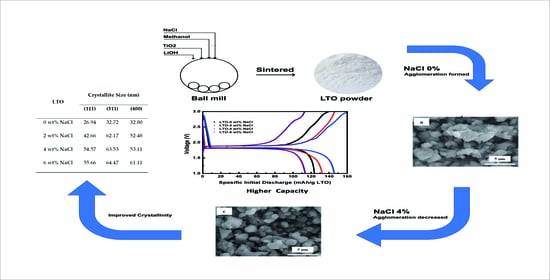
High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Li4Ti5O12
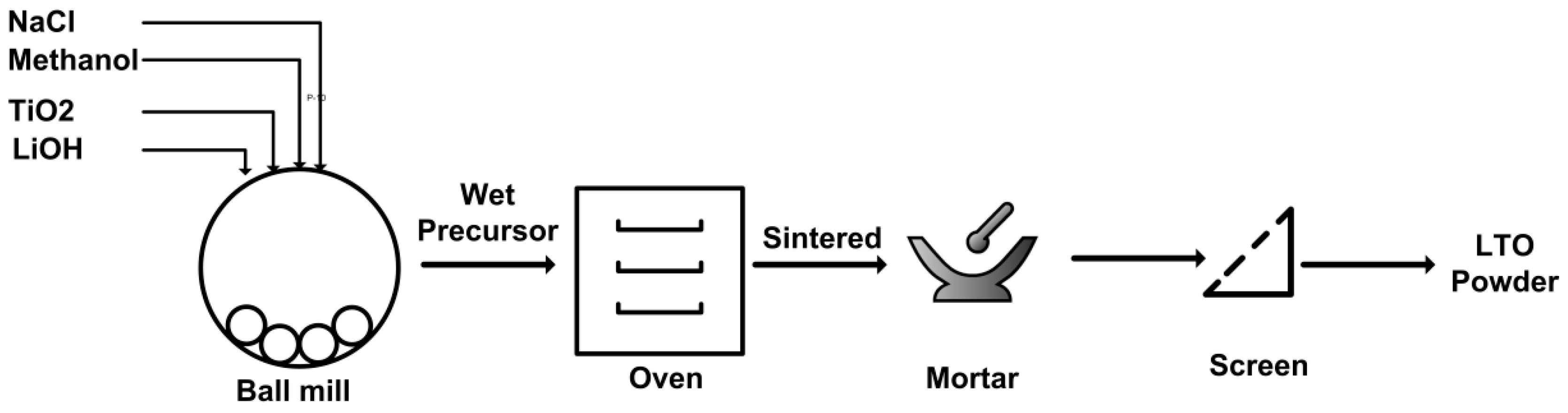
2.2. Li4Ti5O12 Preparation
2.3. Li4Ti5O12 Characterization
2.4. Li4Ti5O12/LiFePO4 Cylindrical Cell Assembly
3. Results and Discussion
3.1. Structural Analysis of LTO
3.2. Morphology Analysis of LTO
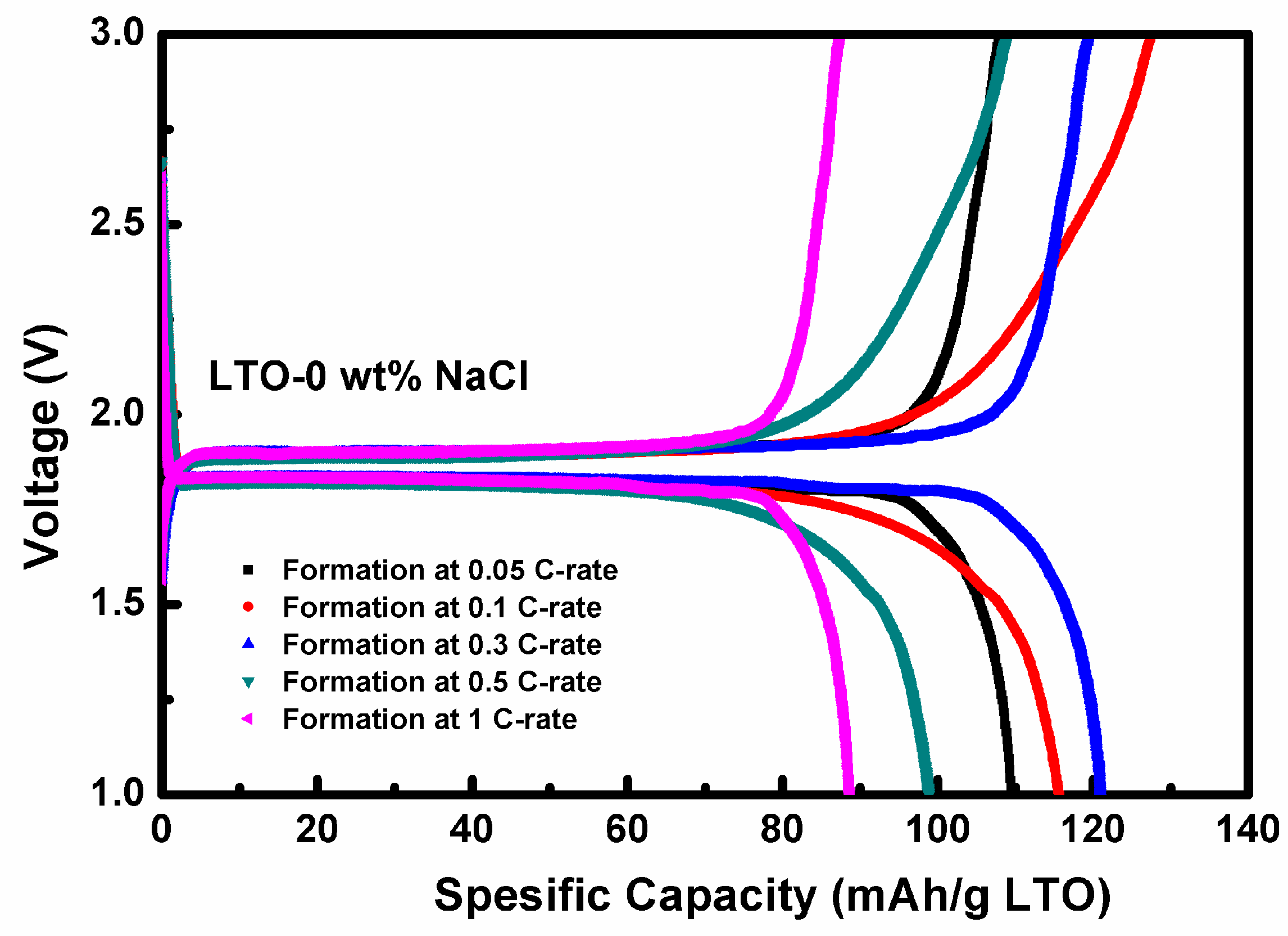
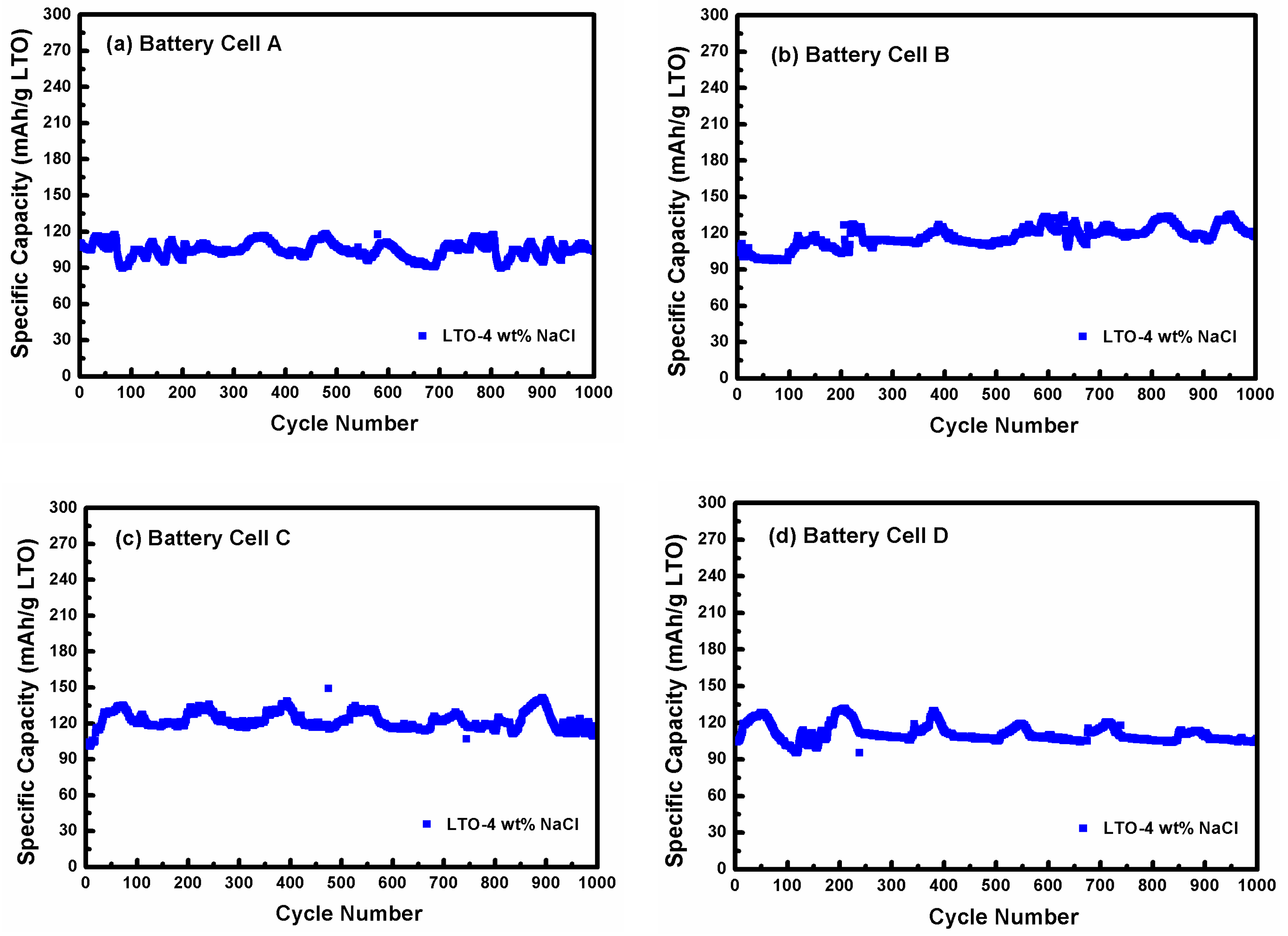
3.3. Electrochemical Performance Measurement of LTO in LTO/LFP Battery
3.4. Post Cycle Analysis of LTO
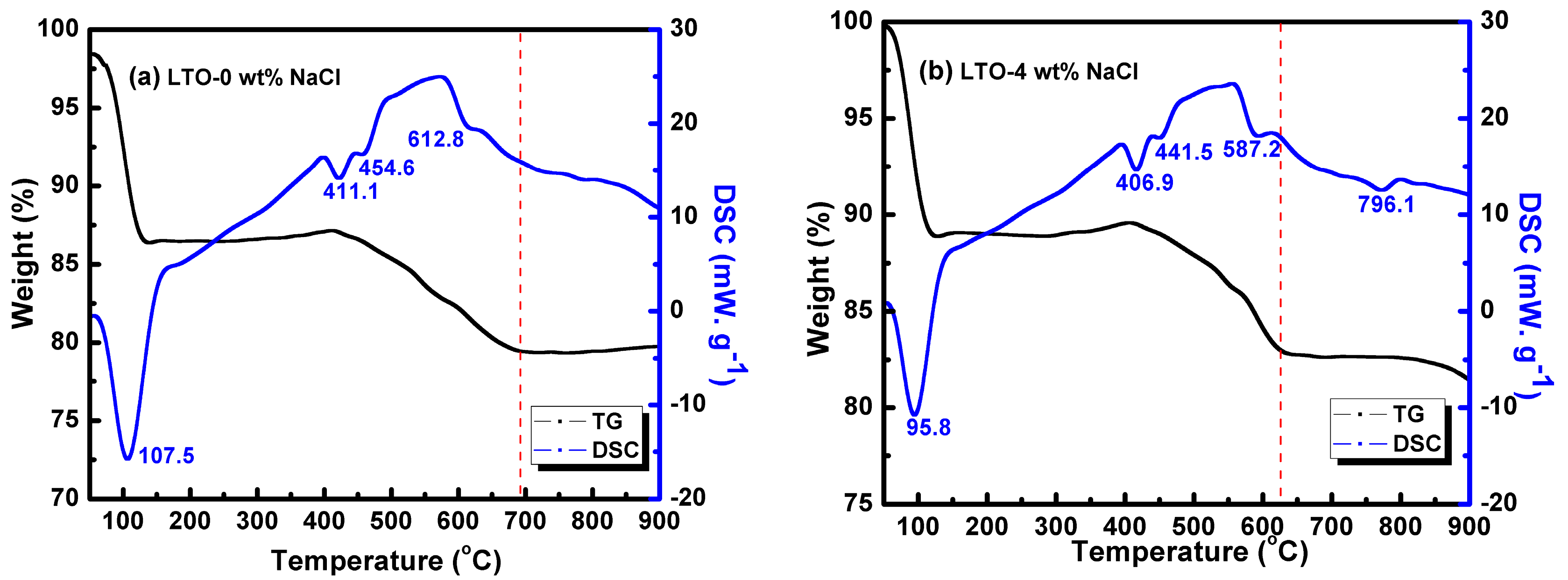
3.5. TG-DSC Study of Salt-Assisted LTO Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ganesan, M. Li4Ti2.5Cr2.5O12 as anode material for lithium battery. Ionics (Kiel) 2008, 395–401. [Google Scholar] [CrossRef]
- Gao, L.; Liu, R.; Hu, H.; Li, G.; Yu, Y. Carbon-decorated Li4Ti5O12/rutile TiO2 mesoporous microspheres with nanostructures as high-performance anode materials in lithium-ion batteries. Nanotechnology 2014, 175402. [Google Scholar] [CrossRef]
- Jung, H.; Jang, M.W.; Hassoun, J.; Sun, Y.; Scrosati, B. A high-rate long-life Li4Ti5O12/Li[Ni0.45Co0.1Mn1.45]O4 lithium-ion battery. Nat. Commun. 2011, 1–5. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Jia, D.; Bao, S.; Guo, Z.P. Preparation and characterization of novel spinel Li4Ti5O12—x Brx anode materials. Electrochim. Acta 2009, 54, 4772–4776. [Google Scholar] [CrossRef]
- Yao, X.L.; Xie, S.; Chen, C.H.; Wang, Q.S.; Sun, J.H.; Li, Y.L.; Lu, S.X. Comparisons of graphite and spinel Li1.33Ti1.67O4 as anode materials for rechargeable lithium-ion batteries. Electrochim. Acta 2005, 50, 4076–4081. [Google Scholar] [CrossRef]
- Molkenova, A.; Taniguchi, I. Preparation and characterization of SiO2/C nanocomposites by a combination of mechanochemical-assisted sol-gel and dry ball milling processes. Adv. Powder Technol. 2015, 26, 377–384. [Google Scholar] [CrossRef]
- Saito, G.; Zhu, C.; Han, C.; Sakaguchi, N.; Akiyama, T. Solution combustion synthesis of porous Sn-C composite as anode material for lithium ion batteries. Adv. Powder Technol. 2016, 3. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Yang, J. Cluster structure of SnO2/SnCo composites as anodes for lithium ion batteries. Adv. Powder Technol. 2014, 1–7. [Google Scholar] [CrossRef]
