Strain Analysis on Electrochemical Failures of Nanoscale Silicon Electrode Based on Three-Dimensional In Situ Measurement
Abstract
1. Introduction
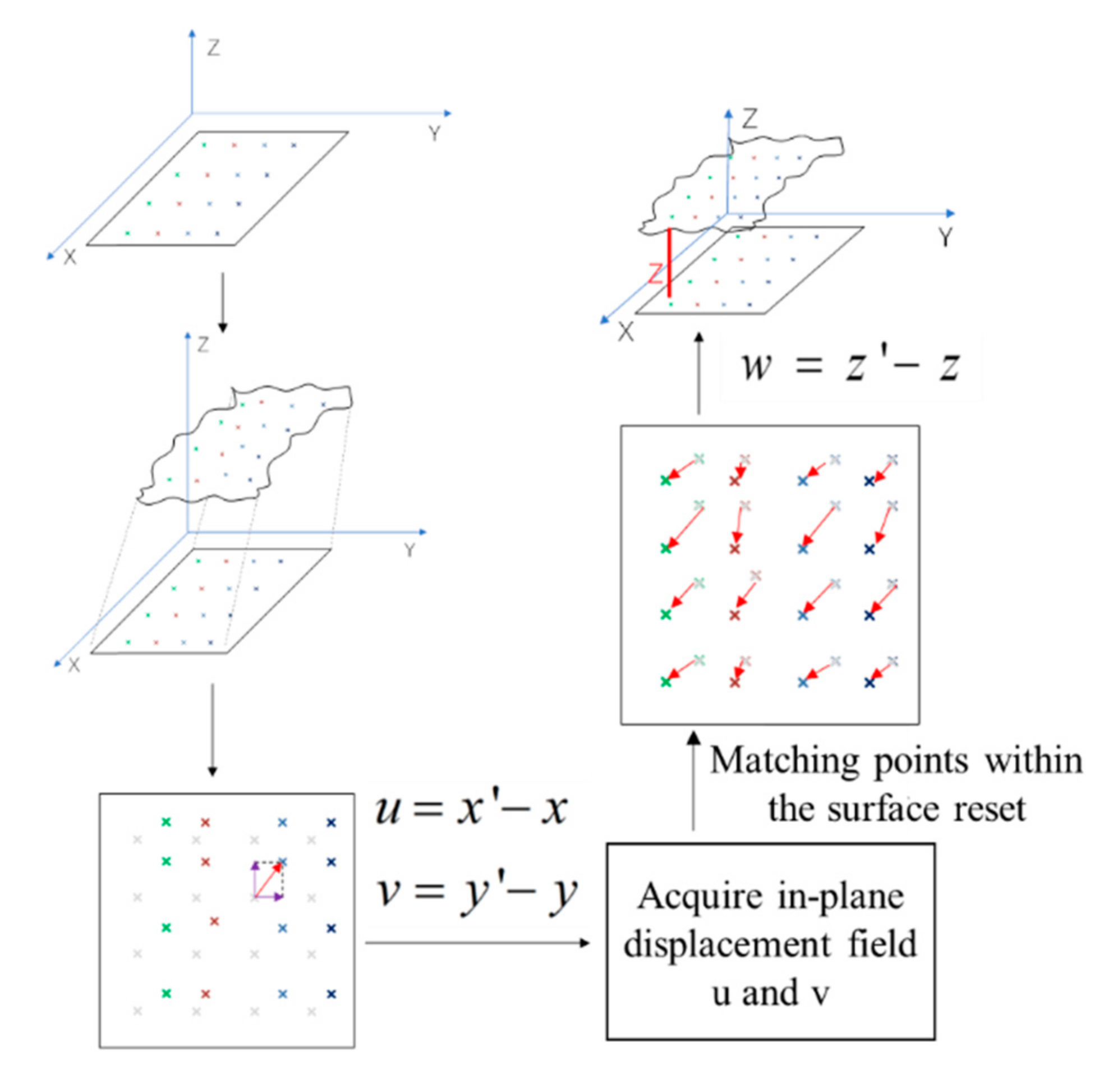
2. Measurement Principle
- (1)
- Images of 256 bit grey-level in laser mode and the surface shape data of the tested electrode are simultaneously captured with an LCSM before the electrode deforms, expressed as and , respectively. Note that which denotes the grey-level values distribution and which denotes the height values distribution are the functions of the in-plane coordinate values .
