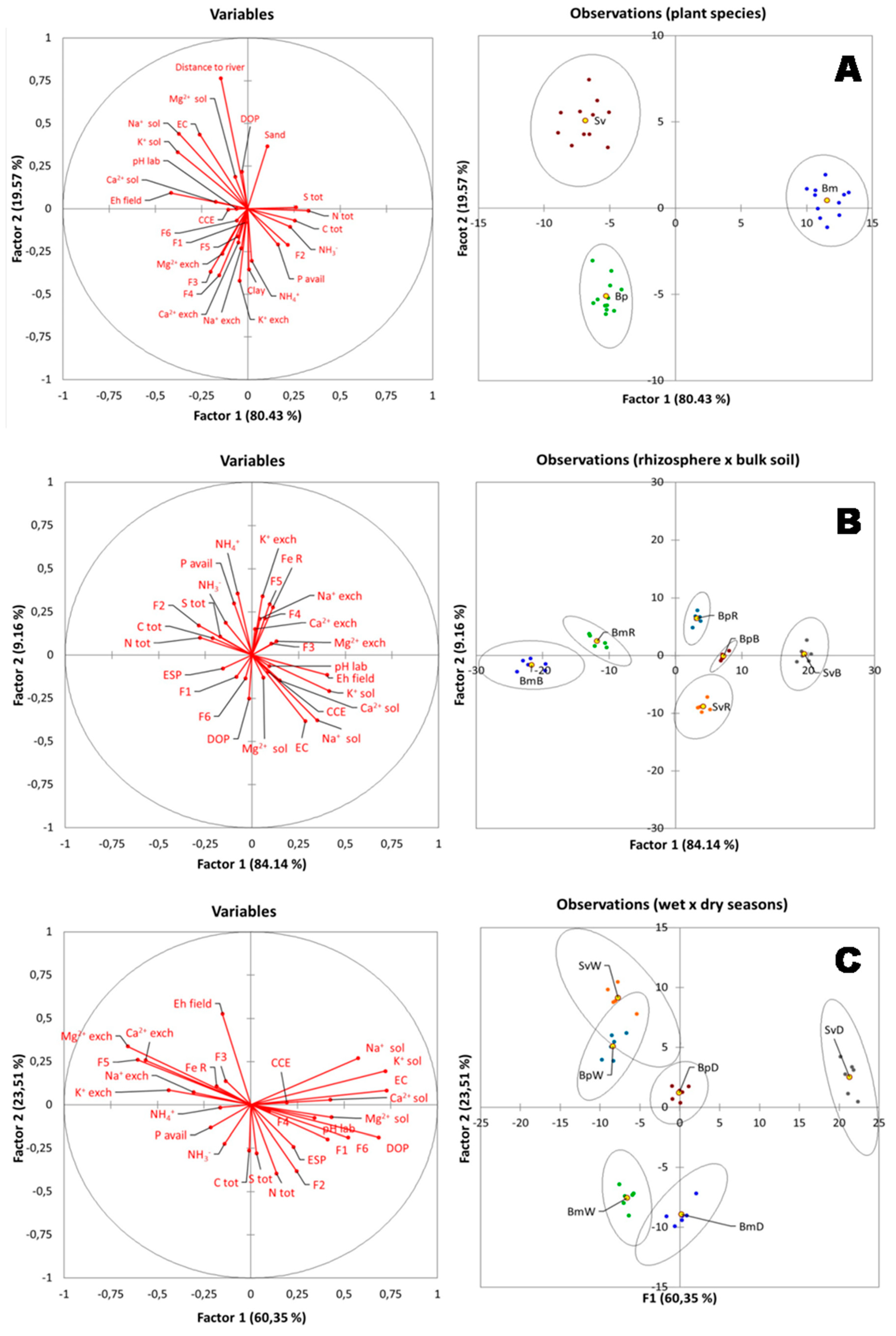
Soil chemical characteristics associated with rhizospheric soils were different than bulk soils for the tree plant species (Figure 6B). Based on the total variance of 84.14% in the DA, one can conclude that the rhizosphere of the plants BmR, BpR, and SvR were considerably different. In contrast, Sv and Bp bulk soils presented a slight difference, with a variance of 9.16% (Figure 6B). Moreover, the Bm and Bp rhizospheric soil samples (BmR and BpR, respectively) are associated with higher contents of NH4+, exchangeable K+, crystalline Fe oxides (F5) and available-P (Figure 6B). On the other hand, the rhizospheric soil from Sv (SvR) is associated with soil salt-related properties (EC, ESP and soluble Na+), and to soluble cations (Na+, Ca2+, Mg2+, K+) (Figure 6B).
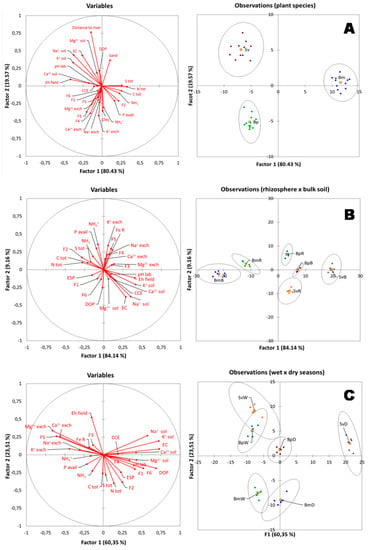
Figure 6.
