The Purpose of Patient-Reported Outcome (PRO) Post Its Digitalization and Integration into Clinical Practice: An Interdisciplinary Redefinition Resembling PROs Theoretical and Practical Evolvement
Abstract
1. Introduction
2. Materials and Methods
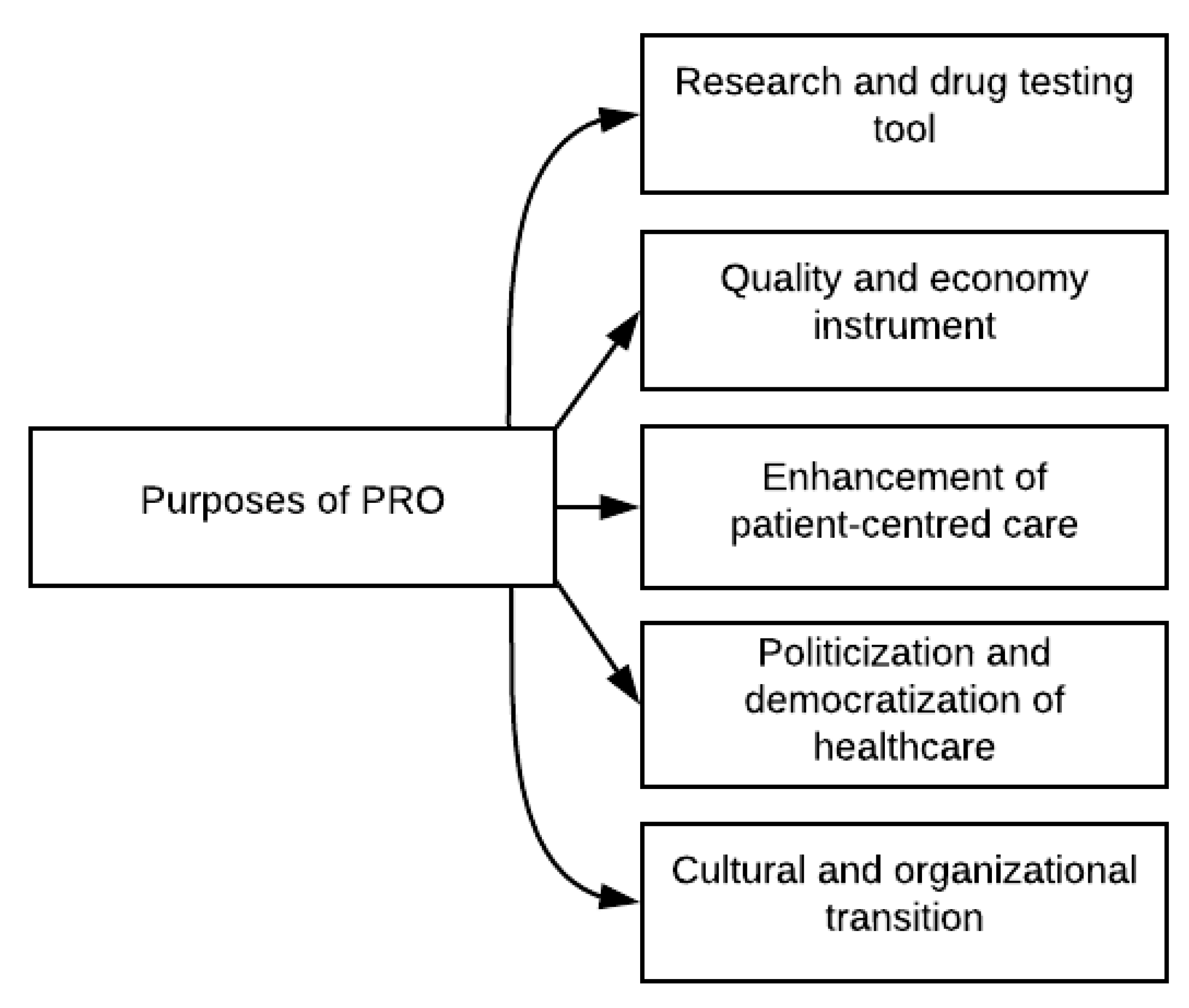
3. Results
3.1. PRO as a Research and Drug Testing Tool
3.2. PRO as a Quality and Economy Instrument
3.3. PRO as Patient-Centred Care
3.4. PRO as a Political and Democratic Tool
3.5. PRO as an Organizational and Cultural Shift
4. Discussion
4.1. Realization of PRO’s Patient-Oriented and Economic Purposes?
4.2. PRO—Information or Outcome Data?
4.3. Directly from the Patient?
4.4. PRO as a Democratic or Political Tool?
4.5. An Interdisciplinary Redefinition of PRO
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HRQoL | Health-Related Quality of Life |
| ISOQOL | International Society for Quality of Life Research |
| PBF | Performance-Based Financing |
| PRO | Patient-reported outcome |
| PROM | Patient-reported outcome measures |
| QoL | Quality of Life |
| VBHC | Value-Based Healthcare |
| ViBIS | The Danish Knowledge Center for User Involvement in Health Care |
References
- Glouberman, S. PROMs: A critical step, but only one of many. In New Models for the New Healthcare—The Case for Routine Patient-Reported Outcome Measurement; Leatt, P., Smith, T., Foster-Kent, D., Harrison, S., Bryant, S., Eds.; Healthcare Papers; Longwoods Publishing: Toronto, ON, Canada, 2012; Volume 11, pp. 29–33. [Google Scholar]
- Højgaard, B.; Kjellberg, J. Fem Megatrends der Udfordrer Fremtidens Sundhedsvæsen; Det Nationale Institut for Kommuners og Regioners Analyse og Forskning; KORA: København, Denmark; Available online: https://www.vive.dk/da/udgivelser/fem-megatrends-der-udfordrer-fremtidens-sundhedsvaesen-8760/ (accessed on 3 September 2020).