- (2)
- Images of 256 bit grey-level (in color mode) and the surface shape data (in laser mode) of the tested the electrode are simultaneously captured after the electrode deforms, expressed as and , respectively.
- (3)
- In-plane displacement distribution of the electrode surface can be determined with color mode images by DIC technique. The displacement along two mutually perpendicular directions can be expressed as and , respectively.
- (4)
- The 3D surface is shifted back to its original location (before it deforms) by subtracting the in-plane displacement distribution values, rewritten as .
- (5)
- Decoupled out-of-plane displacement by subtracting from . The decoupled out-of-plane displacement is written as .
3. Experimental
3.1. Sample Preparation
3.2. Electrochemical Cycling Experiments
3.3. Imaging Processing Parameters
4. Results and Discussion
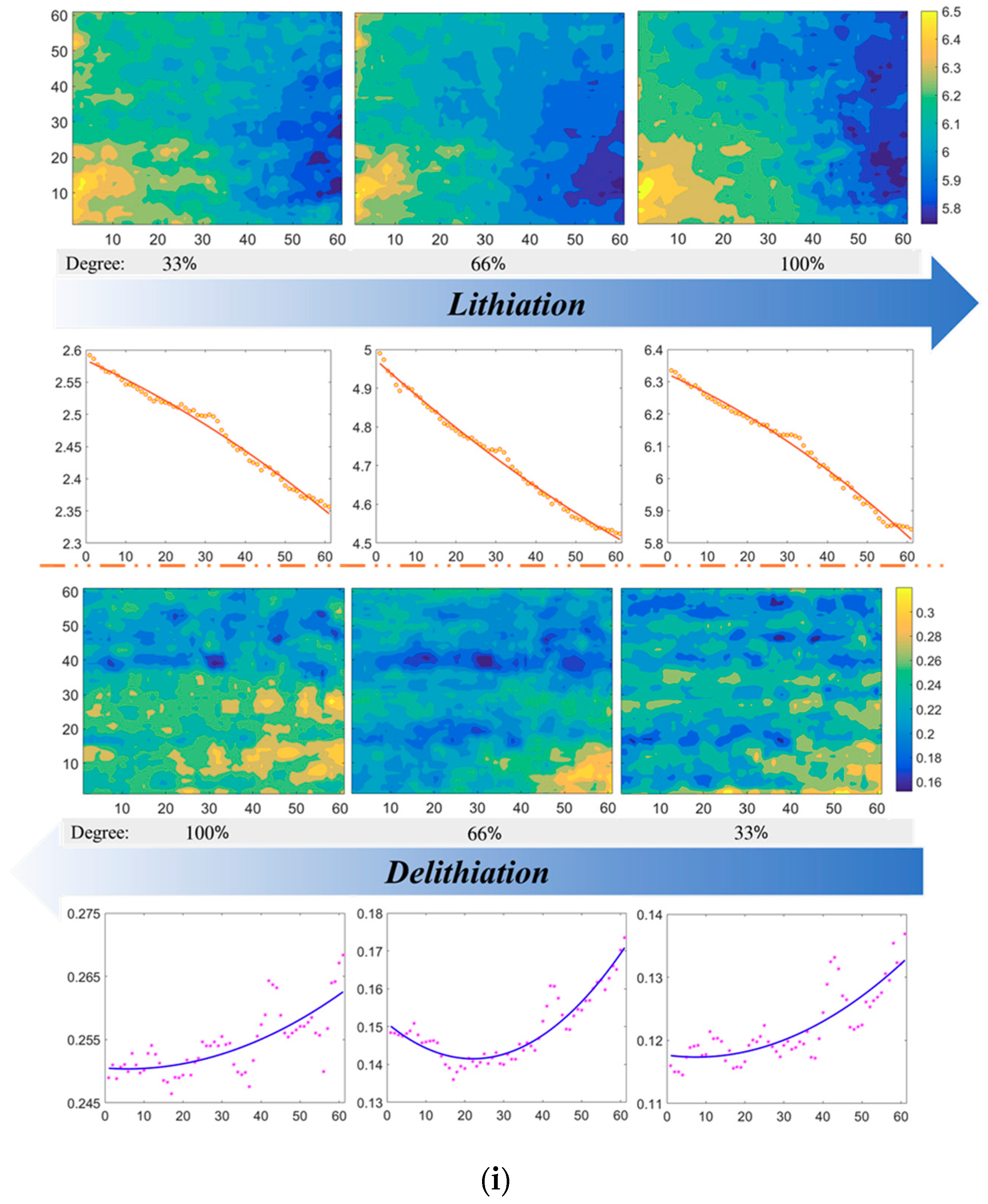
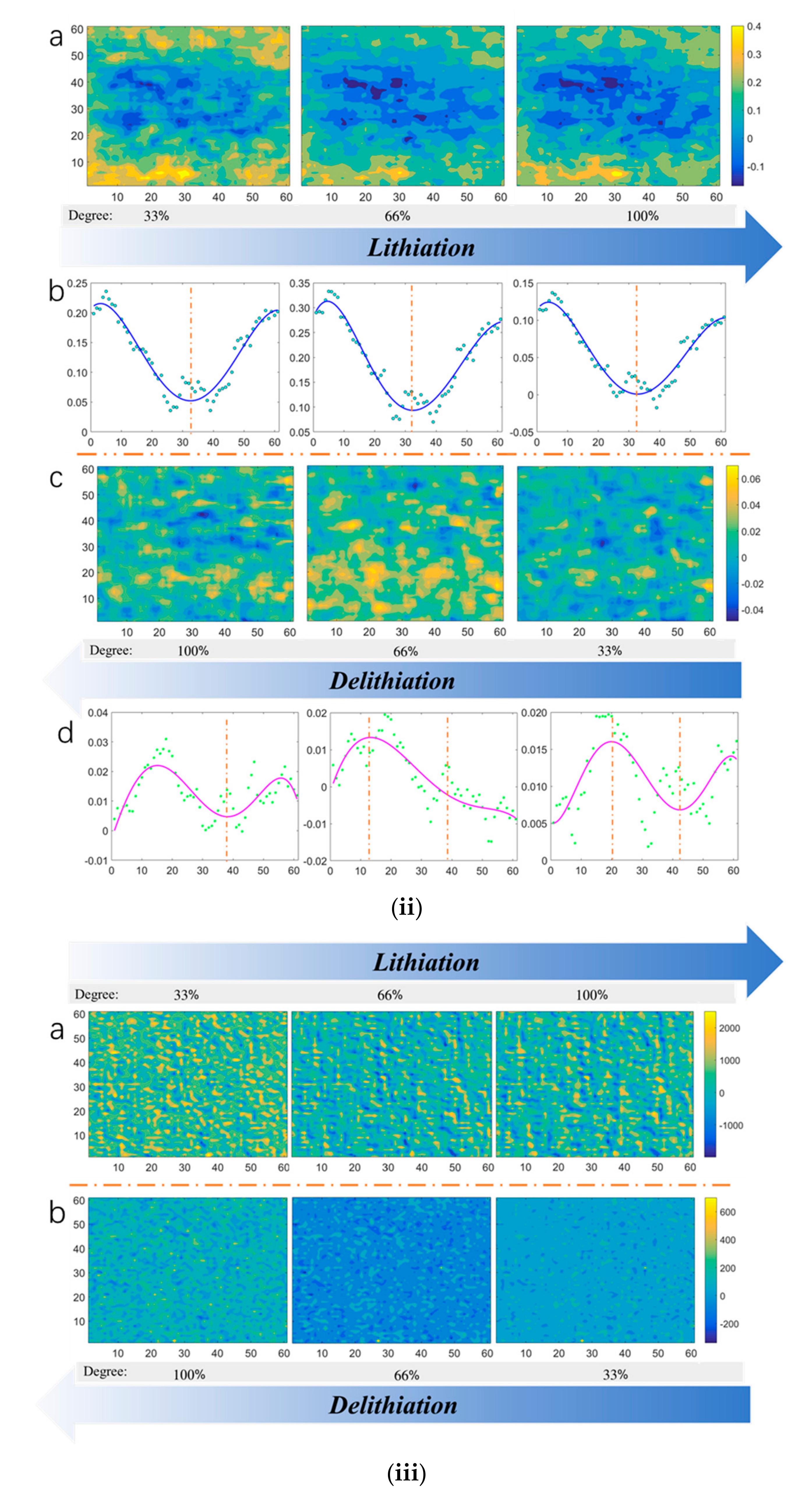
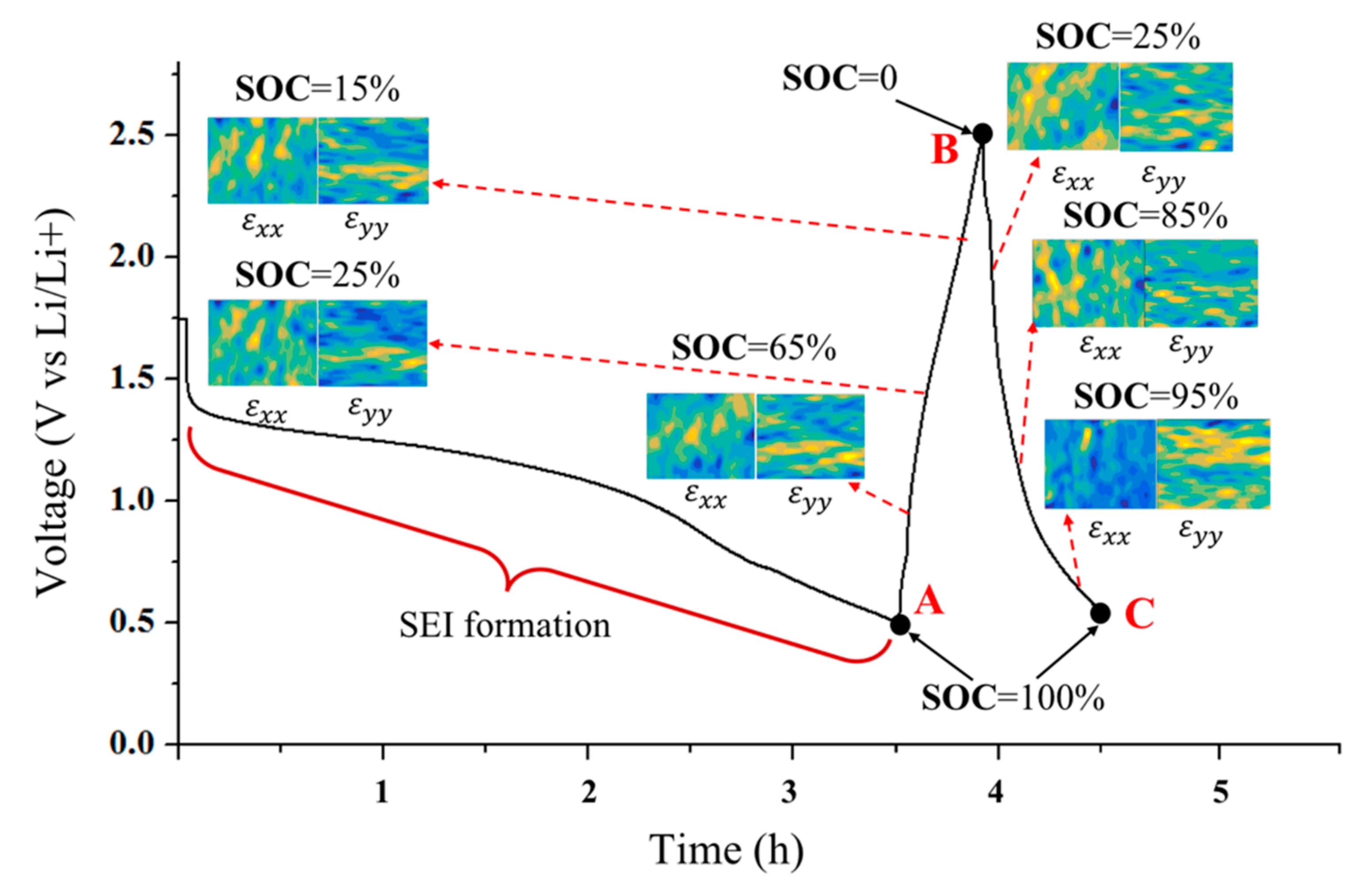
4.1. Surface Shape/strain Distribution of the Si Electrode
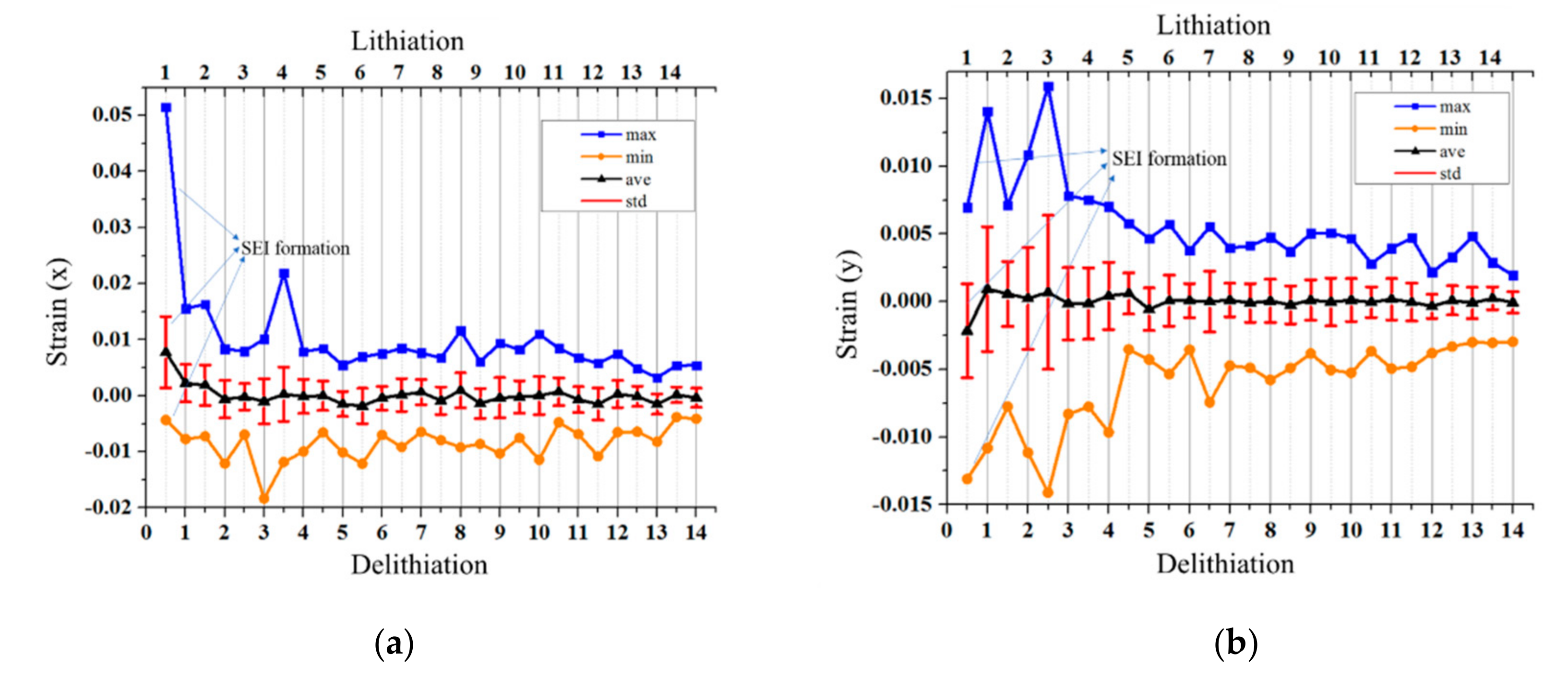
4.2. Strain Criterion of Electrochemical/Mechanical Failures
- (i)
- Both the out-of-plane displacement and the in-plane strain during the lithiation/delithiation process show great discreteness over a large surface area. Positive strains and negative strains exist simultaneously, which indicates that the stress distribution is inhomogeneous.
- (ii)