(A) Discriminant analysis (DA) of plant species distribution and soil properties in the hypersaline tidal flat (HTF). (B) Discriminant analysis (DA) contrasting rhizospheric and bulk soils properties. (C) Discriminant analysis (DA) for rhizospheric and bulk soils properties during dry and wet season. Plant species are represented by Bm: Batis maritima; Bp: Blutaparon portulacoides; Sv: Sporobolus virginicus. Soil proprieties are Exch.: exchangeable cations (Na+ exch; K+ exch; Ca2+ exch; Mg2+ exch); Sol.: soluble cations (Na+ sol; K+ sol; Ca2+ sol; Mg2+ sol); Tot: total (C tot; N tot; S tot); CEC: cations exchangeable capacity; ESP: exchangeable sodium percentage; EC: electrical conductivity; CCE: Calcium Cabonate Equivalent. Iron (Fe) geochemical fractionation, with the following fractions: F1: Exchangeable Fe; F2: Fe bound to Carbonate; F3: ferrihydrite Fe; F4: lepidocrocite Fe; F5: Crystalline Fe; F6: Pyritic Fe; DOP: Degree of Pyritization.
3.2. Soil Characteristics among the Three Plant Species in HTF
We observed statistically differences (p < 0.05) in redox potential (Eh) for Bp and Sv compared to Bm in the dry season for both rhizospheric (R) and bulk (B) soils (Figure 3). On the other hand, there were no differences in pH, and total contents of C, N, and S (TOC, TN, and TS) among the soils vegetated by the three halophytic plants (Figure 3). Nutrients contents showed some changes among the different soil-plant systems (Figure 3). NH4+ contents were higher in BmB (Bm in bulk soil) than BpB and SvB (Bp and Sv in bulk soils); but NO3−, available-P, and calcium carbonate equivalent (CCE) did not differ statistically (Figure 3). Regarding the exchangeable cations, we observed higher K+ and lower Mg2+ in BmR and BpR when compared to BpR (R for rhizospheric soils of Bm, Bp, and Bp), but higher K+ in BpB than BmB and SvB (Figure 4). For the soluble cations (Na+, Ca2+, Mg2+, K+), the monovalent soluble-K+ and -Na+ contents were higher in Sv than Bm and Bp in both rhizospheric (R) and bulk (B) soils, but no differences in the divalent soluble-Mg2+ and -Ca2+ and in the values for CEC and ESP (Figure 4).
The three studied plant species were efficient in altering the soil Fe fractions especially during the dry season (Figure 5). In the dry season, Bp presented the greatest amount of pseudo-total Fe (sum of all Fe fractions), followed by Bm and then Sv, demonstrating a predominance of oxyhydroxides (F3, F4, and F5) because of the drier environment (Figure 5).
Furthermore, differences in soil characteristics were observed among the three plant species by the discriminant analysis (DA) in the studied HTF (Figure 6A). For instance, the Bp plants are associated with soils containing higher values of exchangeable cations (mainly Ca2+, Mg2+, and K+) and low-crystallinity Fe-oxyhydroxides (fractions F3 and F4). On the other hand, the Sv are associated with higher salt contents (e.g., EC and soluble cations; Figure 6A). The Bm is associated with sites with higher contents of TOC and TN, and with lower Eh values, when compared to the other plant species (Figure 6A).
3.3. Seasonality and Soil Physicochemical Changes in HTF
The wet and dry seasons of the Brazilian tropical semiarid coast generated changes in most of the soil physicochemical properties (Figure 3 and Figure 4), mainly for the bulk soils. In contrast, TOC, TN, and CCE were steadier when facing season changes, with no significant differences observed (Figure 3). The pH and exchangeable cations seasonal changes on bulk soils were noteworthy (Figure 3). The pH for the bulk soils was more alkaline in dry seasonal (BmB: 8.2 ± 0.07; BpB: 8.4 ± 0.1; SvB: 8.4 ± 0.5) compared to the wet season (BmB: 8.09 ± 0.1; BpB: 8.01 ± 0.1; SvB: 7.9 ± 0.1). The EC in bulk soils ranged from 17.6 to 29.9 mS cm-1 (Figure 3). During the dry season (lower soil moisture contents), higher EC values were observed (Bm: 22.1 ± 5.7; Bp: 22.7 ± 1.4; Sv: 28.9 ± 1.5 mS cm−1) when compared to the wet season (Bm: 18.1 ± 1.8; Bp: 17.1 ± 1.4; Sv: 18.9 ± 0.5 mS cm−1; Figure 3).
For the rhizospheric soils, some properties were influenced by seasons in a lower magnitude. A similar trend of bulk soils was registered, mainly related to Eh and EC values (Figure 3). Suboxic soil conditions prevailed both during the dry season (BmR: 111 ± 9.8; BpR: 208 ± 21.5; SvR: 263 ± 26.8 mV) and the wet season (BmR: 296 ± 28.5; BpR: 258 ± 12.7; SvR: 249 ± 13.4 mV). Additionally, seasonal changes also significantly affected (p < 0.05) the soluble contents (K+, Na+, Mg2+) in bulk soils (Figure 4), so that during the dry season the contents of soluble K+, Na+, and Mg2+ for Bm decreased respectively from 370 ± 78, 9783 ± 860, and 1666 ± 232 mg L−1 to 297 ± 12, 8545 ± 449 and 1088 ± 133 mg L−1. On the other hand, the bulk soils of Bp and Sv presented the opposite trend, with higher soluble- K+ (Bp: 377 ± 20 mg L−1; Sv: 556 ± 51 mg L−1), soluble-Na+ (Bp: 9783 ± 860 mg L-1; Sv: 13,083 ± 1342 mg L−1) and soluble-Mg2+ (Bp: 1593 ± 165 mg L−1; Sv: 1741 ± 388 mg L−1) were registered during the dry season (Figure 4).