- Groen, W.G.; Kuijpers, W.; Oldenburg, H.S.A.; Wouters, M.W.J.M.; Aaronson, N.K.; Van Harten, W.H. Empowerment of cancer survivors through information technology: An integrative review. J. Med. Internet Res. 2015, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Danish Regions. Plan for Borgernes Sundhedsvæsen—Vores Sundhedsvæsen; Danish Regions; Region Hovedstaden: København, Denmark; Available online: https://www.regioner.dk/media/3047/borgernes-sundhedv-final-let.pdf (accessed on 3 September 2020).
- Ministry of Health (SUM); Danish Regions (DR); Local Government Denmark (KL). Nationale mål for Sunhedsvæsenet; SUM, DR and KL: Copenhagen, Denmark. Available online: https://www.sum.dk/~/media/Filer%20-%20Publikationer_i_pdf/2019/Nationale-maal-for-sundhedsvaesenet-2019/Nationale-Maal-2019-pub.pdf (accessed on 3 September 2020).
- The Danish Health Data Authority (SDS). Strategi 2020—Helhed, Sammenhæng, Tryghed. Available online: file:///C:/Users/je/AppData/Local/Temp/SDS%20Strategi%202020-1.pdf (accessed on 3 September 2020).
- The Danish Knowledge Center for User Involvement in Health Care (VibIS). Program PRO. Available online: https://danskepatienter.dk/vibis (accessed on 3 September 2020).
- The PRO Secretariat. PRO-Landskab. Available online: https://pro-danmark.dk/da/pro-landskab (accessed on 3 September 2020).
- Bland, J.; Khan, H.; Loder, J.; Westlake, S.; Symons, T. NHS in 2030 a People-Powered and Knowledge-Powered Health System; NESTA: London, UK; Available online: https://www.nesta.org.uk/report/the-nhs-in-2030-a-people-powered-and-knowledge-powered-health-system/ (accessed on 3 September 2020).