- Average strains slightly fluctuate with the cycles evolving, which indicates that electrochemical failures are not uncorrelated with average strains. In other words, single point analysis of strain/stress is not feasible in investigating electrochemical failures of electrode.
- (iii)
- MSG values mainly decreases with the cycle number increasing. The trend indicates that the strain distribution tends to be uniform with the electrochemical reaction of working electrode. Furthermore, the trend of MSGs are opposite in lithiation and delithiation processes, which demonstrates that MSG is sensible to the movement directions of Li-ions.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Li, J.; Daniel, C.; Wood, D. Materials Processing for Lithium-Ion Batteries. J. Power Sources 2011, 196, 2452–2460. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef]
- Hsu, C.M.; Connor, S.T.; Tang, M.X.; Cui, Y. Wafer-scale silicon nanopillars and nanocones by Langmuir–Blodgett assembly and etching. Appl. Phys. Lett. 2008, 93, 85. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.; Huggins, R.A.; CUI, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Larcher, D.; Beattie, S.; Morcrette, M.; Edström, K.; Jumas, J.-C.; Tarascon, J.-M. Recent findings and prospects in the field of pure metals as negative electrodes for Li-ion batteries. J. Mater. Chem. 2007, 17, 3759. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Eberman, K.W.; Turner, R.L.; Krause, L.J.; Dahn, J.R. Colossal Reversible Volume Changes in Lithium Alloys. Electrochem. Solid-State Lett. 2001, 4, A137. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103. [Google Scholar] [CrossRef]