Moreover, plant species at the studied HTF seem to be associated with different soil properties within each season (Figure 6C). Samples collected over the dry period (BpD; SvD and BmD) were correlated to soluble cations and EC, and the Fe forms F1 (Exchangeable and soluble Fe), F2 (Fe associated with carbonates), and F4 (Fe in the low-crystalline form, Lepidocrocite). On the other hand, samples from the wet period (BpW, BmW, and SvW) were mostly associated with high Eh values, exchangeable cations, and more stable Fe forms, i.e., crystalline Fe-oxyhydroxides (F5). The soil properties that favored the clustering related to Bm species, in both seasons, were correlated to available P and organic carbon (Figure 6C). While Sv and Bp was correlated to soluble cations in the dry season, and to exchangeable cations in the wet season (Figure 6C).
4. Discussion
4.1. Rhizospheric and Bulk Soils in HTF
We observed that plants in HTF under seasonal fluctuation were forced to invest in surviving mechanisms by altering soil’s physicochemical conditions at the rhizosphere to favor better nutrient utilization. Thus, the rhizosphere may concentrate elevated rates of chemical and biological activity, which controls nutrient biogeochemical cycles in HTF [50]. As the vegetation succeeds and colonizes the bulk soil, biogeochemical changes are promoted in the rhizosphere-soil interface, by decreasing soil electrical conductivity (EC) and bulk density, and increasing the C and N contents [3]. For instance, in an HTF of coastal China, the rhizopheric soil demonstrated greater diversity of bacterial communities compared to the nearby bulk soil, and diversity also increased in the lowlands of the tidal flats [51]. Thus, studies comparing the rhizospheric and bulk soils are important to increase the understanding for the plant’s effects in changing natural occurring soil processes and the mechanisms for ecological succession.
In our study, the Sv and Bp plants presented similar results for bulk soils, but their rhizospheric soils were strongly separated based on the discriminant analysis (Figure 6B). The Bp samples were more related to NH4+ availability and exchangeable K+, while Sv more related to soluble K+ (Figure 6B). These distinct correlations demonstrate that Sv and Bp plants probably use different strategies to survive in an HTF, which could be related to a facilitation process [34,35,52]. Facilitation is a positive interaction resulting from amelioration in the abiotic environment or interference by other organisms [52]. In arid and semi-arid environments, the amelioration of microenvironmental conditions is a key process for structuring plant communities [53].
Bulk soil characteristics of our study were consistent with the soil conditions found in other HTF soils, i.e., neutral-basic pH, hypersaline, sandy-textured soils [1,28]. The predominance of sandy textures was also reported in other studies of similar environments [28], and related to the transportation of sandy material by wind, tides, and inputs from higher inland (terrestrial) areas [54]. The high contents of CCE (Figure 3) are correlated to the precipitation of CaCO3, as a less soluble salt, at the soil surface [55]. The observed TOC and TN rarely varied over ±0.5%, which was also observed by in the superficial horizons of other HTF soils [28]. This low C and N contents is related to the low plant-derived inputs, since the area of occurrence for the bulk soils is completely barren of vegetation (Figure 1 and Figure 2).
Another characteristic for the observed bulk soils in HTF is related to the predominance of Fe-oxyhydroxides of low-crystallinity (F3 and F4 fractions of Fe) and high-crystallinity (F5 fraction of Fe) and the low DOP (<1%) values (Figure 5). This Fe geochemistry fractionation reflects the suboxic environmental conditions in response to the low frequency of tidal flooding. Another study also found a dominance of Fe-oxyhydroxides in the fractions F3 to F5, which ranged between 79 to 143 μmol g−1 at upper 15 cm of soil profiles), and DOP values below 1% [28].
Further studies should be conducted to understand the mechanisms of plant colonization in HTF, and to provide support for environmental restoration practices in highly saline areas. Consequently, HTF are heterogeneous environments, highly susceptible to spatial and temporal soil physicochemical changes, due to plant rhizospheric and climatic seasonal influences.
4.2. Plant Effects on Biogeochemical Soil Properties in HTF
Our results demonstrated distinct edaphic processes ruled by the three dominant plant species in the studied HTF (Bm, Bp, and Sv), indicating changes in both bulk and rhizospheric soils. The first noteworthy rhizospheric process is the increase in nutrient retention capacity, which was also observed in other studies [56]. Another one is a clear decrease in pH within the rhizosphere, as a physicochemical process occurring at the soil-root interface [38,57,58]. The decrease in pH for the rhizospheric soils (when compared to bulk soils) is primarily promoted by the root’s release of H+, root exudation of organic acids, respiration, redox-coupled reactions, and by environmental constraints [57]. Indeed, Bm and Sv are plants capable of growing within a large range of pH (from 5.2 to 8.8), due to their ability to alter in-situ soil conditions [36].
The organic matter incorporation and nutrient bioavailability at the rhizosphere [3,51] is another remarkable process observed in our study (Figure 3). These processes were specific to each plant species and reflected on the TOC and NH4+ differences in rhizosphere when compared to bulk soils. The strong Bm-TOC association (Figure 6A) suggests that Bm is the plant contributing the most to build up soil organic matter contents, compared to Sv and Bp.