- Ministry of Health (SUM); Ministry of Finance (FM); Danish Regions (DR); Local Government Denmark (KL). A Coherent and Trustworthy Health Network for All: Digital Health Strategy 2018–2022. Available online: file:///C:/Users/je/AppData/Local/Temp/Digital%20Health%20Strategy%202018_2022.pdf (accessed on 3 September 2020).
- U.S. Department of Health and Human Services; Food and Drug Administration (FDA). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available online: https://www.fda.gov/media/77832/download (accessed on 3 September 2020).
- Tambuyzer, E.; Van Audenhove, C. Is perceived patient involvement in mental health care associated with satisfaction and empowerment? Health Expect. 2015, 18, 516–526. [Google Scholar] [CrossRef]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A Systematic Review of the Literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Yarlas, A.; Bayliss, M.; Cappelleri, J.C.; Maher, S.; Bushmakin, A.G.; Chen, L.A.; Manuchehri, A.; Healey, P. Psychometric validation of the SF-36 Health Survey in Ulcerative Colitis: Results from a systematic literature review. Qual. Life Res. 2018, 27, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Spertus, J.; Dudley, R.A.; Wu, A.; Chuahan, C.; Cohen, P.; Smith, M.L.; Black, N.; Crawford, A.; Christensen, K.; et al. Methods for Developing Patient-Reported Outcome-Based Performance Measures (PRO-PMs). Value Health 2015, 18, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Kane, P.M.; Ellis-Smith, C.; Daveson, B.A.; Ryan, K.; Mahon, N.G.; McAdam, B.; McQuillan, R.; Tracey, C.; Howley, C.; O’Gara, G.; et al. Understanding how a palliative-specific patient-reported outcome intervention works to facilitate patient-centred care in advanced heart failure: A qualitative study. Palliat. Med. 2018, 32, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.-J.; Feeny, D. Framework to assess the effects of using patient-reported outcome measures in chronic care management. Qual. Life Res. 2014, 23, 1505–1513. [Google Scholar] [CrossRef]
- Kaltoft, M.K.; Nielsen, J.B.; Salkeld, G.; Dowie, J. Enhancing healthcare provider feedback and personal health literacy: Dual use of a decision quality measure. In Studies in Health Technology and Informatics; Christian, N., Kuziemsky, C.E., Kushniruk, A.W., Borycki, E.M., Eds.; IOS Press: Amsterdam, The Netherlands, 2015; Volume 218, p. 74. ISBN 09269630. [Google Scholar]
- Eriksen, J.; Bygholm, A.; Bertelsen, P. The association between patient-reported outcomes (PROs) and patient participation in clinical practice: A scoping review. Patient Educ. Couns. 2020. (under review). [Google Scholar]
- Eriksen, J.; Bertelsen, P.; Bygholm, A. The Digital Transformation of Patient-Reported Outcomes’ (PROs’) Functionality within Healthcare. In Digital Personalized Health and Medicine, Proceedings of the MIE 2020, Medical Informatics Europe 2020, Geneve, Switzerland, 28 April–1 May 2020; Pape-Haugaard, L.P., Lovis, C., Madsen, I.C., Weber, P., Nielsen, P.H., Scott, P., Eds.; Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Booth, A.; Sutton, A.; Papaioannou, D. Systematic Approaches to a Successful Literature Review, 2nd ed.; Sage: Los Angeles, CA, USA, 2016. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2008, 3, 77–101. [Google Scholar] [CrossRef]
- Antunes, B.; Rodrigues, P.P.; Higginson, I.J.; Ferreira, P.L. Outcome measurement—A scoping review of the literature and future developments in palliative care clinical practice. Ann. Palliat. Med. 2018, 8, 196–206. [Google Scholar] [CrossRef]
