Characterization of Gait and Postural Regulation in Late-Onset Pompe Disease
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Analysis
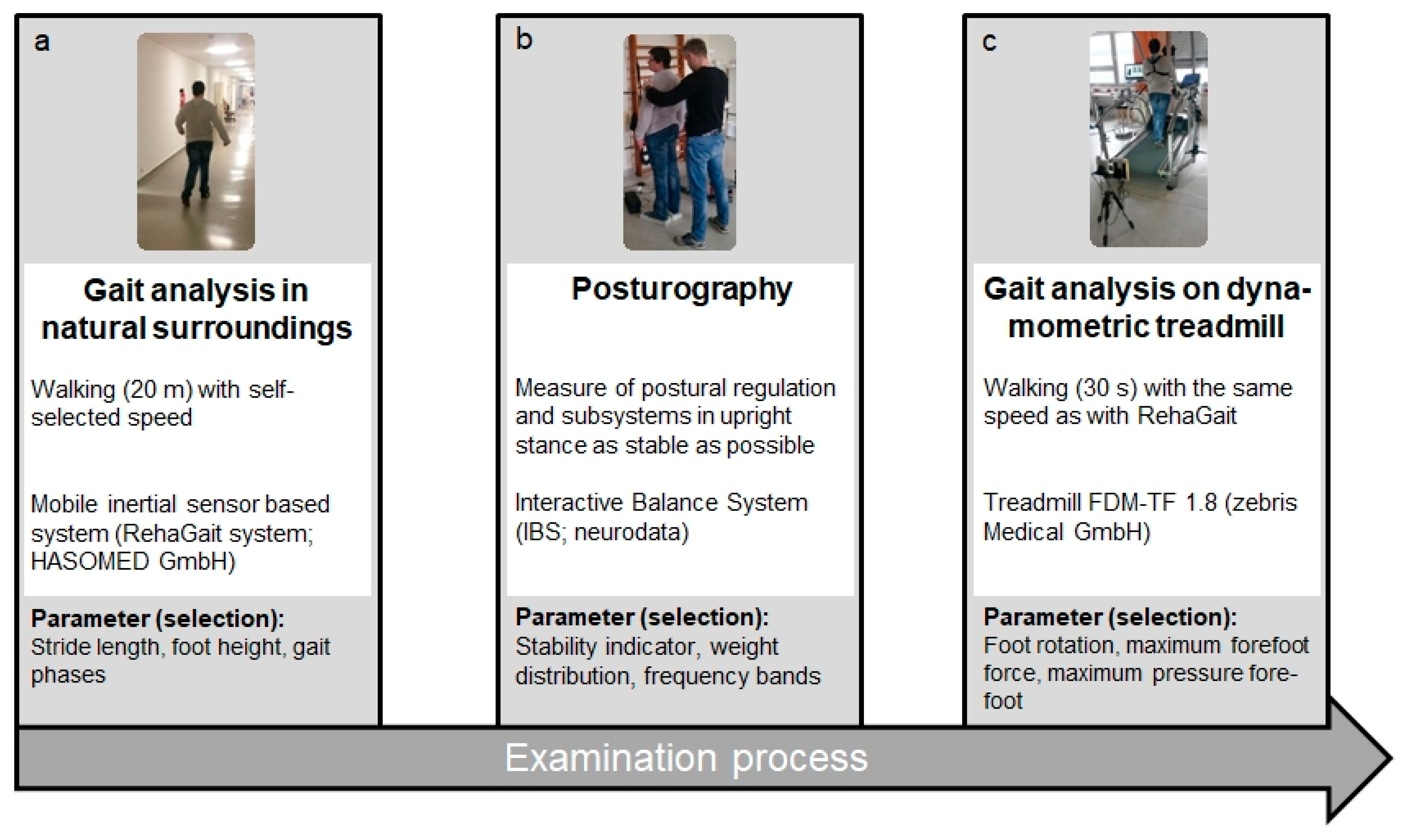
2.3. Mobile Gait Analysis Using an Inertial Sensor Based System
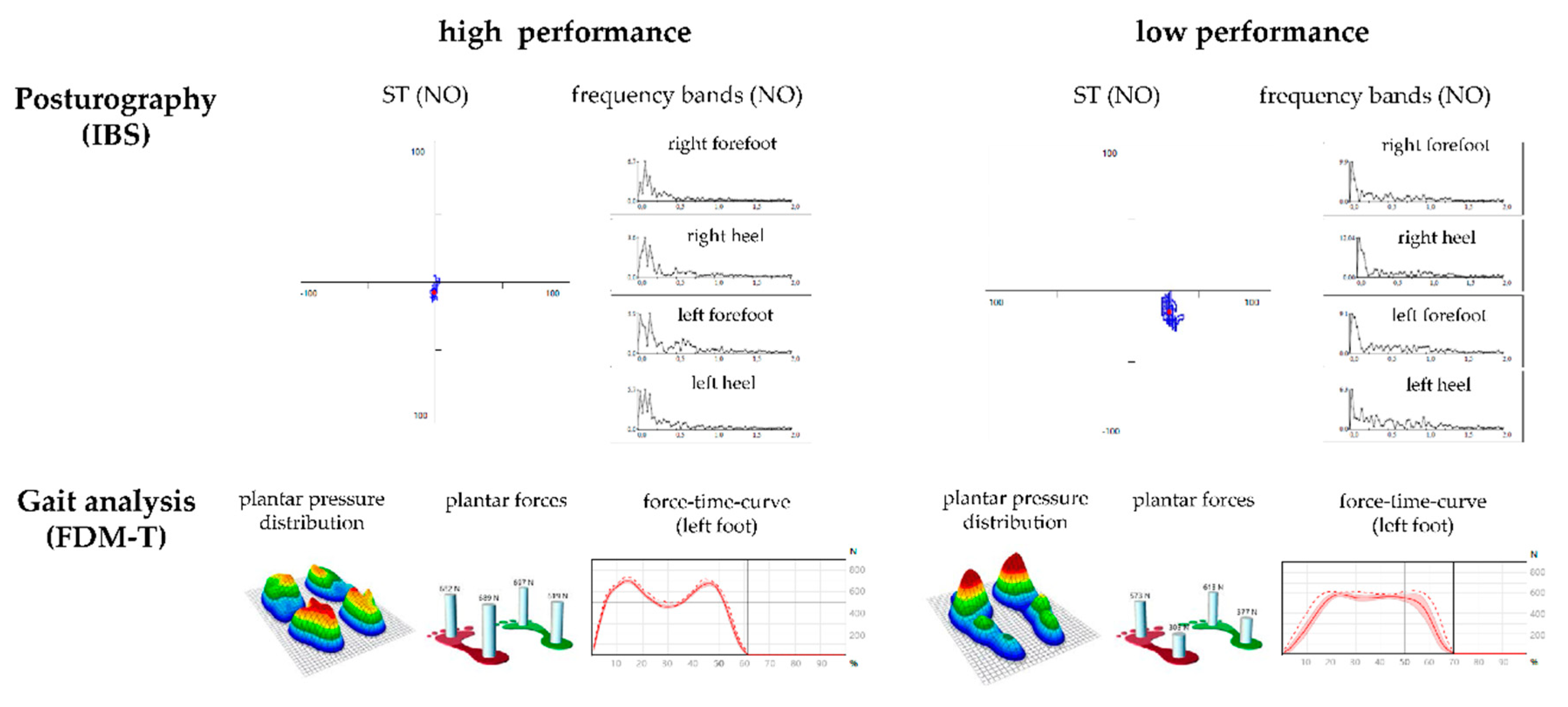
2.4. Balance Measurement Using Posturography
- F1: Frequency band 1 (0.01–0.03 Hz)—visual and nigrostriatal system;
- F2–4: Frequency band 2–4 (0.03–0.5 Hz)—peripheral-vestibular system;
- F5–6: Frequency band 5–6 (0.5–1.0 Hz)—somatosensory system;
- F7–8: Frequency band 7–8 (>1.0 Hz)—cerebellar system.
- Stability indicator (ST): The root mean square of successive differences in pressure signals. Greater instability is indicated by a greater ST.
