Effect of Aeration Mode on Microbial Structure and Efficiency of Treatment of TSS-Rich Wastewater from Meat Processing
Abstract
1. Introduction
2. Materials and Methods
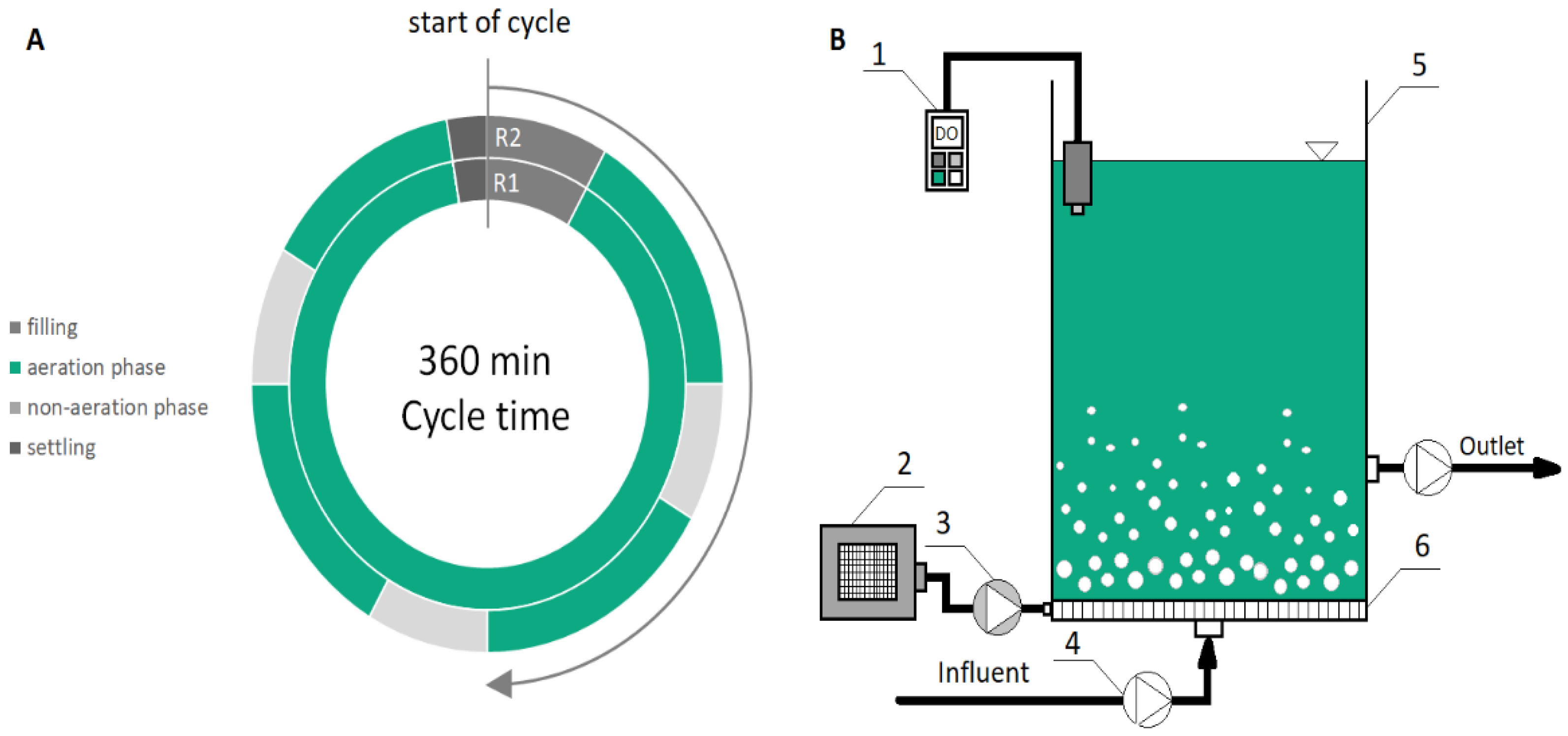
2.1. Experimental Design
2.2. Analytical Methods
2.3. Next-Generation Sequencing
2.4. Real-Time PCR
2.5. Statistics
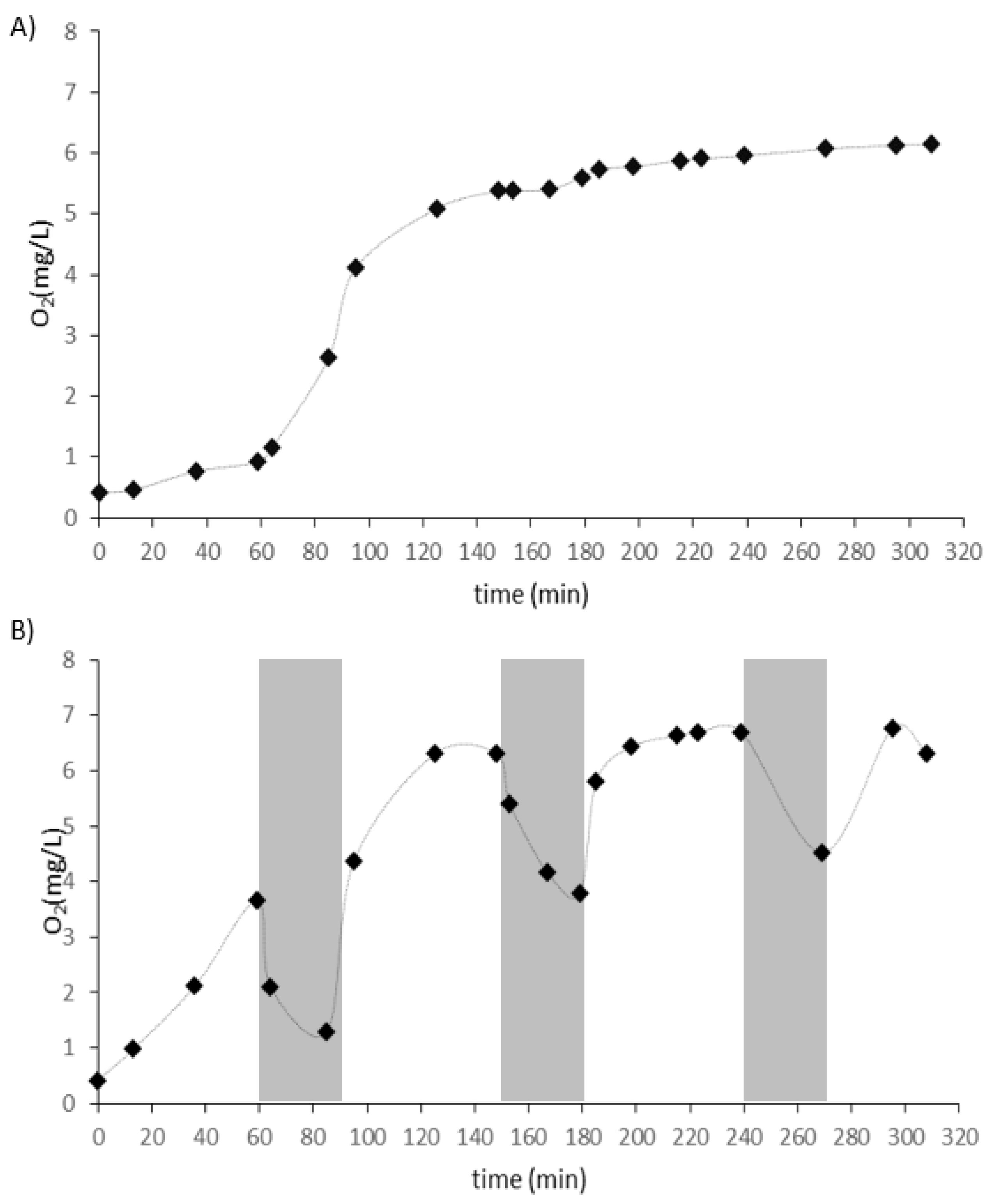
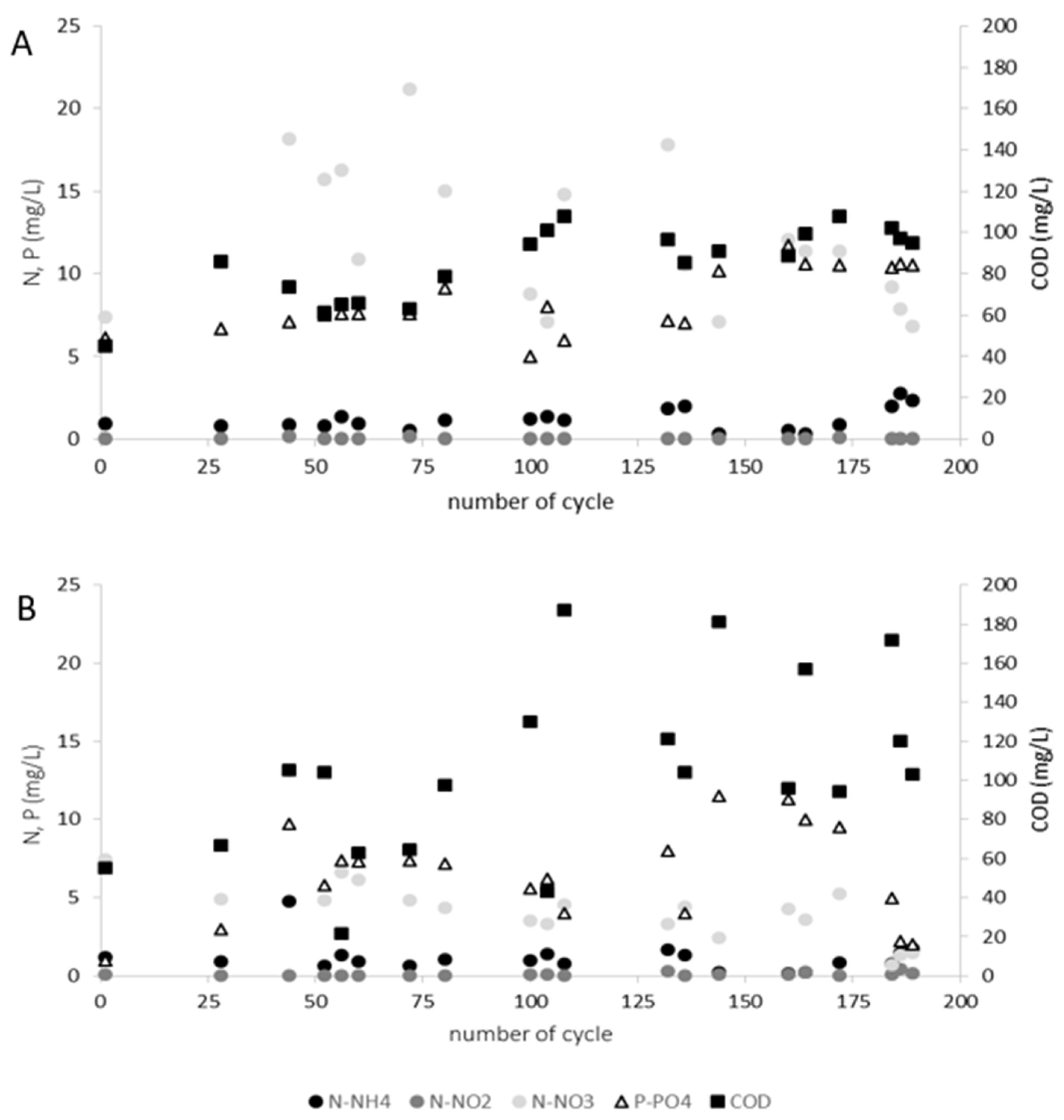
3. Results and Discussion
4. Conclusions
- -
- In an intermittently aerated reactor fed with high-TSS wastewater from meat industry higher N and P removal and a higher concentration of biomass was observed, but also a higher concentration of suspended solids in treated wastewater, smaller diameters of granules and deteriorated settling properties of biomass mainly as a result of the reduction of EPS content in biomass was noted;
- -
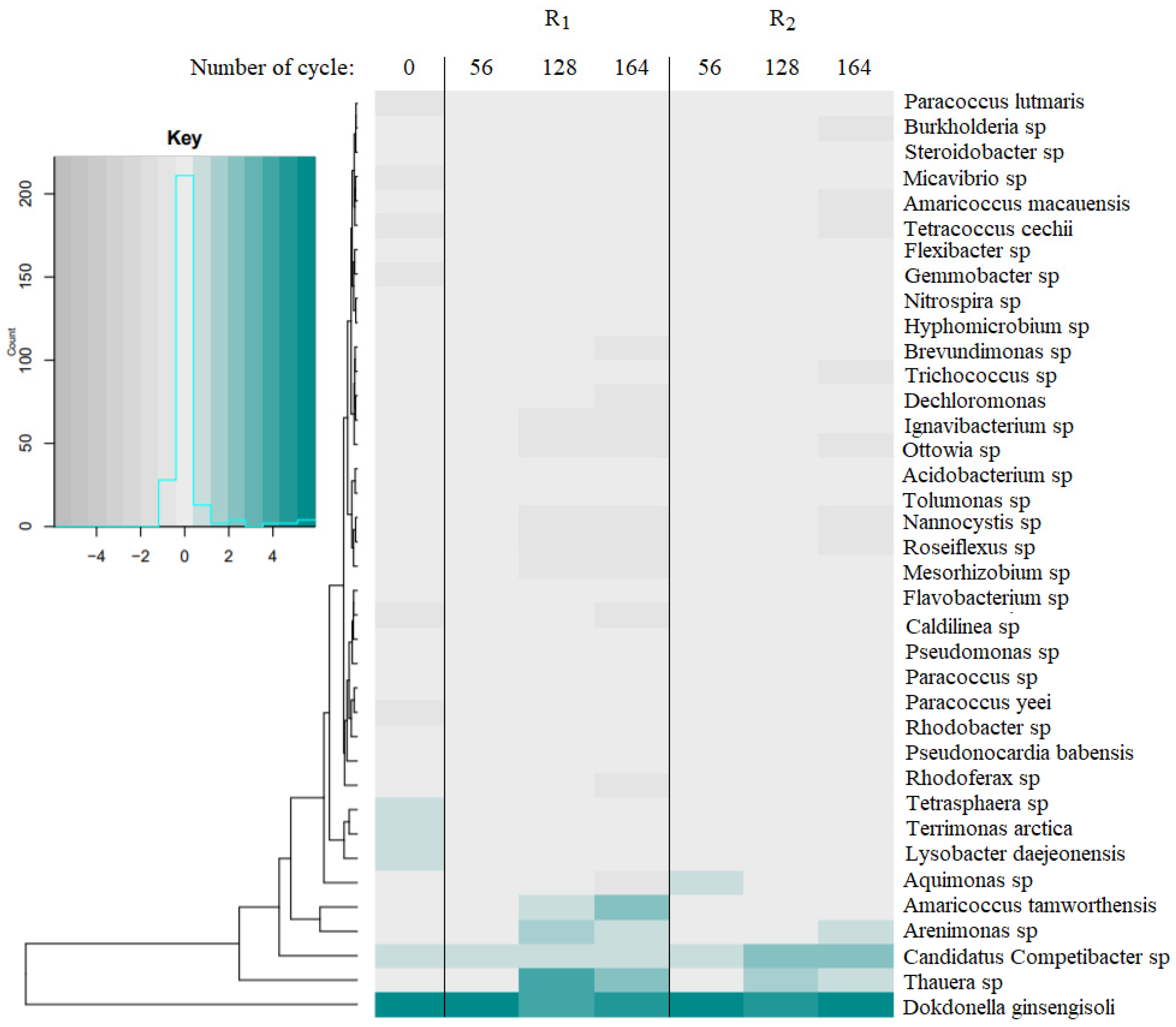
- CCA analysis showed that microbial structure significantly changed in time; an increase in abundances of Arenimonas sp., Thauera sp., and Dokdonella sp. characterized mature granules in period of stable treatment of meat-processing wastewater;
- -
- Constant aeration in the cycle increases the number of nitrifying and denitrifying genes in biomass (amoA and nosZ analysis) while intermittent aeration increases microbial diversity of granules;
- -
- Constant aeration favored growth of microorganisms from the Rhodanobacteraceae, Rhodobacteraceae and Xanthomonadaceae families while in intermittently aerated reactors Competibacteraceae family was more abundant (7.8%) and appeared.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Gene, Primer | Primer Concentration | Size of Amplicon (bp) | Thermal Profile | Reference |
|---|---|---|---|---|
| amoA (amoA1Fa/amoA2R) | 100 nM | ~500 | 94 °C/15 s, 52 °C/45 s, 60 °C/45 s | [59] |
| Bacterial 16S rRNA (519F/907R) | 100 nM | ~600 | 94 °C/15 s, 50 °C/40 s, 60 °C, 40 s | [60] |
| nosZ (NosZ-F/NosZ1622R) | 200 nM | ~500 | 94 °C/15 s, 60 °C/2.5 min | [61,62] |