- Maddipatla, R.; Loka, C.; Choi, W.J.; Lee, K.S. Nanocomposite of Si/C anode material prepared by hybrid process of high-energy mechanical milling and carbonization for Li-ion secondary batteries. Appl. Sci. 2018, 8, 2140. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Bai, Z. The progress of cobalt-based anode materials for lithium ion batteries and sodium ion batteries. Appl. Sci. 2020, 10, 3098. [Google Scholar] [CrossRef]
- Shi, H.; Liu, X.; Wu, R.; Zheng, Y.; Li, Y.; Cheng, X.; Pfleging, W.; Zhang, Y. In situ SEM observation of structured Si/C anodes reactions in an ionic-liquid-based lithium-ion battery. Appl. Sci. 2019, 9, 956. [Google Scholar] [CrossRef]
- Qi, Z.; Shan, Z.; Ma, W.; Li, L.; Wang, S.; Li, C.; Wang, Z. Strain analysis on electrochemical failures of nanoscale silicon electrode based on three-dimensional in situ measurement. Appl. Sci. 2020, 10, 468. [Google Scholar] [CrossRef]
- Zuniga, L.; Gonzalez, G.; Chavez, R.O.; Myers, J.C.; Lodge, T.P.; Alcoutlabi, M. Centrifugally spun α-Fe2O3/TiO2/carbon composite fibers as anode materials for lithium-ion batteries. Appl. Sci. 2019, 9, 4032. [Google Scholar] [CrossRef]
- Lu, L.; Hu, Y.; Dai, K. The advance of fiber-shaped lithium ion batteries. Mater. Today Chem. 2017, 5, 24–33. [Google Scholar] [CrossRef]
- Rutkowska, A.; Konrad, S.; Sitarz, M. Hierarchically structured lithium titanate for ultrafast charging in long-life high capacity batteries. Nat. Commun. 2017, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, L.; Pan, H.; Lu, X.; Gu, L.; Hu, Y.; Li, H.; Armand, M.; Ikuhara, Y.; Chen, L.; et al. Direct atomic-scale confirmation of three-phase storage mechanism in Li4Ti5O12 anodes for room-temperature sodium-ion batteries. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Zhao, B.; Ran, R.; Cai, R.; Shao, Z. Synthesis of well-crystallized Li4Ti5O12 nanoplates for lithium-ion batteries with oustanding rate capability and cycling stability. J. Mater. Chem. A 2013, 13233–13243. [Google Scholar] [CrossRef]
- Shen, Y.; Eltzholtz, J.R.; Iversen, B.B. Controlling Size, Crystallinity, and Electrochemical Performance of Li 4 Ti 5 O 12 Nanocrystals. Chem. Mater. 2013, 25, 5023–5030. [Google Scholar] [CrossRef]