- Howell, D.; Liu, G. Can Routine Collection of Patient Reported Outcome Data Actually Improve Person-Centered Health? In New Models for the New Healthcare—The Case for Routine Patient-Reported Outcome Measurement; Leatt, P., Smith, T., Foster-Kent, D., Harrison, S., Bryant, S., Eds.; Healthcare Papers; Longwoods Publishing: Toronto, ON, Canada, 2012; Volume 11, pp. 42–47. [Google Scholar]
- Wang, M.C.; Bellows, J. Quality of Life and Patient-Centered Outcomes. In Chronic Illness Care: Principles and Practice; Daaleman, T.P., Helton, M.R., Eds.; Springer Science+Business Media: New York, NY, USA, 2018; pp. 95–107. ISBN 9783319718118. [Google Scholar]
- McHorney, C.A. Health status assessment methods for adults: Past accomplishments and future challenges. Annu. Rev. Public Health 1999, 20, 309–335. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, J.; Gotay, C.C.; Snyder, C.F. Patient-reported outcomes in cancer: A review of recent research and policy initiatives. CA Cancer J. Clin. 2007, 57, 278–300. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.P.; Wilburn, J. Patient value: Its nature, measurement, and role in real world evidence studies and outcomes-based reimbursement. J. Med. Econ. 2018, 21, 474–480. [Google Scholar] [CrossRef]
- Donabedian, A. An Introduction to Quality Assurance in Health Care; Oxford University Press Inc.: Oxford, UK, 2003; p. 200. [Google Scholar]
- Velikova, G.; Booth, L.; Smith, A.B.; Brown, P.M.; Lynch, P.; Brown, J.M.; Selby, P.J. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J. Clin. Oncol. 2004, 22, 714–724. [Google Scholar] [CrossRef]
- Black, N. Patient reported outcome measures could help transform healthcare. BMJ 2013, 346, f167. [Google Scholar] [CrossRef]
- Weldring, T.; Smith, S.M.S. Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, 61–68. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.; Dunn, G.; Todd, C. Empowerment: Qualitative underpinning of a new clinical genetics-specific patient-reported outcome. Eur. J. Hum. Genet. 2011, 19, 125–130. [Google Scholar] [CrossRef]
- Huebner, J.; Rosé, C.; Geissler, J.; Gleiter, C.H.; Prott, F.J.; Muenstedt, K.; Micke, O.; Muecke, R.; Buentzel, J.; Bottomley, A.; et al. Integrating cancer patients’ perspectives into treatment decisions and treatment evaluation using patient-reported outcomes—A concept paper. Eur. J. Cancer Care 2014, 23, 173–179. [Google Scholar] [CrossRef]
- Frost, M.H.; Bonomi, A.E.; Cappelleri, J.C.; Schünemann, H.J.; Moynihan, T.J.; Aaronson, N.K.; Cella, D.; Chassany, O.; Fairclough, D.L.; Ferrans, C.E.; et al. Applying quality-of-life data formally and systematically into clinical practice. Mayo Clin. Proc. 2007, 82, 1214–1228. [Google Scholar] [CrossRef]
- Dean, S.; Mathers, J.M.; Calvert, M.; Kyte, D.G.; Conroy, D.; Folkard, A.; Southworth, S.; Murray, P.I.; Denniston, A.K. “The patient is speaking”: Discovering the patient voice in ophthalmology. Br. J. Ophthalmol. 2017, 101, 700–708. [Google Scholar] [CrossRef]
- LeBlanc, T.W.; Abernethy, A.P. Patient-reported outcomes in cancer care-hearing the patient voice at greater volume. Nat. Rev. Clin. Oncol. 2017, 14, 763–772. [Google Scholar] [CrossRef]
- Marquis, P.; Arnould, B.; Acquadro, C.; Roberts, W.M. Patient-reported outcomes and health-related quality of life in effectiveness studies: Pros and cons. Drug Dev. Res. 2006, 67, 193–201. [Google Scholar] [CrossRef]