| Reactor | Number of Cycle | Number of Sequences | Number of OTU | Shannon-Wiener Index (H’) |
|---|---|---|---|---|
| Inoculum | 0 | 9675 | 994 | 3.67 |
| R1 | 56 | 20,360 | 873 | 3.22 |
| 128 | 18,336 | 654 | 2.26 | |
| 164 | 26,594 | 901 | 3.39 | |
| R2 | 56 | 21,699 | 947 | 3.02 |
| 128 | 7237 | 740 | 3.59 | |
| 164 | 14,678 | 1031 | 3.67 |
References
- Gerbens-Leenes, P.W.; Mekonnen, M.M.; Hoekstra, A.Y. The water footprint of poultry, pork and beef: A comparative study in different countries and production systems. Water Resour. Ind. 2013, 1–2, 25–36. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization. Available online: http://www.fao.org/faostat/en (accessed on 1 August 2020).
- Pingali, P. Westernization of Asian diets and the transformation of food systems: Implications for research and policy. Food Policy 2007, 32, 281–298. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.F.; Mehrvar, M.; Quinones-Bolanos, E. Cost-effectiveness analysis of TOC removal from slaughterhouse wastewater using combined anaerobic-aerobic and UV/H2O2 processes. J. Environ. Manag. 2014, 134, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kiepper, B.H.; Merka, W.C.; Fletcher, D.L. Proximate composition of poultry processing wastewater particulate matter from broiler slaughter plants. Poult. Sci. 2008, 87, 1633–1636. [Google Scholar] [CrossRef]
- Cammarota, M.C.; Freire, D.G. A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresour. Technol. 2006, 97, 2195–2210. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Liu, L.; Liang, H.; Wu, W.M. Aerobic granular sludge: Characterization, mechanism of granulation and application to wastewater treatment. Crit. Rev. Biotechnol. 2011, 31, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.F.; Tay, J.H. Improved stability of aerobic granules by selecting slow-growing nitrifying bacteria. J. Biotechnol. 2004, 108, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [PubMed]