- Weight distribution index (WDI): Standard deviation of the weight distribution score.
- Synchronization (Synch): Six values that describe the relationship of vibration patterns between plates calculated as a scalar product: 1000—complete coactivity; −1000—complete compensation; 0—no coactivity or compensation.
- Forefoot–hindfoot ratio (Heel): Percentage of load distribution between the forefoot and hindfoot with an emphasis on heel loading.
- Left–right ratio (Left): Percentage of load distribution between the left and right feet with an emphasis on left side loading.
- Head straight, eyes open, without foam pads (NO);
- Head straight, eyes closed, without foam pads (NC);
- Head straight, eyes open, on foam pads (PO);
- Head straight, eyes closed, on foam pads (PC);
- Head rotated 45° to the right, eyes closed, without foam pads (HR);
- Head rotated 45° to the left, eyes closed, without foam pads (HL);
- Head up (dorsiflexed), eyes closed, without foam pads (HB);
- Head up (plantarflexed), eyes closed, without foam pads (HF).
2.5. Gait Analysis Using a Dynamometric Treadmill
2.6. Statistics
3. Results
3.1. Gait Analysis with Mobile Device (Rehagait) and Dynamometric Treadmill Analysis
3.2. Posturographic Analysis
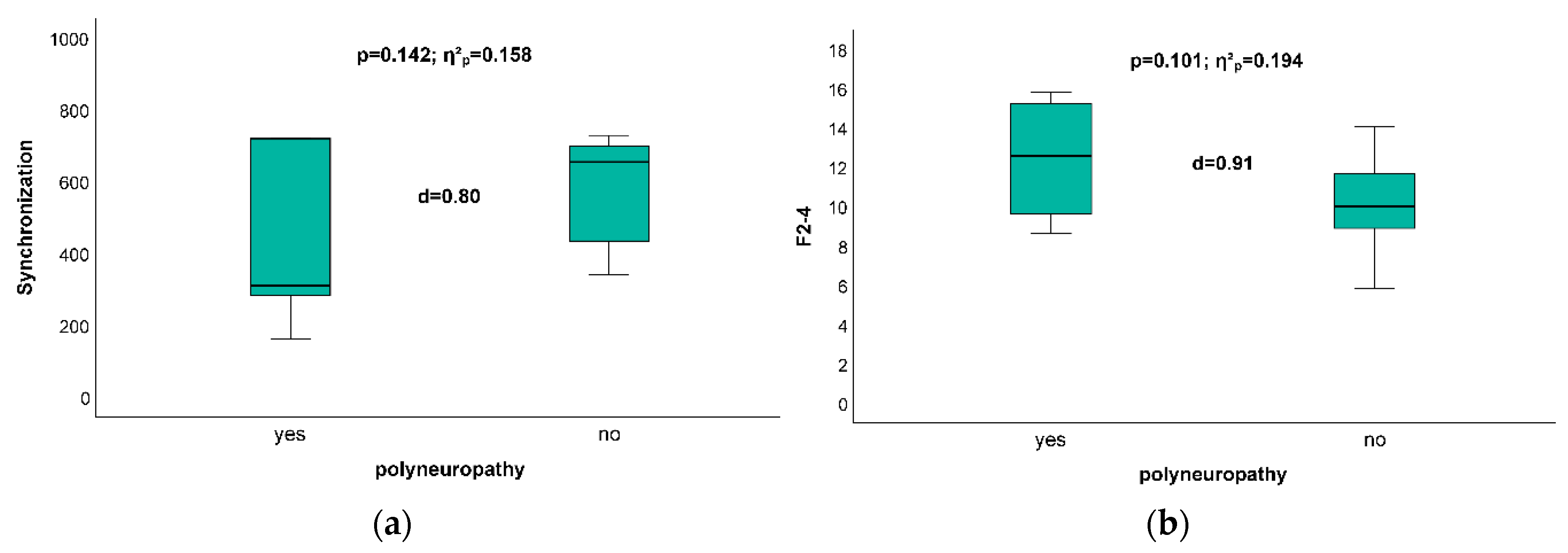
3.3. Impact of Existence of Polyneuropathy on Posturographic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical standards
References
- Hobson-Webb, L.D.; Proia, A.D.; Thurberg, B.L.; Banugaria, S.; Prater, S.N.; Kishnani, P.S. Autopsy findings in late-onset Pompe disease: A case report and systematic review of the literature. Mol. Genet. Metab. 2012, 106, 462–469. [Google Scholar] [CrossRef]
- Kohler, L.; Puertollano, R.; Raben, N. Pompe Disease: From Basic Science to Therapy. Neurotherapeutics 2018, 15, 928–942. [Google Scholar] [CrossRef]
- Meena, N.K.; Ralston, E.; Raben, N.; Puertollano, R. Enzyme Replacement Therapy Can Reverse Pathogenic Cascade in Pompe Disease. Mol. Ther. Methods Clin. Dev. 2020, 18, 199–214. [Google Scholar] [CrossRef]
- Cupler, E.J.; Berger, K.I.; Leshner, R.T.; Wolfe, G.I.; Han, J.J.; Barohn, R.J.; Kissel, J.T. AANEM Consensus Committee on Late-onset Pompe Disease. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve 2012, 45, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Cammarata, G.; Colomba, P.; Sciarrino, S.; Zizzo, C.; Francofonte, D.; Zora, M.; Scalia, S.; Brando, C.; Lo Curto, A.; et al. Pompe disease: Pathogenesis, molecular genetics and diagnosis. Aging (Albany N. Y.) 2020, 12, 15856–15874. [Google Scholar] [CrossRef]