- Yuan, T.; Cai, R.; Ran, R.; Zhou, Y.; Shao, Z. A mechanism study of synthesis of Li4Ti5O12 from TiO2 anatase. J. Alloys Compd. 2010, 505, 367–373. [Google Scholar] [CrossRef]
- Guerfi, A.; Charest, P.; Kinoshita, K.; Perrier, M.; Zaghib, K. Nano electronically conductive titanium-spinel as lithium ion storage negative electrode. J. Power Sources 2004, 126, 163–168. [Google Scholar] [CrossRef]
- Shin, J.; Hong, C.; Yoon, D. Effects of TiO2 Starting Materials on the Solid-State Formation of Li4Ti5O12. J. Am. Ceram. Soc. 2012, 7, 1–7. [Google Scholar] [CrossRef]
- Hao, Y.; Lai, Q.; Lu, J.; Wang, H.; Chen, Y.; Ji, X. Synthesis and characterization of spinel Li4Ti5O12 anode material by oxalic acid-assisted sol-gel method. J. Inorg. Mater. 2006, 158, 1358–1364. [Google Scholar] [CrossRef]
- Rho, Y.H.K. Li+ ion diffusion in Li4Ti5O12 thin film electrode prepared by PVP sol-gel method. Solid State Chem. 2004, 177, 2094–2100. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, X.; Zhou, Y.; Li, H. Preparation and characterization of nanocrystalline Li4Ti5O12 by sol-gel method. Mater. Chem. Phys. 2002, 78, 437–441. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Zhang, F. In situ growth of Li4Ti5O12 on multi-walled carbon nanotubes: Novel coaxial nanocables for high rate lithium ion batteries. J. Mater. Chem. 2011, 761–767. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.; Zhang, X.; Yang, H. Microwave solid-state synthesis of spinel Li4Ti5O12 nanocrystallites as anode material for lithium-ion batteries. Solid State Ion. 2007, 178, 1590–1594. [Google Scholar] [CrossRef]
- Nowack, L.V.; Waser, O.; Yarema, O.W. Rapid, microwave-assisted synthesis of battery-grade lithium titanate (LTO). RSC Adv. 2013, 3, 15618. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef]
- Ju, S.H.; Kim, J.H.; Kang, Y.C. Electrochemical Properties of LiNi0.8Co0.2-xAlxO2 (0≤x≤0.1) Cathode Particles Prepared by Spray Pyrolysis from the Spray Solutions With and Without Organic. Met. Mater. Int. Vol. 2010, 16, 299–303. [Google Scholar] [CrossRef]
- Nugroho, A.; Jin, S.; Yoon, K.; Cho, B.; Lee, Y.; Kim, J. Electrochemistry Communications Facile synthesis of nanosized Li4Ti5O12 in supercritical water. Electrochem. Commun. 2011, 13, 650–653. [Google Scholar] [CrossRef]