- Carrera, P.; Campo, R.; Méndez, R.; Di Bella, G.; Campos, J.L.; Mosquera-Corral, A.; Val del Río, A. Does the feeding strategy enhance the aerobic granular sludge stability treating saline effluents? Chemosphere 2019, 226, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Liu, Y.Q.; Tay, J.H.; Ning, P. Alternating anoxic/oxic condition combined with step-feeding mode for nitrogen removal in granular sequencing batch reactors (GSBRs). Sep. Purif. Technol. 2013, 105, 63–68. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Wojnowska-Baryła, I. Nitrogen-converting communities in aerobic granules at different hydraulic retention times (HRTs) and operational modes. World J. Microbiol. Biotechnol. 2015, 31, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kang, X.; Li, X.; Yuan, Y. Performance of aerobic granular sludge in a sequencing batch bioreactor for slaughterhouse wastewater treatment. Bioresour. Technol. 2015, 190, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, D.P.; Belia, E. Nitrogen and phosphorus removal from an abattoir wastewater in a SBR with aerobic granular sludge. Water Res. 2005, 39, 4817–4823. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Neu, T.R.; Flemming, H.C. (Eds.) Microbial Extracellular Polymeric Substances: Characterization, Structures and Function; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–18. [Google Scholar]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Kang, S.T.; Nam, S.Y. Effect of carbohydrate and protein in the EPS on sludge settling characteristics. Water Sci. Technol. 2001, 43, 193–196. [Google Scholar] [CrossRef]
- Sponza, D.T. Investigation of extracellular polymer substances (EPS) and physicochemical properties of different activated sludge flocs under steady-state conditions. Enzym. Microb. Technol. 2003, 32, 375–385. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A. Performance and microbial characteristics of biomass in a full-scale aerobic granular sludge wastewater treatment plant. Environ. Sci. Pollut. 2017, 25, 1655–1669. [Google Scholar] [CrossRef]