- Toscano, A.; Rodolico, C.; Musumeci, O. Multisystem late onset Pompe disease (LOPD): An update on clinical aspects. Ann. Transl. Med. 2019, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Schoser, B.; Stewart, A.; Kanters, S.; Hamed, A.; Jansen, J.; Chan, K.; Karamouzian, M.; Toscano, A. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: A systematic review and meta-analysis. J. Neurol. 2017, 264, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Gungor, D.; Kruijshaar, M.E.; Plug, I.; Rizopoulos, D.; Kanters, T.A.; Wens, S.C.; Reuser, A.J.J.; van Doorn, P.A.; van der Ploeg, A.T. Quality of life and participation in daily life of adults with Pompe disease receiving enzyme replacement therapy: 10 years of international follow-up. J. Inherit. Metab. Dis. 2016, 39, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, G.; Collaud, F.; Laforet, P.; Mingozzi, F. Progress and challenges of gene therapy for Pompe disease. Ann. Transl. Med. 2019, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, P.T.; Case, L.E.; Chan, J.M.; Austin, S.L.; Kishnani, P. Characterization of gait in late onset Pompe disease. Mol. Genet. Metab. 2015, 116, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.S.; Casabona, A.; Fiumara, A.; Castiglione, D.; Sorge, G.; Cioni, M. Quantitative analysis of upright standing in adults with late-onset Pompe disease. Sci. Rep. 2016, 6, 37040. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Brehme, K.; Pyschik, M.; Pollak, R.; Schaffrath, N.; Schulze, S.; Delank, K.S.; Laudner, K.G.; Schwesig, R. Postural stability and regulation before and after anterior cruciate ligament reconstruction—A two-years longitudinal study. Phys. Ther. Sport 2019, 38, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Brehme, K.; Bartels, T.; Pyschik, M.; Jenz, M.; Delank, K.S.; Laudner, K.G.; Schwesig, R. Postural stability and regulation before and after high tibial osteotomy and rehabilitation. Appl. Sci. 2020, 10, 6517. [Google Scholar] [CrossRef]
- Schwesig, R.; Leuchte, S.; Fischer, D.; Ullmann, R.; Kluttig, A. Inertial sensor based reference gait data for healthy subjects. Gait Posture 2011, 33, 673–678. [Google Scholar] [CrossRef]
- Lauenroth, A.; Laudner, K.G.; Schulze, S.; Delank, K.S.; Fieseler, G.; Schwesig, R. Treadmill-based gait reference data for healthy subjects. Dependence on functional and morphologic parameters. Man. Med. 2018, 56, 182–187. [Google Scholar] [CrossRef]
- Schwesig, R.; Fischer, D.; Kluttig, A. Are there changes in postural regulation across the lifespan? Somatosens. Mot. Res. 2013, 30, 167–174. [Google Scholar] [CrossRef]
- Regnery, C.; Kornblum, C.; Hanisch, F.; Vielhaber, S.; Strigl-Pill, N.; Grunert, B.; Müller-Felber, W.; Glocker, F.X.; Spranger, M.; Deschauer, M.; et al. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J. Inherit. Metab. Dis. 2012, 35, 837–845. [Google Scholar] [CrossRef]
- Schwesig, R.; Kauert, R.; Wust, S.; Becker, S.; Leuchte, S. Reliability of the novel gait analysis system RehaWatch. Biomed. Tech. (Berl) 2010, 55, 109–115. [Google Scholar] [CrossRef]
- Donath, L.; Faude, O.; Lichtenstein, E.; Nüesch, C.; Mündermann, A. Validity and reliability of a portable gait analysis system for measuring spatiotemporal gait characteristics: Comparison to an instrumented treadmill. J. Neuroeng. Rehabil. 2016, 13, 6. [Google Scholar] [CrossRef]