- Golmon, S.; Maute, K.; Lee, S.H.; Dunn, M.L. Stress generation in silicon particles during lithium insertion. Appl. Phys. Lett. 2010, 97, 033111. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Chu, T.C.; Ranson, W.F.; Sutton, M.A. Applications of digital-image-correlation techniques to experimental mechanics. Exp. Mech. 1985, 25, 232–244. [Google Scholar] [CrossRef]
- Choi, Y.S.; Pharr, M.; Oh, K.H.; Vlassak, J.J. A simple technique for measuring the fracture energy of lithiated thin-film silicon electrodes at various lithium concentrations. J. Power Sources 2015, 294, 159–166. [Google Scholar] [CrossRef]
- Demirkan, M.T.; Trahey, L.; Karabacak, T. Low-density silicon thin films for lithium-ion battery anodes. Thin Solid Film. 2016, 600, 126–130. [Google Scholar] [CrossRef]
- Beattie, S.D.; Larcher, D.; Morcrette, M.; Simon, B.; Tarascon, J.-M. Si Electrodes for Li-Ion Batteries—A New Way to Look at an Old Problem. J. Electrochem. Soc. 2008, 2, A158–A163. [Google Scholar] [CrossRef]
- Deshpande, R.; Deshpande, R.; Verbrugge, M.; Cheng, Y.-T.; Wang, J.; Liu, P. Battery Cycle Life Prediction with Coupled Chemical Degradation and Fatigue Mechanics. J. Electrochem. Soc. 2012, 159, A1730–A1738. [Google Scholar] [CrossRef]
- Lu, B.; Song, Y.; Guo, Z.; Zhang, J. Modeling of progressive delamination in a thin film driven by diffusion-induced stresses. Int. J. Solids Struct. 2013, 50, 2495–2507. [Google Scholar] [CrossRef]