- Strong, L.E. The past, present, and future of patient-reported outcomes in oncology. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.O., III; Noonan, V.K.; Auger, C.; Feldman, D.E.; Ahmed, S.; Bartlett, S.J. Montreal Accord on Patient-Reported Outcomes (PROs) use series—Paper 4: Patient-reported outcomes can inform clinical decision making in chronic care. J. Clin. Epidemiol. 2017, 89, 136–141. [Google Scholar] [CrossRef]
- Boyce, M.B.; Browne, J.P.; Greenhalgh, J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research. BMJ Qual. Saf. 2014, 23, 508–518. [Google Scholar] [CrossRef]
- Chang, S.; Newton, P.J.; Inglis, S.; Luckett, T.; Krum, H.; Macdonald, P.; Davidson, P.M. Are all outcomes in chronic heart failure rated equally? An argument for a patient-centred approach to outcome assessment. Heart Fail. Rev. 2014, 19, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, S.; Karnon, J.; Chen, G.; Nair, R.; Salter, A.B. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs). Qual. Life Res. 2018, 28, 567–592. [Google Scholar] [CrossRef]
- Lewis, S. Realizing the PROMise of PROMs. In New Models for the New Healthcare—The Case for Routine Patient-Reported Outcome Measurement; Healthcare Papers; Leatt, P., Smith, T., Foster-Kent, D., Harrison, S., Bryant, S., Eds.; Longwoods Publishing: Toronto, ON, Canada, 2012; Volume 11, pp. 20–23. [Google Scholar]
- Wiering, B.; de Boer, D.; Delnoij, D. Asking what matters: The relevance and use of patient-reported outcome measures that were developed without patient involvement. Wiley 2017, 20, 1330–1341. [Google Scholar] [CrossRef]
- Hostetter, M.; Klein, S. Using Patient-Reported Outcomes to Improve Health Care Quality. Available online: https://www.commonwealthfund.org/publications/newsletter-article/using-patient-reported-outcomes-improve-health-care-quality (accessed on 3 September 2020).
- Nelson, E.C.; Hvitfeldt, H.; Reid, R.; Grossman, D.; Lindblad, S.; Mastanduno, M.P. Using Patient-Reported Information to Improve Health Outcomes and Health Care Value: Case Studies from Dartmouth, Karolinska and Group Health; The Dartmouth Institute for Health Policy and Clinical Practice and Centre for Population Health: Darmount, NS, Canada, 2012; pp. 1–54. [Google Scholar]
- Porter, M.E. What is value in health care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef]
- Mejdahl, C.T.; Schougaard, L.M.V.; Hjollund, N.H.; Riiskjær, E.; Thorne, S.; Lomborg, K. PRO-based follow-up as a means of self-management support—An interpretive description of the patient perspective. JPRO 2018, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Zimlichman, E. Using patient-reported outcomes to drive patientcentered care. In Information Technology for Patient Empowerment in Healthcare; Grando, M.A., Rozenblum, R., Bates, D.W., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2015; pp. 241–256. ISBN 9781614514343. [Google Scholar]
- Ayers, D.C. Implementation of patient-reported outcome measures in total knee arthroplasty. J. Am. Acad. Orthop. Surg. 2017, 25, S48–S50. [Google Scholar] [CrossRef]
- Rademakers, J.; Delnoij, D.; De Boer, D. Structure, process or outcome: Which contributes most to patients’ overall assessment of healthcare quality? BMJ Qual. Saf. 2011, 20, 326–331. [Google Scholar] [CrossRef] [PubMed]
- National Quality Forum (NQF). Patient Reported Outcomes (PROs) in Performance Measurement. Available online: https://www.qualityforum.org/Patient-Reported_Outcomes.aspx (accessed on 3 September 2020).