- Auvinet, B.; Berrut, G.; Touzard, C.; Moutel, L.; Collet, N.; Chaleil, D.; Barrey, E. Reference data for normal subjects obtained with an accelerometric device. Gait Posture 2002, 16, 124–134. [Google Scholar] [CrossRef]
- Reinhardt, L.; Heilmann, F.; Teicher, M.; Wollny, R.; Lauenroth, A.; Delank, K.S.; Schwesig, R.; Kurz, E. Comparison of posturographic outcomes between two different de-vices. J. Biomech. 2019, 86, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Grein, H.J.; Wicher, C.; Schuetze, J.; Mueller, A.; Lauenroth, A.; Hottenrott, K.; Schwesig, R. Influence of pathologic and simulated visual dysfunctions on the postural system. Exp. Brain Res. 2008, 186, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, U.; Kohen-Raz, R.; Alex, D.; Kohen-Raz, A.; Azarya, M. Postural characteristics of diabetic neuropathy. Diabetes Care 1999, 22, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Schwesig, R.; Goldich, Y.; Hahn, A.; Muller, A.; Kohen-Raz, R.; Kluttig, A.; Morad, Y. Postural control in subjects with visual impairment. Eur. J. Ophthalmol. 2011, 21, 303–309. [Google Scholar] [CrossRef]
- Schwesig, R.; Becker, S.; Fischer, D. Intraobserver reliability of posturography in healthy subjects. Somatosens. Mot. Res. 2014, 31, 16–22. [Google Scholar] [CrossRef]
- Schwesig, R.; Fischer, D.; Becker, S.; Lauenroth, A. Intraobserver reliability of posturography in patients with vestibular neuritis. Somatosens. Mot. Res. 2014, 31, 28–34. [Google Scholar] [CrossRef]
- Schwesig, R.; Hollstein, L.; Plontke, S.K.; Delank, K.S.; Fieseler, G.; Rahne, T. Comparison of intraobserver single-task reliabilities of the Interactive Balance System (IBS) and Vertiguard in asymptomatic subjects. Somatosens. Mot. Res. 2017, 34, 9–14. [Google Scholar] [CrossRef]
- Schulze, S.; Schwesig, R.; Edel, M.; Fieseler, G.; Delank, K.S.; Hermassi, S.; Laudner, K.G. Treadmill based reference running data for healthy subjects is dependent on speed and morphological parameters. Hum. Mov. Sci. 2017, 55, 269–275. [Google Scholar] [CrossRef]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Schwesig, R.; Becker, S.; Lauenroth, A.; Kluttig, A.; Leuchte, S.; Esperer, H.D. A novel posturographic method to differentiate sway patterns of patients with Parkinson’s disease from patients with cerebellar ataxia. Biomed. Tech. (Berl.) 2009, 54, 347–356. [Google Scholar] [CrossRef]
- Chan, J.; Desai, A.K.; Kazi, Z.B.; Corey, K.; Austin, S.; Hobson-Webb, L.D.; Case, L.E.; Jones, H.N.; Kishnani, P.S. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol. Genet. Metab. 2017, 120, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Zierz, S. Profile of alglucosidase alfa in the treatment of Pompe disease: Safety, efficacy, and patient acceptability. Res. Rep. Endocr. Disord. 2015, 6, 1–9. [Google Scholar] [CrossRef][Green Version]