- Feng, H.; Daining, F. Diffusion-Induced Stresses of Spherical Core-Shell Electrodes. J. Electrochem. Soc. 2013, 4, A595–A600. [Google Scholar]
- Brassart, L.; Suo, Z. Reactive flow in solids. J. Mech. Phys. Solids 2013, 61, 61–77. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, F.; Qu, J. A finite deformation stress-dependent chemical potential and its applications to lithium ion batteries. J. Mech. Phys. Solids 2012, 60, 1280–1295. [Google Scholar] [CrossRef]
- Brassart, L.; Suo, Z. Reactive flow in large-deformation electrodes of lithium-ion batteries. Int. J. Appl. Mech. 2012, 3, 1250023. [Google Scholar] [CrossRef]
- He, Y.-L.; Hu, H.J.; Song, Y.C.; Song, Y.-C.; Guo, Z.-S.; Liu, C.; Zhang, J.-Q. Effects of concentration-dependent elastic modulus on the diffusion of lithium ions and diffusion induced stress in layered battery electrodes. J. Power Sources 2014, 248, 517–523. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Q.; Song, H.; Shi, B.; Kang, Y. Modeling and in situ characterization of lithiation-induced stress in electrodes during the coupled mechano-electro-chemical process. J. Power Sources 2017, 342, 896–903. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Hu, J.; Lu, B.; Cheng, Y.; Zhang, J. In situ measurement of mechanical property and stress evolution in a composite silicon electrode. J. Power Sources 2017, 366, 80–85. [Google Scholar] [CrossRef]