- Nugroho, A.; Jin, S.; Yoon, K.; Kim, J. Synthesis of Li4Ti5O12 in supercritical water for Li-ion batteries: Reaction mechanism and high-rate performance. Electrochim. Acta 2012, 78, 623–632. [Google Scholar] [CrossRef]
- Bach, S.; Pereira-Ramos, J.P.; Baffler, N. Electrochemical properties of sol-gel Li4/3Ti5/3O4. J. Power Sources 1999, 81–82, 273–276. [Google Scholar] [CrossRef]
- Yin, S.Y.; Feng, C.Q.; Wu, S.J.; Liu, H.L.; Ke, B.Q.; Zhang, K.L.; Chen, D.H. Molten salt synthesis of sodium lithium titanium oxide anode material for lithium ion batteries. J. Alloy Compd. 2015, 642, 1–6. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, F.; Wu, F.; Wu, C.; Bao, L. Influence of composite LiCl-KCl molten salt on microstructure and electrochemical performance of spinel Li4Ti5O12. Electrochim. Acta 2008, 54, 322–327. [Google Scholar] [CrossRef]
- Laumann, A.; Bremholm, M.; Hald, P.; Holzapfel, M.; Fehr, K.T.; Brummerstedt, B. Rapid Green Continuous Flow Supercritical Synthesis of High Performance Li4Ti5O12 Nanocrystals for Li Ion Battery Applications. J. Electrochem. Soc. 2012, 159, 166–171. [Google Scholar] [CrossRef]
- Shen, L.; Guo, L.; Bao, N.; Yanagisawa, K. Salt-assisted Solid-state Chemical Reaction. Synthesis of ZnO Nanocrystals. Chem. Lett. 2003, 32, 9–10. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Deng, X.; Huang, Z.; Zhang, Y. Salt-assisted solution combustion synthesis of ZnFe2O4 nanoparticles and photocatalytic activity with TiO2 (P25) as nanocomposite. J. Ceram. Soc. Jpn. 2012, 2, 579–583. [Google Scholar] [CrossRef][Green Version]
- Hwang, T.; An, G.; Cho, J.; Kim, J.; Choa, Y. Effects of Different Salts on Salt-Assisted Ultrasonic Spray Pyrolysis (SA-USP) Calcination for the Synthesis of Strontium Ferrite. J. Nanosci. Nanotechnol. 2015, 15, 8062–8069. [Google Scholar] [CrossRef]
- Purwanto, A.; Widiyandari, H.; Suryana, R.; Jumari, A. Improving the performance of fl uorine-doped tin oxide by adding salt. Thin Solid Films 2015, 586, 41–45. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Li, G.; Zhuang, W.; Yin, G.; Ren, Y.; Ding, Y. Energy management strategy and size optimization of a LFP/LTO hybrid battery system for electric vehicle. SAE Tech. Pap. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Wang, W.E.I.; Choi, D.; Yang, Z. Li-Ion Battery with LiFePO4 Cathode and Li4Ti5O12 Anode for Stationary Energy Storage. Metall. Mater. Trans. A 2013, 44, 21–25. [Google Scholar] [CrossRef]