Halophytes plants are capable of using inorganic nitrogen forms (NH4+ and NO3−) to overcome highly salt environments, as typically observed by the greater contents of NH4+ and NO3− in rhizospheric soils [59]. The NH4+ contents in our study for the rhizospheric soils were up to three times greater than the bulk ones, while NO3− did not present significant differences between areas (Figure 3). Moreover, the NH4+ content in the studied plants was related to rhizosphere activity, due organic matter incorporation, corresponding to Bm soils, which contained the highest values of TOC and NH4+ among species in both seasons (Figure 3).
Plants may favor their growth conditions in a hypersaline habitat by altering soil conditions, and thus increasing the pool of NH4+ and other nutrients [56]. The topmost benefit of using NH4+ rather than NO3− is the decrease in water consumption by plants in this water-stressful hypersaline environment [60,61]. Clonal plants supplied with NH4+ were able to reduce water consumption, buffering the negative effect of water deprivation and causing an increase in plant performance [62]. Thus, the selective use of NH4+, instead of NO3−, by the studied species can ameliorate water use-efficiency and mitigate the negative effects of hypersaline conditions.
Together with plant activity and exudates, microbial communities are also greatly important for the biogeochemical cycling of N and other elements in humid coastal areas [21,26,63]. Microbial communities in HTF are predominantly halotolerant to halophilic microorganisms that may vary along the tidal flat in biodiversity, structure, and richness, as well as in association with different plants [64]. Indeed, investigations regarding microbial functioning and biodiversity in saline and hypersaline soils are rare [65]. The major microbial N pathways in humid coastal areas include nitrification and denitrification. Nitrification refers to the oxidation of ammonium (NH4+) to nitrite (NO2−) and nitrate (NO3−) under aerobic conditions, carried out by chemoautotrophs that uses NH4+ as energy source [66,67]. Denitrification comprises the reduction of NO2− and NO3− to gaseous N forms (such as N2O), which is mediated by heterotrophic bacteria under anaerobic conditions [68]. Some microorganisms also provide important ecosystem services to coastal hypersaline areas by actively fixing nitrogen [69]. Consequently, in these hypersaline environments, plants and microorganisms match up to alter N species to favor survivorship of both communities.
Plants located in areas far from the river, which are less inundated by flooding tides due microrelief variations, are able to live in the presence of high salinity and soluble cations, such as the Sv species (Figure 6). The Sv plants were proven to survive and flourish under high concentrations of Na+ and Mg2+ (125 to 450 mmol L−1), and to selectively absorb and retain K+ [34]. The accumulation and compartmentation of ions and essential nutrients (particularly K+) under high salinity is one of the major physiological strategies for salt tolerance in halophyte plants [70]. Consequently, the great development of Sv in more saline areas is related to more efficient use of K+ (and other nutrients) to improve their expansion under hypersaline conditions. This use efficiency is demonstrated in our data by a significant difference in K+ contents for Sv and other species, and between Sv bulk and rhizospheric soils (Figure 4).
The success of the three species in thriving at HTF systems is probably due to the two following factors: the ability to buffer the adverse conditions by developing nutrient pools (as K+ and NH4+) to overcome the high salinity, and the capacity of allocating resources by physiological integration and adaptation. The physiological integration means that stolon and rhizome connections allow translocation of resources and other substances between connected ramets growing in soil patches differing in quality [71,72]. The connection of ramets has been suggested as a mechanism of toxic dilution by the redistribution throughout the clonal system, decreasing toxicity [73]. This mechanism has also been suggested as a component of halophytes’ resistance to salt stress and could also contribute to explain the growth of clonal plants in the hypersaline conditions of our study.
4.3. Seasonal Effects on Soil Properties in HTF
The HTF soil properties are strongly related to the soil hydrological conditions, the tidal flooding events, and the climatic conditions of the region [1,16]. The soil properties and plant-soil interactions in HTF of the Brazilian semiarid coast are directly influenced by rainfall patterns concentrated within a few months of the year (Figure 1 and Figure 6C) and the low frequency of tidal flooding, which occur only twice a month during the spring tides [6,18]. We observed that soil parameters such as pH, Eh, EC, and exchangeable cations were significantly (p < 0.05) affected by these changes in the freshwater influx during the wet season (Figure 3, Figure 4, and Figure 6C). Consequently, changes in soil properties and an intense soil salinization are due to the extended period without fresh water inputs by rainfall, the elevated evapotranspiration demands in the dry season, and the upper topographic position of the HTF, which rule the tidal flooding frequency [6,7].
Thus, the intensification of salinity, and the large availability of soluble cations, during the dry season (Figure 5) are mainly caused by the low efficiency of these soil system to remove salts. Contrarily, the water input onto the system during the wet season decreases the salinity condition, reflect in changes on EC values and exchangeable cations contents (Figure 6).
Furthermore, the rhizospheric soils are majorly buffered by root’s conditions, so that the HTF plants maintain a certain stability through the wet-dry seasons, despite the significant changes in the pH of bulk soils between seasons. On the other hand, the rhizospheric soil conditions in our study is related to a combined effect of cations-anions exchange (mainly related to N content and dynamics) and redox-coupled process (mainly related to flooding events).
The redox potential (Eh) can be greatly affected by water dynamics and by root respiration in wetland systems [38]. The water input in HTF is very low, compared to other coastal wetlands, because spring tides are the major source of water inputs and rainfall decreases dramatically over the dry season in the Brazilian semiarid coast [6]. The Eh values were constantly positive (around +200 mV), indicating that HTF are under suboxic to oxic conditions. Alternation in redox conditions allows the reduction of Fe oxyhydroxides to Fe2+ and its subsequent oxidation [74,75], favoring the formation of low crystallinity Fe oxyhydroxides phases (F3 and F4) (Figure 5), which was also registered in other HTF of Ceará State (Brazil) [28]. The significant differences in Fe contents between wet-dry seasons indicates redistribution of Fe in the system, with Fe losses during the wet season (by dissolutive Fe reduction) and Fe increments in the dry season due to Fe oxidation and re-precipitation. Thus, the semiarid coastal seasonality has a strong influence in plant-soil dynamics and the fate of nutrients, C, N, and Fe phases.