- Liu, M. Finite element analysis of lithium insertion-induced expansion of a silicon thin film on a rigid substrate under potentiostatic operation. J. Power Sources 2015, 275, 760–768. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Jiang, H.; Wei, Y.-J.; Song, W.I.; Fang, D.-N. Failure mechanism of 2D silicon film anodes: In-situ observations and simulations on crack evolution. Chem. Commun. 2018, 10, 1039. [Google Scholar]
- Chen, J.; Thapa, A.K.; Berfield, T.A. In-situ characterization of strain in lithium battery working electrodes. J. Power Sources 2014, 271, 406–413. [Google Scholar] [CrossRef]
- Jones, E.M.C.; Silberstein, M.N.; White, S.R.; Sottos, N.R. In Situ Measurements of Strains in Composite Battery Electrodes during Electrochemical Cycling. Exp. Mech. 2014, 54, 971–985. [Google Scholar] [CrossRef]
- Haimei, X.; Haibin, S.; Yilan, K.; Jianshan, W. In Situ Experimental Measurement of the Mechanical Properties of Carbon-Based Electrodes during the Electrochemical Process. J. Electrochem. Soc. 2018, 165, A2069–A2074. [Google Scholar]
- Wang, Y.; Liu, Y.; Zheng, J.; Zheng, H.; Mei, Z.; Du, X.; Li, H. Electrochemical performances and volume variation of nano-textured silicon thin films as anodes for lithium-ion batteries. Nanotechnology 2013, 24, 424011. [Google Scholar] [CrossRef] [PubMed]
- Bing, P.; Huimin, X.; Bo-qin, X.; Fu-long, D. Performance of sub-pixel registration algorithms in digital image correlation. Meas. Sci. Technol. 2006, 17, 1615. [Google Scholar] [CrossRef]
- Pan, B.; Qian, K.; Xie, H.; Asundi, A. Two-dimensional digital image correlation for in-plane displacement and strain measurement: A review. Meas. Sci. Technol. 2009, 20, 62001. [Google Scholar] [CrossRef]