- Kataoka, K.; Takahashi, Y.; Kijima, N. Single crystal growth and structure refinement of Li4Ti5O12. J. Phys. Chem. Solids 2008, 69, 1454–1456. [Google Scholar] [CrossRef]
- Zhu, W.; Zhuang, Z.; Yang, Y.; Zhang, R.; Lin, Z. Synthesis and electrochemical performance of hole-rich Li4Ti5O12 anode material for lithium-ion secondary batteries. J. Phys. Chem. Solids 2016, 93, 52–58. [Google Scholar] [CrossRef]
- Wang, S.; Quan, W.; Zhu, Z.; Yang, Y.; Liu, Q.; Ren, Y.; Zhang, X.; Xu, R.; Hong, Y.; Zhang, Z.; et al. Lithium titanate hydrates with superfast and stable cycling cycling in lithium ion batteries. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, N.; Sun, K. A novel grain restraint strategy to synthesize highly crystallized Li4Ti5O12 (~20 nm) for lithium ion batteries with superior high-rate performance. J. Mater. Chem. 2012, 22, 11688. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Yamato, R.; Ohzuku, T. Zero-strain insertion mechanism of Li[Li1/3Ti5/3]O4 for advanced lithium-ion (shuttlecock) batteries. Electrochim. Acta 2005, 51, 1125–1129. [Google Scholar] [CrossRef]
- Yi, T.-F.; Yang, S.-Y.; Xie, Y. Recent Advances of Li4Ti5O12 as Promising Next Generation Anode Material for High Powe Lithium-ion Batteries. J. Mater. Chem. A 2015. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium titanate as anode material for lithium-ion cells: A review. Ionics 2014, 601–620. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Z.; Mao, J. Mechanisms of the decrease in low-temperature electrochemical performance of Li4Ti5O12-based anode materials. Sci. Rep. 2017, 7, 15292. [Google Scholar] [CrossRef]
- Cai, W.; Chen, R.; Yang, Y.; Yi, M.; Xiang, L. Removal of SO42− from Li2CO3 by Recrystallization in in Na2CO3 Solution. Crystals 2018, 8, 19. [Google Scholar] [CrossRef]
- Muzayanha, S.U.; Yudha, C.S.; Nur, A.; Widiyandari, H.; Haerudin, H.; Nilasary, H.; Fathoni, F.; Purwanto, A. A Fast Metals Recovery Method for the Synthesis of Lithium Nickel Cobalt Aluminum Oxide Material from Cathode Waste. Metals (Basel) 2019, 9, 615. [Google Scholar] [CrossRef]