- APHA (Ed.) 1992 Standard Methods for the Examination of Water and Wastewater, 18th ed.; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Rusanowska, P.; Cydzik-Kwiatkowska, A.; Świątczak, P.; Wojnowska-Baryła, I. Changes in extracellular polymeric substances (EPS) content and composition in aerobic granule size-fractions during reactor cycles at different organic loads. Bioresour. Technol. 2019, 272, 188–193. [Google Scholar] [CrossRef]
- Frølund, B.; Griebe, T.; Nielsen, P.H. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 755–761. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A.; Rusanowska, P. Microbiota of anaerobic digesters in a full-scale wastewater treatment plant. Arch. Environ. Prot. 2017, 43, 53–60. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco 5: Software for multivariate data exploration, testing and summarization. In Biometrics; Plant Research International: Wageningen, The Netherlands, 2012. [Google Scholar]
- Mosquera-Corral, A.; De Kreuk, M.K.; Heijnen, J.J.; Van Loosdrecht, M.C. Effects of oxygen concentration on N-removal in an aerobic granular sludge reactor. Water Res. 2005, 39, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Liu, Q.S.; Liu, Y. The effect of upflow air velocity on the structure of aerobic granules cultivated in a sequencing batch reactor. Water Sci. Technol. 2004, 49, 35–40. [Google Scholar] [CrossRef]
- De Kreuk, M.K.; Pronk, M.; Van Loosdrecht, M.C. Formation of aerobic granules and conversion processes in an aerobic granular sludge reactor at moderate and low temperatures. Water Res. 2005, 39, 4476–4484. [Google Scholar] [CrossRef]
- Luo, J.; Chen, H.; Han, X.; Sun, Y.; Yuan, Z.; Guo, J. Microbial community structure and biodiversity of size-fractionated granules in a partial nitritation–anammox process. FEMS Microbiol. Ecol. 2005, 93, fix021. [Google Scholar] [CrossRef]
- Weissbrodt, D.G.; Neu, T.R.; Kuhlicke, U.; Rappaz, Y.; Holliger, C. Assessment of bacterial and structural dynamics in aerobic granular biofilms. Front. Microbiol. 2013, 4, 175. [Google Scholar] [CrossRef]