Tropical HTF are considered strategic areas for mangrove expansion in response to regional and global environmental threads, such as climate change and sea-level-rise scenarios [2,30]. Plants in HTF are greatly capable of modifying their soil rhizospheric conditions, which can be useful to other species (i.e., creating facilitation [52] among species). For example, in coastal wetlands of Belize, the herbaceous vegetation facilitates the colonization of mangroves (Rhizophora mangle) in a disturbed area [76]. Mangrove and associated hypersaline areas are interdependent, but connected by water, sediments, nutrients, and organic matter fluxes, and also by animal populations that move between the different eco-components [2,77]. HTF are formed by a combination of hydric deficit and limited tidal flooding in an evaporative environment backing mangrove forests, and the ecosystems provided [5,27,78].
5. Conclusions
Our study revealed that biogeochemical soil properties in HTF of the Brazilian semiarid coast were affected by seasonality and the plant-rhizosphere-soil conditions. Consequently, the HTF can be characterized as a heterogeneous system that is highly influenced by water availability and by inhabiting plants capable of surviving under the suppressing hypersaline conditions.
Comparing bulk and rhizosphere soils, one concludes that HTF plants were able to modify their rhizosphere-soil interface, increasing the availability of nutrients, which brings survival advantage for them and other plants species. Each of the studied plants was developed under different soil conditions, which is probably related to their physiological requirements and metabolism. For example, Bm and Bp where related to NH4+, Bm to soil C and N contents, while Sv was highly correlated to soluble cations (such as K+ and salinity).
Seasonality directly impacted soil dynamics in HTF, such as the contents of exchangeable and soluble cations, EC, pH, and Eh values, mainly because of freshwater inputs. Despite the fact that rhizospheric soils were more affected by these seasonal changes, the plants adapted to maintain their rhizospheric environment were less variable to seasonal changes. Our results suggest that the combined effects of seasonal changes and plant action rule most of the biogeochemical processes in HTF, and that plants had the capacity to change the soil conditions to improve their development. Furthermore, our study provided essential information to improve the success of environmental restoration and preservation in salt affected and coastal soils.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/21/7624/s1, Table S1. Particle size distribution (sand, silt, and clay) in g kg−1 for both rhizospheric (Rz) and bulk soils for the three plant species (Bm, Bp, and Sv). There is no significant statistical difference (at 5% level) between Rhizospheric and Bulk soils for each species for sand, silt, and clay.
Author Contributions
Conceptualization, T.O.F., X.L.O., G.N.N., R.B.Z., and R.L.C.; methodology, T.O.F., X.L.O., S.R.R., R.B.Z., and R.L.C.; software, G.N.N., D.B., and R.L.C.; validation, T.O.F., D.B., S.R.R., X.L.O. and R.L.C.; formal analysis, R.L.C., G.N.N., D.B.; writing—original draft preparation, R.L.C., T.O.F., G.N.N., S.R.R., R.B.Z., X.L.O.; writing—review and editing, D.B. and G.N.N.; funding acquisition, T.O.F., X.L.O. All authors have read and agreed to the published version of the manuscript.
Funding
The first author would like to thank CAPES for the financial support to R.LC., T.O.F, and X.L.O. We thank the members of Núcleo de Pesquisa em Pedologia (Nuppe/UFC) for field and laboratory support and María J. Santiso for laboratory assistance. The authors are grateful for the financial support provided by National Council for Scientific and Technology Development (CNPq, process 305996/2018-5; 409593/2018-4); Consellería de Innovación e Industria-Xunta de Galicia (PGIDIT08MDS036000PR); Cross-Research in Environmental Technologies of the Santiago de Compostela University (CRETUS) strategic group (AGRUP2015/02), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (GNN, JCNE Grant FAPERJE-26/202.757/2019), and São Paulo Research Foundation (FAPESP, DB: Grant # 2019/02855-0).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sartor, L.R.; Graham, R.C.; Ying, S.C.; Otero, X.L.; Montes, C.R.; Ferreira, T.O. Role of Redox Processes in the Pedogenesis of Hypersaline Tidal Flat Soils on the Brazilian Coast. Soil Sci. Soc. Am. J. 2018, 82, 1217–1230. [Google Scholar] [CrossRef]
- Schaeffer-Novelli, Y.; Soriano-Sierra, E.J.; Vale, C.C.D.; Bernini, E.; Rovai, A.S.; Pinheiro, M.A.A.; Schmidt, A.J.; De Almeida, R.; Júnior, C.C.; Menghini, R.P.; et al. Climate changes in mangrove forests and salt marshes. Braz. J. Oceanogr. 2016, 64, 37–52. [Google Scholar] [CrossRef]