- Tseng, W.J.; Kao, S. Effect of seed particles on crystallization and crystallite size of anatase TiO2 nanocrystals by solvothermal treatment. Adv. Powder Technol. 2015, 26, 1225–1229. [Google Scholar] [CrossRef]
- Kong, J.; Chao, B.; Wang, T.; Yan, Y. Preparation of ultra fine spherical AlOOH and Al2O3 powders by aqueous precipitation method with mixed surfactants. Powder Technol. 2012, 229, 7–16. [Google Scholar] [CrossRef]
- Yang, C.; Hu, H.; Lin, S.J.; Chien, W. Electrochemical performance of V-doped spinel Li4Ti5O12/C composite anode in Li-half and Li4Ti5O12/LiFePO4-full cell. J. Power Sources 2014, 258, 424–433. [Google Scholar] [CrossRef]
- Gao, J.; Ying, J.; Jiang, C. Preparation and characterization of spherical La-doped Li4Ti5O12 anode material for lithium ion batteries. Ionics (Kiel) 2009, 15, 597–601. [Google Scholar] [CrossRef]
- Ju, S.H.; Kang, Y.C. Characteristics of spherical-shaped Li4Ti5O12 anode powders prepared by spray pyrolysis. Phys. Chem. Solids 2009, 70, 40–44. [Google Scholar] [CrossRef]
- Mahmoud, A.; Saadoune, I.; Lippens, P.; Chamas, M. The design and study of new Li-ion full cells of LiCo2/3Ni1/6Mn1/6O2 positve electrode paired with MnSn2 and Li4Ti5O12 negative electrodes. Solid State Ion. 2017, 300, 175–181. [Google Scholar] [CrossRef]
- Bai, X.; Li, T.; Bai, Y.J. Capacity degradation of Li4Ti5O12 during long-term cycling in terms of composition and structure. Dalton Trans. 2020, 49, 10003–10010. [Google Scholar] [CrossRef]
- Jian, Z.; Hwang, S.; Li, Z.; Hernandez, A.S.; Wang, X.; Xing, Z.; Su, D.; Ji, X. Hard–Soft Composite Carbon as a Long-Cycling and High-Rate Anode for Potassium-Ion Batteries. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Yang, X.; Tang, W.; Liu, Z.; Makita, Y.; Kasaishi, S. Co-Precipitation Synthesis of Acetylene Black Õ Li-Birnessite Composite Suitable for a Li-Rechargeable Battery. J. Electrochem. Soc. 2002, 191–194. [Google Scholar] [CrossRef]