- De Sousa Rollemberg, S.; De Oliveira, L.; Barros, A.; Melo, V.; Firmino, P.; Dos Santos, A. Effects of carbon source on the formation, stability, bioactivity and biodiversity of the aerobic granule sludge. Bioresour. Technol. 2019, 278, 195–204. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; Erguder, T.H. The effect of seed sludge type on aerobic granulation via anoxic–aerobic operation. Environ. Technol. 2014, 35, 2928–2939. [Google Scholar] [CrossRef]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Rusanowska, P.; Zielińska, M.; Bernat, K.; Wojnowska-Baryła, I. Microbial structure and nitrogen compound conversions in aerobic granular sludge reactors with non-aeration phases and acetate pulse feeding. Environ. Sci. Pollut. Res. 2016, 23, 24857–24870. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.C.; Stratton, H.M.; Seviour, R.J. Environmental factors contributing to 385 the “G bacteria” population in full-scale EBPR plants. Water Sci. Technol. 2002, 4–5, 185–192. [Google Scholar] [CrossRef]
- Zeng, R.J.; Yuan, Z.; Keller, J. Improved understanding of the interactions and complexities of biological nitrogen and phosphorus removal processes. Rev. Environ. Sci. Biol. 2004, 3, 265–272. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Mielczarek, A.T.; Kragelund, C. A conceptual ecosystem model of microbial communities in enhanced biological phosphorus removal plants. Water Res. 2010, 44, 5070–5088. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Batstone, D.J.; Keller, J. Biological phosphorus removal from abattoir wastewater at very short sludge ages mediated by novel PAO clade Comamonadaceae. Water Res. 2015, 69, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.; Zekker, I.; Jaagura, M.; Tenno, T. Enhancement of anoxic phosphorus uptake of denitrifying phosphorus removal process by biomass adaption. Int. J. Environ. Sci. Technol. 2019, 16, 5965–5978. [Google Scholar] [CrossRef]