- Schwartz, C.E.; Sprangers, M.A.G. An introduction to quality of life asssessment in oncology: The value of measuring patient-reported outcomes. Am. J. Manag. Care 2002, 8, S550–S559. [Google Scholar] [PubMed]
- Hjollund, N.H.I. Fifteen years’ use of patient-reported outcome measures at the group and patient levels: Trend analysis. J. Med. Internet Res. 2019, 21, e15856. [Google Scholar] [CrossRef]
- Talib, T.L.; DeChant, P.; Kean, J.; Monahan, P.O.; Haggstrom, D.A.; Stout, M.E.; Kroenke, K. A qualitative study of patients’ perceptions of the utility of patient-reported outcome measures of symptoms in primary care clinics. Qual. Life Res. 2018, 27, 3157–3166. [Google Scholar] [CrossRef]
- Jayadevappa, R.; Chhatre, S. Patient centered care—A conceptual model and review of the state of the art. Open Health Serv. Policy J. 2011, 4, 15–25. [Google Scholar] [CrossRef]
- du Plessis, D.; Sake, J.-K.; Halling, K.; Morgan, J.; Georgieva, A.; Bertelsen, N. Patient Centricity and Pharmaceutical Companies: Is It Feasible? Ther. Innov. Regul. Sci. 2017, 51, 460–467. [Google Scholar] [CrossRef]
- Cook, D.J.; Manning, D.M.; Holland, D.E.; Prinsen, S.K.; Rudzik, S.D.; Roger, V.L.; Deschamps, C. Patient engagement and reported outcomes in surgical recovery: Effectiveness of an e-health platform. J. Am. Coll. Surg. 2013, 217, 648–655. [Google Scholar] [CrossRef]
- Fagerlind, H.; Ring, L.; Brülde, B.; Feltelius, N.; Lindblad, A.K. Patients’ understanding of the concepts of health and quality of life. Patient Educ. Couns. 2010, 78, 104–110. [Google Scholar] [CrossRef]
- Sartorius, N. Patient-reported outcomes in psychiatry. Dialogues Clin. Neurosci. 2014, 16, 123–124. [Google Scholar]
- Lavallee, D.C.; Chenok, K.E.; Love, R.M.; Petersen, C.; Holve, E.; Segal, C.D.; Franklin, P.D. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff. 2016, 35, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Trillingsgaard, C.; Nielsen, B.K.; Hjøllund, N.H.; Lomborg, K. Use of patient-reported outcomes in outpatient settings as a means of patient involvement and self-management support—A qualitative study of the patient perspective. Eur. J. Pers. Cent. Healthc. 2016, 4, 359–367. [Google Scholar] [CrossRef]
- Marshall, S.; Haywood, K.; Fitzpatrick, R. Impact of patient-reported outcome measures on routine practice: A structured review. J. Eval. Clin. Pract. 2006, 12, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Noonan, V.K.; Lyddiatt, A.; Ware, P.; Jaglal, S.B.; Riopelle, R.J.; Bingham, C.O., III; Figueiredo, S.; Sawatzky, R.; Santana, M.; Bartlett, S.J.; et al. Montreal Accord on Patient-Reported Outcomes (PROs) use series—Paper 3: Patient-reported outcomes can facilitate shared decision-making and guide self-management. J. Clin. Epidemiol. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Chen, J.; Ou, L.; Hollis, S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv. Res. 2013, 13, 211. [Google Scholar] [CrossRef]
- Greenhalgh, J. The applications of PROs in clinical practice: What are they, do they work, and why? Qual. Life Res. 2009, 18, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Ohueri, C.W.; Schryver, E.; Bozic, K.J.; Koenig, K.M. Patient-identified Barriers and Facilitators to Pre-Visit Patient-Reported Outcomes Measures Completion in Patients with Hip and Knee Pain. J. Arthroplast. 2018, 33, 643–649.e1. [Google Scholar] [CrossRef]