- Gao, J.; Gong, B.; Zhang, Q.; Wang, G. Study of the surface reaction mechanism of Li4Ti5O12 anode for lithium-ion cells. Ionics (Kiel) 2016, 21. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H. Gassing in Li4Ti5O12-based batteries and its remedy. Sci. Rep. 2012, 2, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xue, L.; Zhang, W.; Fan, X.; Yan, Y.; Li, Q.; Huang, Y.; Zhang, W. Hydrothermal Synthesis of Li4Ti5O12/TiO2 Nano-composite As High Performance Anode Material for Li-Ion Batteries. Electrochim. Acta 2014, 147, 506–512. [Google Scholar] [CrossRef]
- Sato, Y.; Takeda, O. Hydrogen Storage and Transportation System through Lithium Hydride Using Molten Salt Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 451–470. [Google Scholar]
- Qiu, Z.; Zhang, Y.; Xia, S.; Yao, Y. A facile method for synthesis of LiNi0.8Co0.15Al0.05O2 cathode material. Solid State Ion. 2017, 307, 73–78. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl-NaCl system Condensation in the KCl-NaCl system. Fuel Process. Technol. 2011, 105, 142–148. [Google Scholar] [CrossRef]
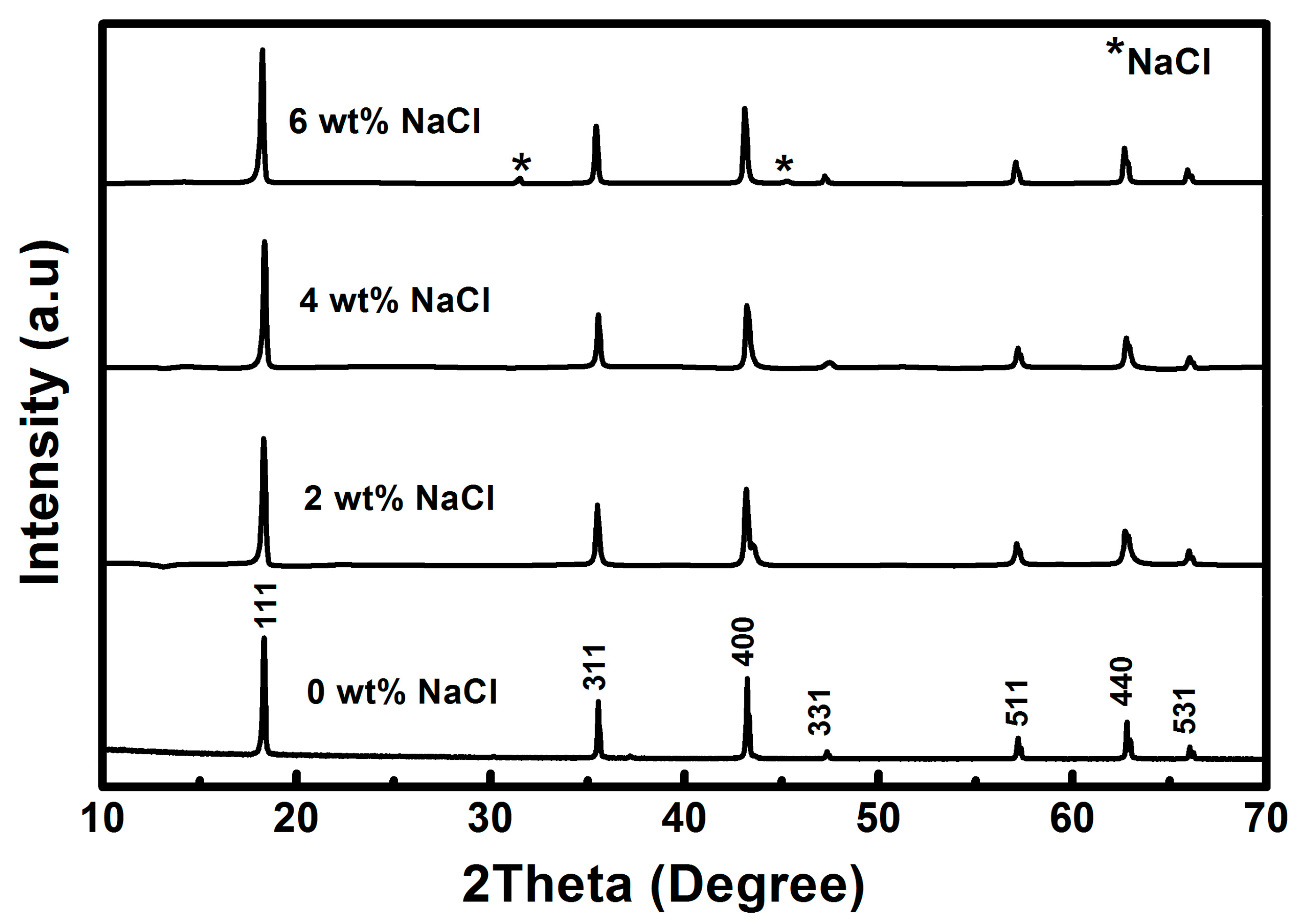
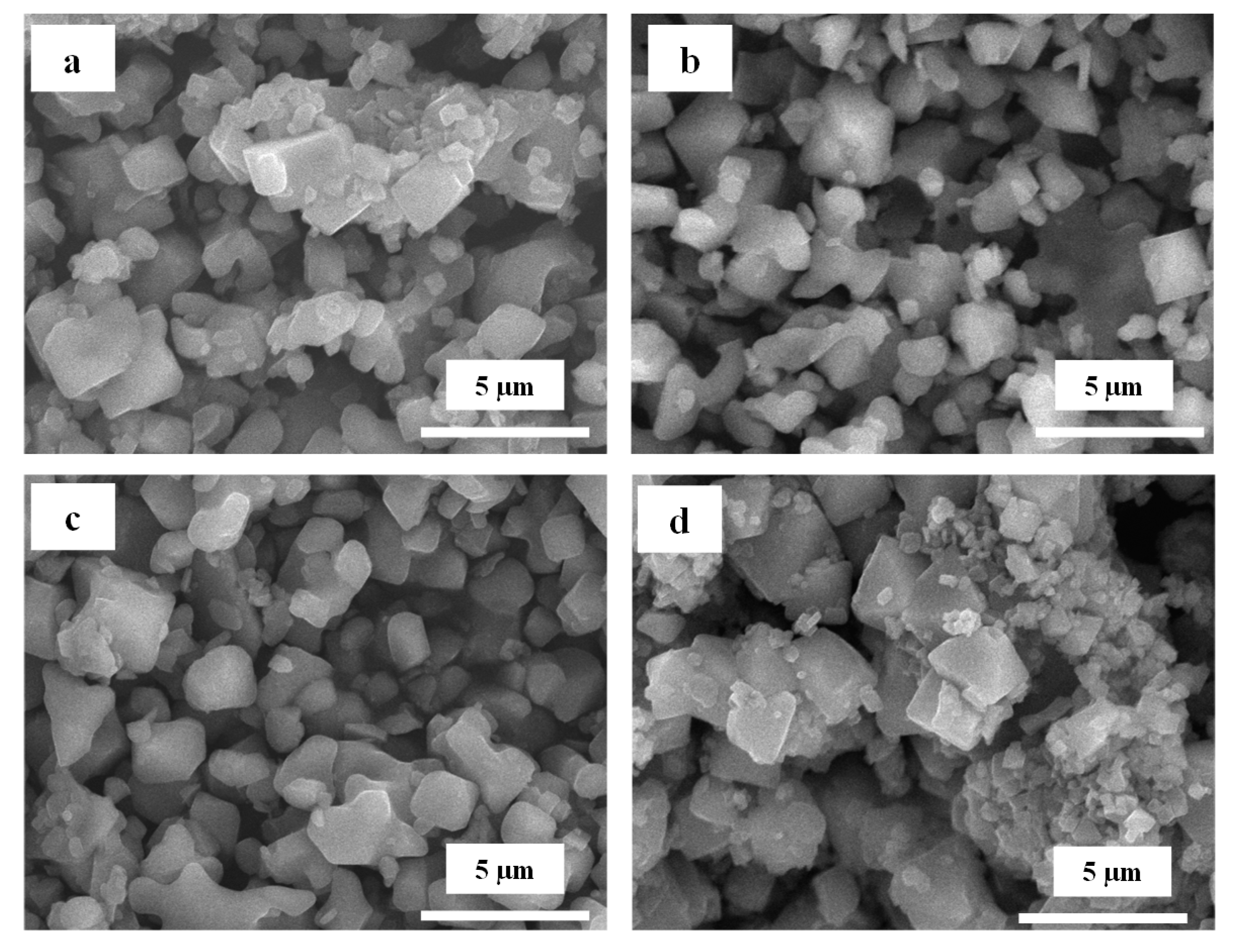
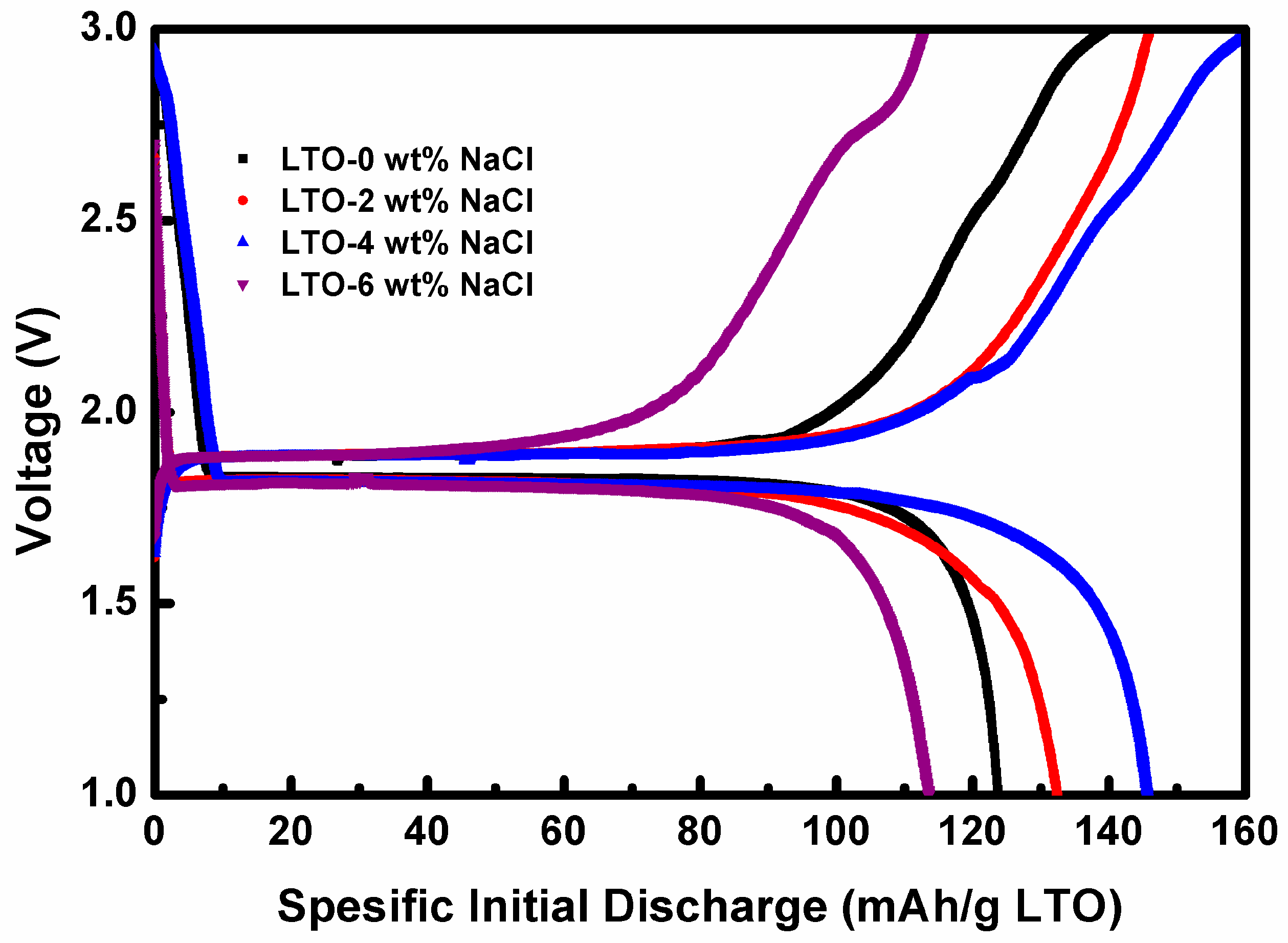

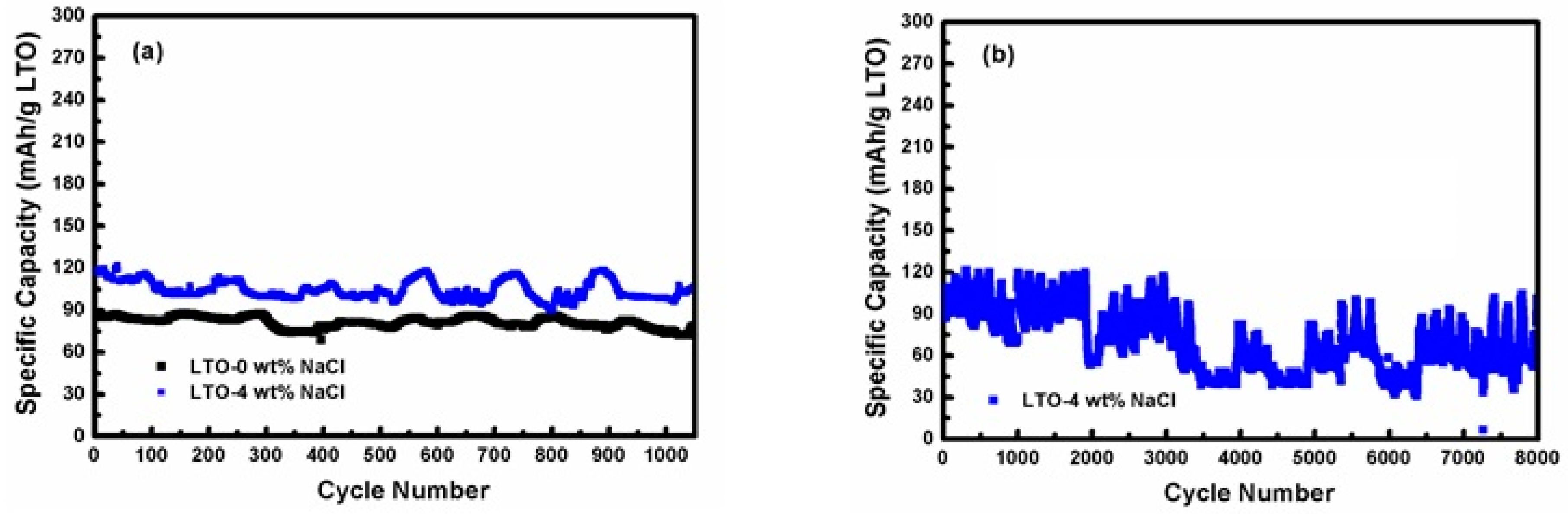
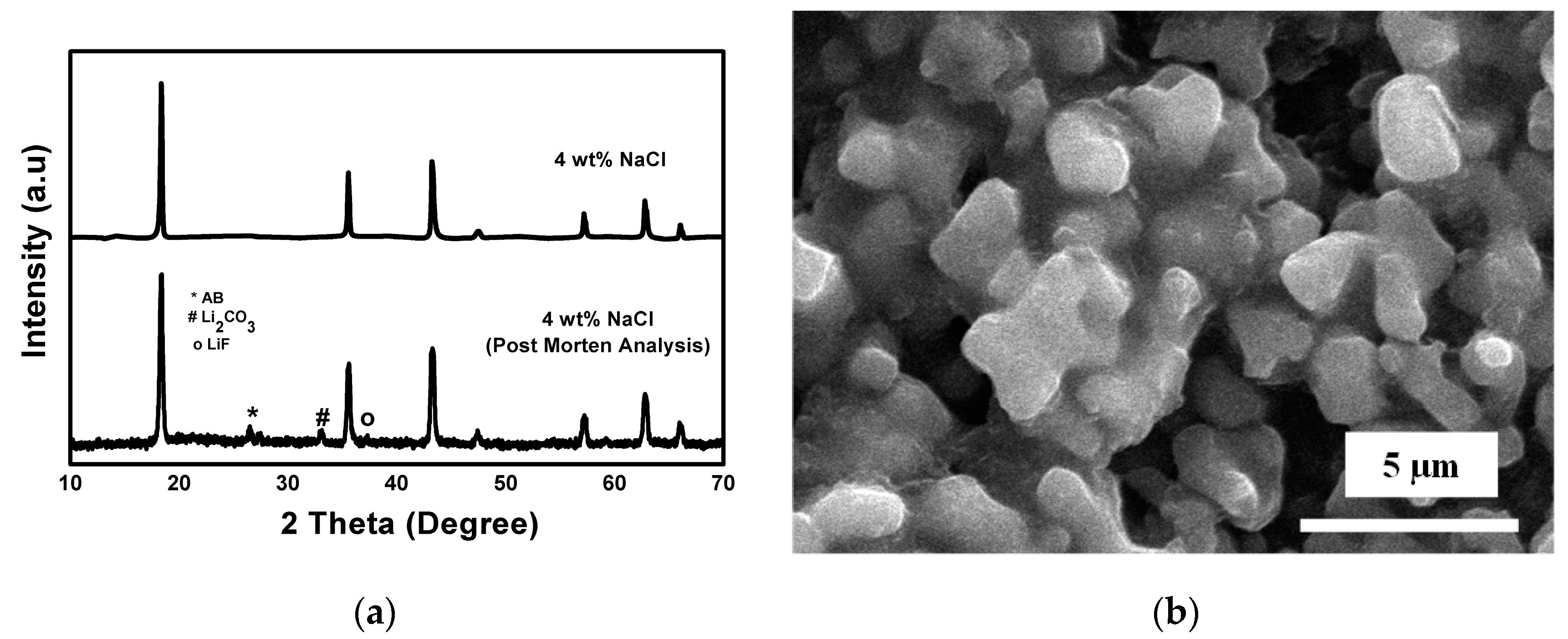
| LTO | Lattice Constant (Å) | FWHM (°) | Crystallite Size (nm) | ||||
|---|---|---|---|---|---|---|---|
| (111) | (311) | (400) | (111) | (311) | (400) | ||
| 0 wt % NaCl | 8.377 | 0.321 | 0.266 | 0.272 | 26.94 | 32.72 | 32.80 |
| 2 wt % NaCl | 8.373 | 0.197 | 0.140 | 0.170 | 42.66 | 62.17 | 52.48 |
| 4 wt % NaCl | 8.362 | 0.154 | 0.137 | 0.168 | 54.57 | 63.53 | 53.11 |
| 6 wt % NaCl | 8.384 | 0.151 | 0.135 | 0.146 | 55.66 | 64.47 | 61.11 |
| LTO | Capacity Retention (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0.3 C | 1 C | 3 C | 5 C | 7 C | 10 C | 0.3 C | |
| LTO-0 wt % NaCl | 100 | 97.1 | 90.3 | 80.3 | 75.0 | 69.2 | 99.7 |
| LTO-4 wt % NaCl | 100 | 97.4 | 91.8 | 86.6 | 84.9 | 81.4 | 99.9 |
| LTO Precursors | Methods | Particles Size | Electrochemical Testing | Initial Specific Capacity (mAh/g) | Retention Capacity | Rate Performances | Ref. |
|---|---|---|---|---|---|---|---|
| TiCl4 + Li2CO3 | Sol–gel | 5–20 µm | Half Cell | 158 mAh/g at 0.1 C | 75.95% after 50 cycles at 0.1 C | 70 mAh/g at 1 C | [56] |
| LiNO3 + TTIP | Spray pyrolysis | 1.5 µm | Half Cell | 175 mAh/g at 0.1 C | 81.14% after 30 cycles at 0.1 C | 105 mAh/g at 1C | [57] |
| LiOH.H2O + TTIP | Supercritical Hydrothermal | 20–50 nm | Half Cell | 149.6 mAh/g at 1 C | 95.52% after 50 cycles at 1C | 108 mAh/g at 8C 98 mAh/g at 10 C | [31] |