- Falvo, A.; Levantesi, C.; Rossetti, S.; Seviour, R.J.; Tandoi, V. Synthesis of intracellular storage polymers by Amaricoccus kaplicensis, a tetrad forming bacterium present in activated sludge. J. Appl. Microbiol. 2001, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Oehmen, A.; Blackall, L.L. The effect of GAOs (glycogen accumulating organisms) on anaerobic carbon requirements in full-scale Australian EBPR (enhanced biologicalphosphorus removal) plants. Water Sci. Technol. 2003, 47, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.K.; Knowles, R. Production of nitrous oxide by Nitrosomonas europaea: Effects of acetylene, pH, and oxygen. Can. J. Microbiol. 1984, 30, 1397–1404. [Google Scholar] [CrossRef]
- Wang, Z.P.; Liu, L.L.; Yao, H.; Cai, W.M. Effects of extracellular polymeric substances on aerobic granulation in sequencing batch reactors. Chemosphere 2006, 63, 1728–1735. [Google Scholar] [CrossRef]
- Al-Halbouni, D.; Traber, J.; Lyko, S.; Wintgens, T.; Melin, T.; Tacke, D.; Hollender, J. Correlation of EPS content in activated sludge at different sludge retention times with membrane fouling phenomena. Water Res. 2008, 42, 1475–1488. [Google Scholar] [CrossRef]
- McSwain, B.S.; Irvine, R.L.; Hausner, M.; Wilderer, P.A. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ. Microbiol. 2005, 71, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D.J.; Tay, J.H. Extracellular polymeric substances and structural stability of aerobic granule. Water Res. 2008, 42, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, Y.; Khanal, S.K.; Lu, H.; Fang, H.; Zhao, Q. Understanding the role of extracellular polymeric substances on ciprofloxacin adsorption in aerobic sludge, anaerobic sludge, and sulfate-reducing bacteria sludge systems. Environ. Sci. Technol. 2018, 52, 6476–6486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Lu, Z.X. Optimization of processing parameters for the mycelial growth and extracellular polysaccharide production by Boletus spp. ACCC 50328. Process Biochem. 