- Li, C.; Xie, H.; Liu, Z.; Liu, S. Microscopic 3D structural inversion technique for multilevel periodic structures with SEM moiré method. J. Opt. 2014, 16, 102001. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Xie, H. Novel scanning electron microscope bulge test technique integrated with loading function. Rev. Sci. Instrum. 2014, 85, 103709. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Xie, H.; Wu, D. Statistics-based electron Moiré technique: A novel method applied to the characterization of mesoporous structures. Nanoscale 2014, 6, 13409–13415. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Xie, H.; Wu, D. Novel 3D SEM Moire method for micro height measurement. Opt. Express 2013, 21, 15734–15746. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Xie, H. A measurement method for micro 3D shape based on grids-processing and stereovision technology. Meas. Sci. Technol. 2013, 24, 045401. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Z.; Li, C.; He, X.; Rong, J.; Huang, X.; Xie, H. Centrosymmetric 3D Deformation Measurement using Grid Method with a Single-Camera. Exp. Mech. 2017, 57, 537–546. [Google Scholar] [CrossRef]
- Pan, B.; Lu, Z.; Xie, H. Mean intensity gradient: An effective global parameter for quality assessment of the speckle patterns used in digital image correlation. Opt. Lasers Eng. 2010, 48, 469–477. [Google Scholar] [CrossRef]
- Vogel, Y.B.; Zhang, J.; Nadim, D.; Ciampi, S. Switching of Current Rectification Ratios within a Single Nanocrystal by Facet-Resolved Electrical Wiring. ACS Nano 2018, 12, 8071–8080. [Google Scholar] [CrossRef] [PubMed]