- Li, N.; Shao, T.; Zhu, T.; Long, X.-H.; Gao, X.; Liu, Z.; Shao, H.; Rengel, Z. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci. Rep. 2018, 8, 9728. [Google Scholar] [CrossRef]
- Sartor, L.R.; Graham, R.C.; Ying, S.C.; Andrade, G.R.; Montes, C.R.; Ferreira, T.O. Are hypersaline tidal flat soils potential silicon sinks in coastal wetlands? Geoderma 2019, 337, 215–224. [Google Scholar] [CrossRef]
- Soares, R.H.R.D.M.; De Assunção, C.A.; Fernandes, F.D.O.; Marinho-Soriano, E. Identification and analysis of ecosystem services associated with biodiversity of saltworks. Ocean Coast. Manag. 2018, 163, 278–284. [Google Scholar] [CrossRef]
- Albuquerque, A.G.B.M.; Ferreira, T.O.; Cabral, R.L.; Nóbrega, G.N.; Romero, R.E.; Meireles, A.J.D.A.; Otero, X.L. Hypersaline tidal flats (apicum ecosystems): The weak link in the tropical wetlands chain. Environ. Rev. 2014, 22, 99–109. [Google Scholar] [CrossRef]
- Hadlich, G.; Ucha, J.; Celino, J. Apicuns na Baía de Todos os Santos: Distribuição espacial, descrição e caracterização física e química. In Avaliação de Ambientes na Baía de Todos os Santos: Aspectos Geoquímicos, Geofísicos e Biológicos; Queiroz, A., Celino, J., Eds.; UFBA: Salvador, Brazil, 2008; pp. 59–72. [Google Scholar]
- Augspurger, C.K. Light Requirements of Neotropical Tree Seedlings: A Comparative Study of Growth and Survival. J. Ecol. 1984, 72, 777. [Google Scholar] [CrossRef]
- Budowski, G. The distinction between old secondary and climax species in tropical Central American lowlands. Trop. Ecol. 1970, 11, 1–32. [Google Scholar]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Haoliang, L.; Chongling, Y.; Liu, J. Low-molecular-weight organic acids exuded by Mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environ. Exp. Bot. 2007, 61, 159–166. [Google Scholar] [CrossRef]
- Dayton, P.K. Experimental Evaluation of Ecological Dominance in a Rocky Intertidal Algal Community. Ecol. Monogr. 1975, 45, 137–159. [Google Scholar] [CrossRef]
- Bertness, M.D.; Hacker, S.D. Physical Stress and Positive Associations Among Marsh Plants. Am. Nat. 1994, 144, 363–372. [Google Scholar] [CrossRef]
- Caçador, M.I.; Madureira, M.J.; Vale, C. Effects of plant roots on salt-marsh sediment geochemistry. In Proceedings in Marine Science; Flemming, B.W., Delafontaine, M.T., Liebezeit, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 2, pp. 197–204. [Google Scholar]
- Araújo, E.D.S.; Da Silva, J.B.; Oliveira, T.D.S.; De Santana, N.M.G.; Freire, M.B.G.D.F. Apicum do estuário de Barra de Gramame-PB: Análises físicas e químicas. Rev. Bras. Geogr. Fís. 2019, 12, 112–123. [Google Scholar] [CrossRef]
- Lebigre, J. Les Marais à Mangrove et lês Tannes. Available online: http://www.futura-sciences.com/magazines/voyage/infos/dossiers/d/geographie-marais-mangrove-tannes-683/ (accessed on 20 August 2020).
- Barcellos, D.; Queiroz, H.M.; Nóbrega, G.N.; Filho, R.L.D.O.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Otero, X.L.; Macías, F.; Ferreira, T.O. Phosphorus geochemistry in a Brazilian semiarid mangrove soil affected by shrimp farm effluents. Environ. Monit. Assess. 2014, 186, 5749–5762. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, H.M.; Artur, A.G.; Taniguchi, C.A.K.; Da Silveira, M.R.S.; Nascimento, J.C.D.; Nóbrega, G.N.; Otero, X.L.; Ferreira, T.O. Hidden contribution of shrimp farming effluents to greenhouse gas emissions from mangrove soils. Estuar. Coast. Shelf Sci. 2019, 221, 8–14. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ferreira, T.O.; Taniguchi, C.A.K.; Barcellos, D.; Nascimento, J.C.D.; Nóbrega, G.N.; Otero, X.L.; Artur, A.G. Nitrogen mineralization and eutrophication risks in mangroves receiving shrimp farming effluents. Environ. Sci. Pollut. Res. 2020, 27, 34941–34950. [Google Scholar] [CrossRef]
- Cohen, M.C.; Rodrigues, E.; Rocha, D.O.; Freitas, J.; Fontes, N.A.; Pessenda, L.C.; De Souza, A.V.; Gomes, V.L.; França, M.C.; Bonotto, D.M.; et al. Southward migration of the austral limit of mangroves in South America. Catena 2020, 195, 104775. [Google Scholar] [CrossRef]
- Feher, L.C.; Osland, M.J.; Griffith, K.T.; Grace, J.B.; Howard, R.J.; Stagg, C.L.; Enwright, N.M.; Krauss, K.W.; Gabler, C.A.; Day, R.H.; et al. Linear and nonlinear effects of temperature and precipitation on ecosystem properties in tidal saline wetlands. Ecosphere 2017, 8, e01956. [Google Scholar] [CrossRef]
- Vidal-Torrado, P.; Ferreira, T.; Otero, X.; Souza, V., Jr.; Ferreira, F.; Andrade, G.; Macías, F. Pedogenetic processes in mangrove soils. In Biogeochemistry and Pedogenetic Process in Saltmarsh and Mangrove Systems; Otero, X., Macías, F., Eds.; MDPI: Basel, Switzerland, 2010; p. 27. [Google Scholar]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Mendonça, E.D.S.; Romero, R.E.; Otero, X.L. The importance of blue carbon soil stocks in tropical semiarid mangroves: A case study in Northeastern Brazil. Environ. Earth Sci. 2019, 78, 369. [Google Scholar] [CrossRef]