2005, 40, 1043–1051. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Li, T. Impact of extracellular polymeric substances on the settlement ability of aerobic granular sludge. Environ. Technol. 2010, 31, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, B.J.; Reeve, P.; Dinesh, N.; Short, M.D.; van den Akker, B. Comparison of an anaerobic feed and split anaerobic–aerobic feed on granular sludge development, performance and ecology. Chemosphere 2017, 172, 408–417. [Google Scholar] [CrossRef]
- Jones, C.M.; Stres, B.; Rosenquist, M.; Hallin, S. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol. Biol. Evol. 2008, 25, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Pishgar, R.; Dominic, J.A.; Sheng, Z.; Tay, J.H. Denitrification performance and microbial versatility in response to different selection pressures. Bioresour. Technol. 2019, 281, 72–83. [Google Scholar] [CrossRef]
- Srinandan, C.S.; Jadav, V.; Cecilia, D.; Nerurkar, A.S. Nutrients determine the spatial architecture of Paracoccus sp. biofilm. Biofouling 2010, 26, 449–459. [Google Scholar] [CrossRef]
- Foglar, L.; Briški, F. Wastewater denitrification process—The influence of methanol and kinetic analysis. Process Biochem. 2003, 39, 95–103. [Google Scholar] [CrossRef]
- Brooksbank, A.M.; Latchford, J.W.; Mudge, S.M. Degradation and modification of fats, oils and grease by commercial microbial supplements. World J. Microbiol. Biotechnol. 2007, 23, 977–985. [Google Scholar] [CrossRef]
- Al-Khatib, M.A.; Alam, M.Z.; Shabana, H. Isolation of bacterial strain for biodegradation of fats, oil and grease. Malays. J. Anal. Sci. 2015, 19, 138–143. [Google Scholar]
- Teixeira, P.D.; Silva, V.S.; Tenreiro, R. Integrated selection and identification of bacteria from polluted sites for biodegradation of lipids. Int. Microbiol. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Tang, T.; Wang, Y.; Zhi, L.; Lv, J.; Li, J. Function of quorum sensing and cell signaling in the formation of aerobic granular sludge. Rev. Environ. Sci. Bio/Technol. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: New York, NY, USA, 1991; pp. 205–248. [Google Scholar]