- Fleischmann, M.; Vaughan, B. The challenges and opportunities of using patient reported outcome measures (PROMs) in clinical practice. Int. J. Osteopath. Med. 2018, 28, 56–61. [Google Scholar] [CrossRef]
- Schougaard, L.M.V.; Mejdahl, C.T.; Petersen, K.H.; Jessen, A.; De Thurah, A.; Sidenius, P.; Lomborg, K.; Hjollund, N.H. Effect of patient-initiated versus fixed-interval telePRO-based outpatient follow-up: Study protocol for a pragmatic randomised controlled study. BMC Health Serv. Res. 2017, 17, 83. [Google Scholar] [CrossRef]
- Cummings, G. The road to improving patient-reported outcomes: Measures or healthcare reform? In New Models for the New Healthcare—The Case for Routine Patient-Reported Outcome Measurement; Healthcare Papers; Leatt, P., Smith, T., Foster-Kent, D., Harrison, S., Bryant, S., Eds.; Longwoods Publishing: Toronto, ON, Canada, 2012; Volume 11, pp. 24–28. [Google Scholar] [CrossRef]
- Meadows, K.A. Patient-reported outcome measures: An overview. Br. J. Community Nurs. 2011, 16, 146–151. [Google Scholar] [CrossRef]
- Gensheimer, S.G.; Wu, A.W.; Snyder, C.F.; Basch, E.; Gerson, J.; Holve, E.; Hunt, D.R.; Smider, N.; Snyder, C.; Stiefel, M.; et al. Oh, the Places We’ll Go: Patient-Reported Outcomes and Electronic Health Record. Patient 2018, 11, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mejdahl, C.T.; Schougaard, L.M.V.; Hjollund, N.H.; Riiskjær, E.; Lomborg, K. Exploring organisational mechanisms in PRO-based follow-up in routine outpatient care—An interpretive description of the clinician perspective. BMC Health Serv. Res. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Prodinger, B.; Taylor, P. Improving quality of care through patient-reported outcome measures (PROMs): Expert interviews using the NHS PROMs Programme and the Swedish quality registers for knee and hip arthroplasty as examples. BMC Health Serv. Res. 2018, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Bezjak, A. Logistics of collecting patient-reported outcomes (PROs) in clinical practice: An overview and practical examples. Qual. Life Res. 2009, 18, 125–136. [Google Scholar] [CrossRef]
- Basch, E.; Iasonos, A.; Barz, A.; Culkin, A.; Kris, M.G.; Artz, D.; Fearn, P.; Speakman, J.; Farquhar, R.; Scher, H.I.; et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J. Clin. Oncol. 2007, 25, 5374–5380. [Google Scholar] [CrossRef]
- Palfreyman, S. Patient-reported outcome measures and how they are used. Nurs. Older People 2011, 23, 31–36. [Google Scholar] [CrossRef]
- Cannella, L.; Efficace, F.; Giesinger, J. How should we assess patient-reported outcomes in the onco-hematology clinic? Curr. Opin. Support. Palliat. Care 2018, 12, 522–529. [Google Scholar] [CrossRef]
- Kelkar, A.A.; Spertus, J.; Pang, P.; Pierson, R.F.; Cody, R.J.; Pina, I.L.; Hernandez, A.; Butler, J. Utility of Patient-Reported Outcome Instruments in Heart Failure. JACC: Heart Fail. 2016, 4, 165–175. [Google Scholar] [CrossRef]
- Howell, D.; Molloy, S.; Wilkinson, K.; Green, E.; Orchard, K.; Wang, K.; Liberty, J. Patient-reported outcomes in routine cancer clinical practice: A scoping review of use, impact on health outcomes, and implementation factors. Ann. Oncol. 2015, 26, 1846–1858. [Google Scholar] [CrossRef]
- Schougaard, L.M.V.; Larsen, L.P.; Jessen, A.; Sidenius, P.; Dorflinger, L.; de Thurah, A.; Hjollund, N.H. AmbuFlex: Tele-patient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseases. Qual. Life Res. 2016, 25, 525–534. [Google Scholar] [CrossRef]