| LiOH.H2O + TiO2 | Molten salt (LiCl-KCl) | 4–5 µm | Half Cell | 169 mAh/g at 0.2 C | 93.49% after 50 cycles at 0.2 C | 130 mAh/g at 5C | [34] |
| Li2CO3 + TiO2 | Solid state (coated carbon) | 1–3 µm | Full cell (LTO vs. LFP) | 180 mAh/g at 0.1 C | 97.77% after 400 cycles at 1 C charging and 3 C discharging | 80 mAh/g at 0.2 C charging and 10 C discharging | [55] |
| TiCl4 + LiOH.H2O | Sol–gel | 360 nm | Full Cell (LTO vs. NMC) | 149 mAh/g at 1 C | 93.29% after 150 cycles at 1 C | 40 mAh/g at 10 C | [58] |
| Commercial LTO | - | 2 µm | Full Cell (LTO vs. LFP) | 147 mAh/g at 0.1 C | 51% after 700 cycles at 0.5 C | 9 mAh/g at 10 C | [42] |
| LiOH + TiO2 +NaCl | Salt-assisted solid state | 1.83–2.28 µm | Full Cell (LTO vs. LFP) | 145.6 mAh/g at 0.3 C | 98% after 8000 cycles at 10C | 118 mAh/g at 10 C | This study |
| LTO | Lattice Constant (Å) | FWHM | Crystallite Size (nm) | ||||
|---|---|---|---|---|---|---|---|
| 4 wt % NaCl | 8.362 | 0.154 | 0.137 | 0.168 | 54.57 | 63.53 | 53.11 |
| 4 wt % NaCl (post mortem) | 8.357 | 0.161 | 0.142 | 0.175 | 52.23 | 59.22 | 48.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purwanto, A.; Muzayanha, S.U.; Yudha, C.S.; Widiyandari, H.; Jumari, A.; Dyartanti, E.R.; Nizam, M.; Putra, M.I. High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery. Appl. Sci. 2020, 10, 7135. https://doi.org/10.3390/app10207135
Purwanto A, Muzayanha SU, Yudha CS, Widiyandari H, Jumari A, Dyartanti ER, Nizam M, Putra MI. High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery. Applied Sciences. 2020; 10(20):7135. https://doi.org/10.3390/app10207135
Chicago/Turabian StylePurwanto, Agus, Soraya Ulfa Muzayanha, Cornelius Satria Yudha, Hendri Widiyandari, Arif Jumari, Endah Retno Dyartanti, Muhammad Nizam, and Muhamad Iqbal Putra. 2020. "High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery" Applied Sciences 10, no. 20: 7135. https://doi.org/10.3390/app10207135
APA StylePurwanto, A., Muzayanha, S. U., Yudha, C. S., Widiyandari, H., Jumari, A., Dyartanti, E. R., Nizam, M., & Putra, M. I. (2020). High Performance of Salt-Modified–LTO Anode in LiFePO4 Battery. Applied Sciences, 10(20), 7135. https://doi.org/10.3390/app10207135