- Kloos, K.; Mergel, A.; Rösch, C.; Bothe, H. Denitrification within the genus Azospirillum and other associative bacteria. Aust. J. Plant Physiol. 2001, 28, 991–998. [Google Scholar] [CrossRef]
- Throbäck, I.N.; Enwall, K.; Jarvis, Ä.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]

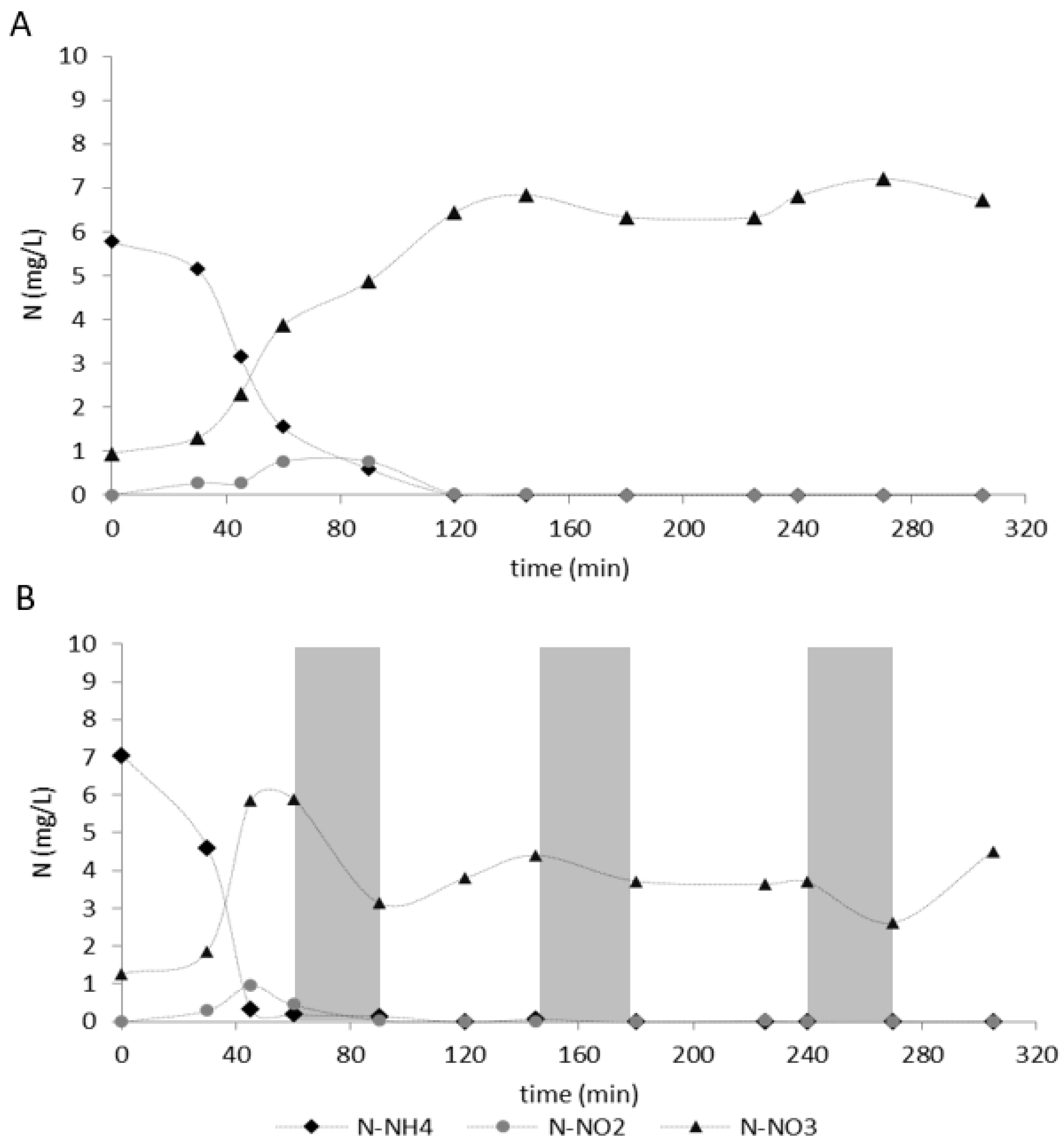

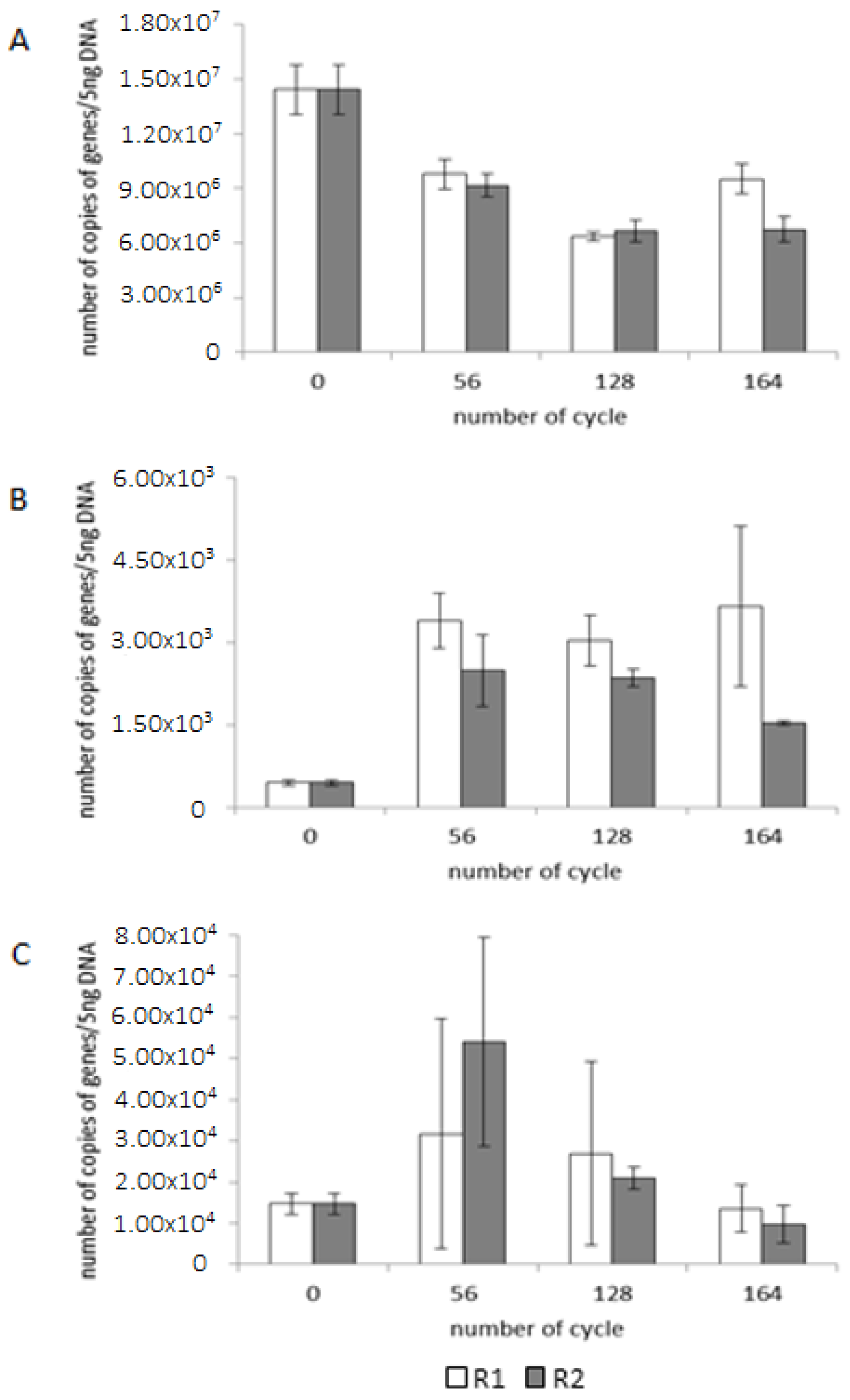
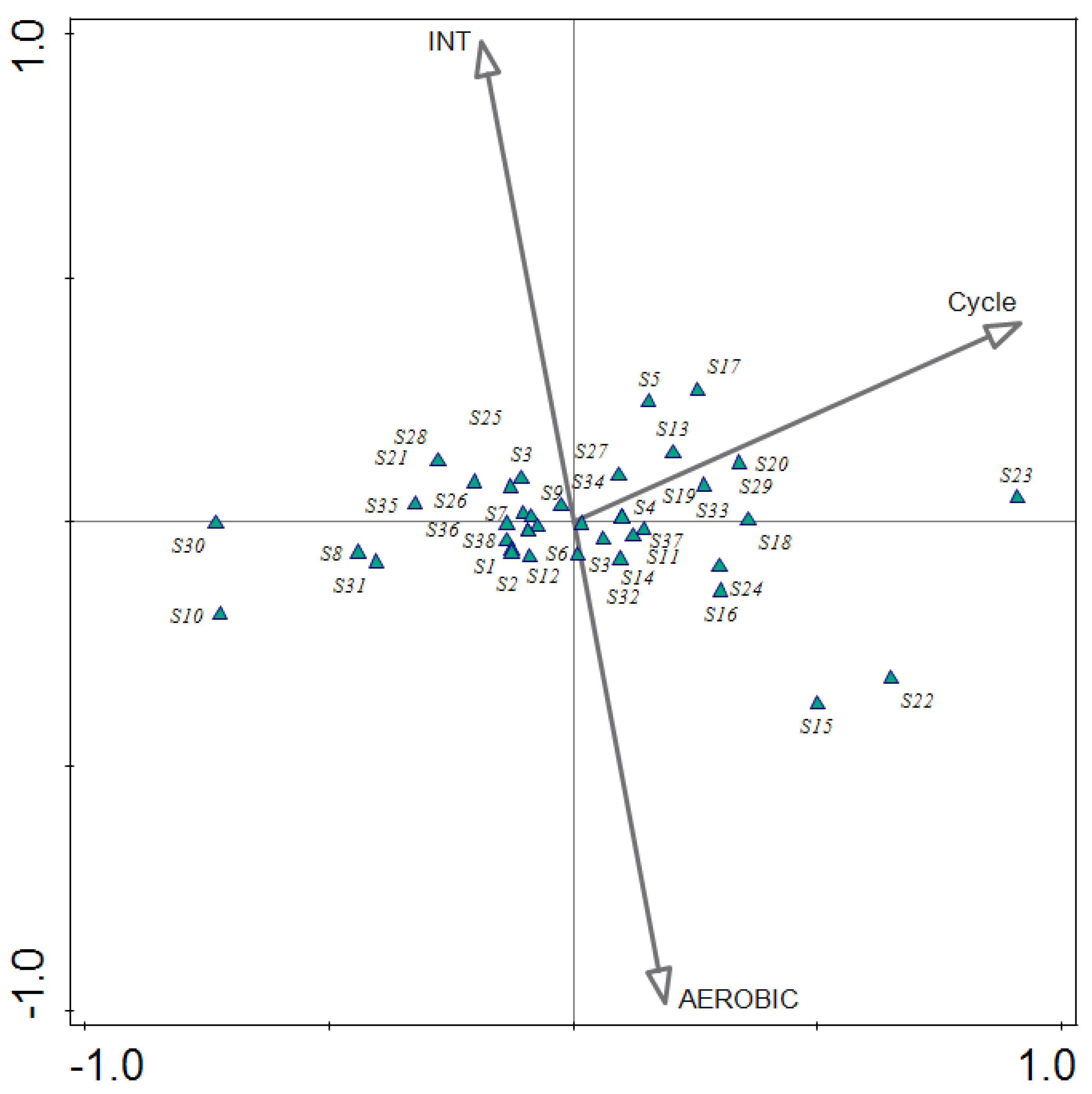
| Parameter | Unit | Value |
|---|---|---|
| COD | mg L−1 | 1686.8 ± 612.0 |
| BOD | mg L−1 | 1268.4 ± 421.5 |
| TKN | mg L−1 | 69.8 ± 19.5 |
| N-NH4+ | mg L−1 | 20.4 ± 13.8 |
| TP | mg L−1 | 23.9 ± 4.3 |
| P-PO43− | mg L−1 | 10.3 ± 14.1 |
| Fats | mg L−1 | 450 ± 37 |
| VFA | mg L−1 | 910 ± 154.2 |
| Acetic acid | mg L−1 | 254 ± 24.6 |
| Alkalinity | - | 9 ± 0.3 |
| pH | - | 8.0 ±0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jachimowicz, P.; Cydzik-Kwiatkowska, A.; Szklarz, P. Effect of Aeration Mode on Microbial Structure and Efficiency of Treatment of TSS-Rich Wastewater from Meat Processing. Appl. Sci. 2020, 10, 7414. https://doi.org/10.3390/app10217414
Jachimowicz P, Cydzik-Kwiatkowska A, Szklarz P. Effect of Aeration Mode on Microbial Structure and Efficiency of Treatment of TSS-Rich Wastewater from Meat Processing. Applied Sciences. 2020; 10(21):7414. https://doi.org/10.3390/app10217414
Chicago/Turabian StyleJachimowicz, Piotr, Agnieszka Cydzik-Kwiatkowska, and Patrycja Szklarz. 2020. "Effect of Aeration Mode on Microbial Structure and Efficiency of Treatment of TSS-Rich Wastewater from Meat Processing" Applied Sciences 10, no. 21: 7414. https://doi.org/10.3390/app10217414
APA StyleJachimowicz, P., Cydzik-Kwiatkowska, A., & Szklarz, P. (2020). Effect of Aeration Mode on Microbial Structure and Efficiency of Treatment of TSS-Rich Wastewater from Meat Processing. Applied Sciences, 10(21), 7414. https://doi.org/10.3390/app10217414