- Otero, X.L.; Araújo, J.M.C.A.; Barcellos, D.; Queiroz, H.M.; Romero, D.J.; Nóbrega, G.N.; Siqueira-Neto, M.; Ferreira, T.O. Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). Appl. Sci. 2020, 10, 2215. [Google Scholar] [CrossRef]
- Zang, Z.; Zou, X.; Zuo, P.; Song, Q.; Wang, C.; Wang, J. Impact of landscape patterns on ecological vulnerability and ecosystem service values: An empirical analysis of Yancheng Nature Reserve in China. Ecol. Indic. 2017, 72, 142–152. [Google Scholar] [CrossRef]
- Albuquerque, A.G.B.M.; Ferreira, T.O.; Nóbrega, G.N.; Romero, R.E.; Júnior, V.S.D.S.; Meireles, A.J.D.A.; Otero, X.L. Soil genesis on hypersaline tidal flats (apicum ecosystem) in a tropical semi-arid estuary (Ceará, Brazil). Soil Res. 2014, 52, 140–154. [Google Scholar] [CrossRef]
- Fatubarin, A.; Olojugba, M.R. Effect of rainfall season on the chemical properties of the soil of a Southern Guinea Savanna ecosystem in Nigeria. J. Ecol. Nat. Environ. 2014, 6, 182–189. [Google Scholar] [CrossRef]
- De Lacerda, L.D.; De Menezes, M.O.T.; Molisani, M.M. Changes in mangrove extension at the Pacoti River estuary, CE, NE Brazil due to regional environmental changes between 1958 and 2004. Biota Neotrop. 2007, 7, 67–72. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Neto, M.S.; Queiroz, H.M.; Artur, A.G.; Mendonça, E.D.S.; Silva, E.O.; Otero, X.L. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 2016, 542, 685–693. [Google Scholar] [CrossRef]
- Freire, G. Etude hydrologique et sedimentologique de l’estuaire du rio pacoti (fortaleza-ceare-bresil). Ph.D. Thesis, Université de Nantes, Nantes, France, 1989. [Google Scholar]
- Albuquerque, A. Pedogênese e evolução De Solos De Apicum Em Clima Tropical Semiárido. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Ceará, Brazil, 2015. [Google Scholar]
- Bell, H.L.; O’Leary, J.W. Effects of salinity on growth and cation accumulation of Sporobolus virginicus (Poaceae). Am. J. Bot. 2003, 90, 1416–1424. [Google Scholar] [CrossRef]
- Cordazzo, C.V.; Seeliger, U. Reproduction and vegetative regeneration in Blutaparon portulacoides (Amaranthaceae) on backshores in southern Brazil. J. Coast. Res. 2003, 481–485. [Google Scholar]
- Lonard, R.I.; Judd, F.W.; Stalter, R. The Biological Flora of Coastal Dunes and Wetlands: Batis maritima C. Linnaeus. J. Coast. Res. 2011, 27, 441. [Google Scholar] [CrossRef]
- Álvarez, E.; Fernández-Sanjurjo, M.J.; Otero, X.L.; Macías, F. Aluminum speciation in the bulk and rhizospheric soil solution of the species colonizing an abandoned copper mine in Galicia (NW Spain). J. Soils Sediments 2010, 11, 221–230. [Google Scholar] [CrossRef]
- Chung, J.-B.; Zasoski, R.J. Ammonium-Potassium and Ammonium-Calcium Exchange Equilibria in Bulk and Rhizosphere Soil. Soil Sci. Soc. Am. J. 1994, 58, 1368–1375. [Google Scholar] [CrossRef]
- Otero, X.L.; Huerta-Diaz, M.A.; De La Peña, S.; Ferreira, T.O. Sand as a relevant fraction in geochemical studies in intertidal environments. Environ. Monit. Assess. 2013, 185, 7945–7959. [Google Scholar] [CrossRef] [PubMed]
- Perlatti, F.; Ferreira, T.O.; Sartor, L.R.; Otero, X.L. Copper Biogeochemistry in Response to Rhizosphere Soil Processes Under Four Native Plant Species Growing Spontaneously in an Abandoned Mine Site in NE Brazil. Water Air Soil Pollut. 2016, 227, 1–15. [Google Scholar] [CrossRef]
- Loveland, P.J.; Whalley, W.R.; Smith, K.; Mullins, C. Particle size analysis. Smith Mullins Soil Anal. Phys. Methods 2000, 281–314. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- EMBRAPA. Manual de Métodos de Análise de Solo; Embrapa Solos: Rio de Janeiro, RJ, Brasil, 1997. [Google Scholar]
- Moore, T.J.; Loeppert, R.H.; West, L.T.; Hallmark, C.T. Routine method for calcium carbonate equivalent of soils. Commun. Soil Sci. Plant Anal. 1987, 18, 265–277. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Kempers, A. Determination of sub-microquantities of ammonium and nitrates in soils with phenol, sodiumnitroprusside and hypochlorite. Geoderma 1974, 12, 201–206. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Morse, J.W. A quantitative method for determination of trace metal concentrations in sedimentary pyrite. Mar. Chem. 1990, 29, 119–144. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary pyrite formation. Am. J. Sci. 1970, 268, 1–23. [Google Scholar] [CrossRef]
- Dantas, J.S.; de Souza, A.P.; de Farias, M.F.; Nogueira, V.d.F.B. Interactions among groups of microorganisms with rhizosphere. Appl. Res. Agrotechnol. 2009, 2, 213–224. [Google Scholar]