- Stover, A.; Irwin, D.E.; Chen, R.C.; Chera, B.S.; Mayer, D.K.; Muss, H.B.; Rosenstein, D.L.; Shea, T.C.; Wood, W.A.; Lyons, J.C.; et al. Integrating Patient-Reported Outcome Measures into Routine Cancer Care: Cancer Patients’ and Clinicians’ Perceptions of Acceptability and Value. eGEMS 2015, 3, 1169. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, M.M.; Patek, S.D.; Sheridan, K.; Lach, J.C.; Goldman, M.D. Remotely engaged: Lessons from remote monitoring in multiple sclerosis. Int. J. Med. Inform. 2017, 100, 26–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, C.-H. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual. Life Res. 2007, 16, 157–166. [Google Scholar] [CrossRef]
- Tevis, S.E.; James, T.A.; Kuerer, H.M.; Pusic, A.L.; Yao, K.A.; Merlino, J.; Dietz, J. Patient-reported outcomes for breast cancer. Ann. Surg. Oncol. 2018, 25, 2839–2845. [Google Scholar] [CrossRef]
- Appleby, J.; Devlin, N.; Parkin, D. Using Patient Reported Outcomes to Improve Health Care; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2016; pp. 1–120. [Google Scholar]
- Brundage, M.D.; Smith, K.C.; Little, E.A.; Bantug, E.T.; Snyder, C.F. The PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: Results from a mixed-methods evaluation. Qual. Life Res. 2015, 24, 2457–2472. [Google Scholar] [CrossRef]
- Greenhalgh, J.; Abhyankar, P.; McCluskey, S.; Takeuchi, E.; Velikova, G. How do doctors refer to patient-reported outcome measures (PROMS) in oncology consultations? Qual. Life Res. 2013, 22, 939–950. [Google Scholar] [CrossRef]
- Snyder, C.; Smith, K.; Holzner, B.; Rivera, Y.M.; Bantug, E.; Brundage, M.; PRO Data Presentation Delphi Panel. Making a picture worth a thousand numbers: Recommendations for graphically displaying patient-reported outcomes data. Qual. Life Res. 2019, 28, 345–356. [Google Scholar] [CrossRef]
- Adams, J.; Chapman, J.; Bradley, S.; Ryan, S.J. Literacy levels required to complete routinely used patient reported outcome measures in rheumatology. Rheumatology 2013, 52, 460–464. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksen, J.; Bygholm, A.; Bertelsen, P. The Purpose of Patient-Reported Outcome (PRO) Post Its Digitalization and Integration into Clinical Practice: An Interdisciplinary Redefinition Resembling PROs Theoretical and Practical Evolvement. Appl. Sci. 2020, 10, 7507. https://doi.org/10.3390/app10217507
Eriksen J, Bygholm A, Bertelsen P. The Purpose of Patient-Reported Outcome (PRO) Post Its Digitalization and Integration into Clinical Practice: An Interdisciplinary Redefinition Resembling PROs Theoretical and Practical Evolvement. Applied Sciences. 2020; 10(21):7507. https://doi.org/10.3390/app10217507
Chicago/Turabian StyleEriksen, Jeppe, Ann Bygholm, and Pernille Bertelsen. 2020. "The Purpose of Patient-Reported Outcome (PRO) Post Its Digitalization and Integration into Clinical Practice: An Interdisciplinary Redefinition Resembling PROs Theoretical and Practical Evolvement" Applied Sciences 10, no. 21: 7507. https://doi.org/10.3390/app10217507
APA StyleEriksen, J., Bygholm, A., & Bertelsen, P. (2020). The Purpose of Patient-Reported Outcome (PRO) Post Its Digitalization and Integration into Clinical Practice: An Interdisciplinary Redefinition Resembling PROs Theoretical and Practical Evolvement. Applied Sciences, 10(21), 7507. https://doi.org/10.3390/app10217507