| Diameter (Millimeter) | Thickness (Micro) | Material/Ingredient | |
|---|---|---|---|
| Working electrode | 8 | 0.8 | Silicon |
| Counter electrode | 15.6 | 450 | Lithium metal |
| Observation window | 6 | 200 | Quartz glass |
| Separator | 19 | 28 | Microporous polypropylene film |
| Gasket | 15.6 | 800 | Stainless steel |
| Top cover | 20 | Stainless steel |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Shan, Z.; Ma, W.; Li, L.; Wang, S.; Li, C.; Wang, Z. Strain Analysis on Electrochemical Failures of Nanoscale Silicon Electrode Based on Three-Dimensional In Situ Measurement. Appl. Sci. 2020, 10, 468. https://doi.org/10.3390/app10020468
Qi Z, Shan Z, Ma W, Li L, Wang S, Li C, Wang Z. Strain Analysis on Electrochemical Failures of Nanoscale Silicon Electrode Based on Three-Dimensional In Situ Measurement. Applied Sciences. 2020; 10(2):468. https://doi.org/10.3390/app10020468
Chicago/Turabian StyleQi, Zhifeng, Zhongqiang Shan, Weihao Ma, Linan Li, Shibin Wang, Chuanwei Li, and Zhiyong Wang. 2020. "Strain Analysis on Electrochemical Failures of Nanoscale Silicon Electrode Based on Three-Dimensional In Situ Measurement" Applied Sciences 10, no. 2: 468. https://doi.org/10.3390/app10020468
APA StyleQi, Z., Shan, Z., Ma, W., Li, L., Wang, S., Li, C., & Wang, Z. (2020). Strain Analysis on Electrochemical Failures of Nanoscale Silicon Electrode Based on Three-Dimensional In Situ Measurement. Applied Sciences, 10(2), 468. https://doi.org/10.3390/app10020468