- Yin, Y.; Yan, Z. Variations of soil bacterial diversity and metabolic function with tidal flat elevation gradient in an artificial mangrove wetland. Sci. Total Environ. 2020, 718, 137385. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Fowler, N. The role of competition in plant communities in arid and semiarid regions. Annu. Rev. Ecol. Syst. 1986, 17, 89–110. [Google Scholar] [CrossRef]
- Meireles, A.J.d.A.; Cassola, R.S.; Tupinambá, S.V.; Queiroz, L.d.S. Impactos Ambientais Decorrentes Das Atividades Da Carcinicultura Ao Longo Do Litoral Cearense, Nordeste Do Brasil. Mercat. Rev. Geogr. UFC 2007, 6, 83–106. [Google Scholar] [CrossRef]
- Van Breemen, N.; Buurman, P. Soil Formation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; p. 408. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and Indirect Effects of Invasive Plants on Soil Chemistry and Ecosystem Function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Yin, H.; Xu, Z.; Chen, Z.; Wei, Y.; Liu, Q. Nitrogen transformation in the rhizospheres of two subalpine coniferous species under experimental warming. Appl. Soil Ecol. 2012, 59, 60–67. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of Ammonium and Nitrate Nutrition On Some Physiological Processes in Higher Plants—Growth, Photosynthesis, Photorespiration, and Water Relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hogh Jensen, H.; Schjoerring, J. Effects of drought and inorganic N form on nitrogen fixation and carbon isotope discrimination in Trifolium repens. Plant Physiol. Biochem. (Paris) 1997, 35, 55–62. [Google Scholar]
- Roiloa, S.R.; Antelo, B.; Retuerto, R. Physiological integration modifies δ15N in the clonal plant Fragaria vesca, suggesting preferential transport of nitrogen to water-stressed offspring. Ann. Bot. 2014, 114, 399–411. [Google Scholar] [CrossRef]
- An, J.; Liu, C.; Wang, Q.; Yao, M.; Rui, J.; Zhang, S.; Li, X. Soil bacterial community structure in Chinese wetlands. Geoderma 2019, 337, 290–299. [Google Scholar] [CrossRef]
- Vogt, J.C.; Abed, R.M.M.; Albach, D.C.; Palinska, K.A. Bacterial and Archaeal Diversity in Hypersaline Cyanobacterial Mats Along a Transect in the Intertidal Flats of the Sultanate of Oman. Microb. Ecol. 2017, 75, 331–347. [Google Scholar] [CrossRef]
- Vera-Gargallo, B.; Ventosa, A. Metagenomic Insights into the Phylogenetic and Metabolic Diversity of the Prokaryotic Community Dwelling in Hypersaline Soils from the Odiel Saltmarshes (SW Spain). Genes 2018, 9, 152. [Google Scholar] [CrossRef]
- Abed, R.M.M.; De Beer, D.; Stief, P. Functional-Structural Analysis of Nitrogen-Cycle Bacteria in a Hypersaline Mat from the Omani Desert. Geomicrobiol. J. 2015, 32, 119–129. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Meyer, R.L.; Allen, D.E.; Schmidt, S. Nitrification and denitrification as sources of sediment nitrous oxide production: A microsensor approach. Mar. Chem. 2008, 110, 68–76. [Google Scholar] [CrossRef]
- Bolhuis, H.; Severin, I.; Confurius-Guns, V.; Wollenzien, U.I.A.; Stal, L.J. Horizontal transfer of the nitrogen fixation gene cluster in the cyanobacterium Microcoleus chthonoplastes. ISME J. 2009, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant. Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Saitoh, T.; Seiwa, K.; Nishiwaki, A. Importance of physiological integration of dwarf bamboo to persistence in forest understorey: A field experiment. J. Ecol. 2002, 90, 78–85. [Google Scholar] [CrossRef]
- Slade, A.J.; Hutchings, M.J. An analysis of the costs and benefits of physiological integration between ramets in the clonal perennial herb Glechoma hederacea. Oecologia 1987, 73, 425–431. [Google Scholar] [CrossRef]
- Roiloa, S.R.; Retuerto, R. Development, photosynthetic activity and habitat selection of the clonal plant Fragaria vesca growing in copper-polluted soil. Funct. Plant Biol. 2006, 33, 961. [Google Scholar] [CrossRef]
- Barcellos, D.; Cyle, K.T.; Thompson, A. Faster redox fluctuations can lead to higher iron reduction rates in humid forest soils. Biogeochemistry 2018, 137, 367–378. [Google Scholar] [CrossRef]
- Chen, C.; Meile, C.; Wilmoth, J.L.; Barcellos, D.; Thompson, A. Influence of pO2 on Iron Redox Cycling and Anaerobic Organic Carbon Mineralization in a Humid Tropical Forest Soil. Environ. Sci. Technol. 2018, 52, 7709–7719. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.L.; Cahoon, D.R.; Feller, I.C. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob. Ecol. Biogeogr. 2007, 16, 545–556. [Google Scholar] [CrossRef]
- Gordon, D.M. Disturbance to mangroves in tropical-arid Western Australia: Hypersalinity and restricted tidal exchange as factors leading to mortality. J. Arid. Environ. 1988, 15, 117–145. [Google Scholar] [CrossRef]
- Xu, C.; Pu, L.; Zhu, M.; Li, J.; Chen, X.; Wang, X.; Xie, X. Ecological Security and Ecosystem Services in Response to Land Use Change in the Coastal Area of Jiangsu, China. Sustainability 2016, 8, 816. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).