- van der Walt, J.D.; Swash, M.; Leake, J.; Cox, E.L. The pattern of involvement of adult-onset acid maltase deficiency at autopsy. Muscle Nerve 1987, 10, 272–281. [Google Scholar] [CrossRef]
- DeRuisseau, L.R.; Fuller, D.D.; Qiu, K.; DeRuisseau, K.C.; Donnelly, W.H., Jr.; Mah, C.; Reier, P.J.; Byrne, B.J. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc. Natl. Acad. Sci. USA 2009, 106, 9419–9424. [Google Scholar] [CrossRef] [PubMed]
- Hensel, O.; Schneider, I.; Wieprecht, M.; Kraya, T.; Zierz, S. Decreased outlet angle of the superior cerebellar artery as indicator for dolichoectasia in late onset Pompe disease. Orphanet J. Rare Dis. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, O.; Marino, S.; Granata, F.; Morabito, R.; Bonanno, L.; Brizzi, T.; Lo Buono, V.; Corallo, F.; Longo, M.; Toscano, A. Central nervous system involvement in late-onset Pompe disease: Clues from neuroimaging and neuropsychological analysis. Eur. J. Neurol. 2019, 26, 442-e35. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.; Rahne, T.; Plontke, S.K. Prevalence of hearing loss in patients with late-onset Pompe disease: Audiological and otological consequences. Int. J. Audiol. 2013, 52, 816–823. [Google Scholar] [CrossRef]
- Wirsching, A.; Muller-Felber, W.; Schoser, B. Are evoked potentials in patients with adult-onset pompe disease indicative of clinically relevant central nervous system involvement? J. Clin. Neurophysiol. 2014, 31, 362–366. [Google Scholar] [CrossRef]
- Montagnese, F.; Thiele, S.; Wenninger, S.; Schoser, B. Long-term whole-body vibration training in two late-onset Pompe disease patients. Neurol. Sci. 2016, 37, 1357–1360. [Google Scholar] [CrossRef]
- Vry, J.; Schubert, I.J.; Semler, O.; Haug, V.; Schonau, E.; Kirschner, J. Whole-body vibration training in children with Duchenne muscular dystrophy and spinal muscular atrophy. Eur. J. Paediatr. Neurol. 2014, 18, 140–149. [Google Scholar] [CrossRef]
- Yang, F.; King, G.A.; Dillon, L.; Su, X. Controlled whole-body vibration training reduces risk of falls among community-dwelling older adults. J. Biomech. 2015, 48, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Corrado, B.; Ciardi, G.; Iammarrone, C.S. Rehabilitation management of Pompe disease, from childhood trough adulthood: A systematic review of the literature. Neurol. Int. 2019, 11, 7983. [Google Scholar] [CrossRef] [PubMed]
| Subject ID (No.) | Sex | GAA-Genotype | Age (y) | Disease Duration (y) | Duration ERT (mo) | NIV | Use of Ambulatory Aids | |
|---|---|---|---|---|---|---|---|---|
| 1 | M | IVS1 (−13T > G) | p.C103G | 58 | 14 | 135 | Yes | None |
| 2 | F | IVS1 (−13T > G) | c.925G > A | 62 | 2 | 9 | No | None |
| 3 | M | IVS1 (−13T > G) | p.G309R | 82 | 6 | 37 | No | Rolling walker |
| 4 | F | IVS1 (−13T > G) | p.L552P | 53 | 17 | 99 | Yes | E-wheelchair |
| 5 | M | IVS1 (−13T > G) | IVS1 (−13T > G) | 62 | 13 | 29 | Yes | Rolling walker |
| 6 | M | IVS1 (−13T > G) | p.P493L | 51 | 15 | 63 | No | Walking sticks |
| 7 | F | IVS1 (−13T > G) | p.P493L | 61 | 21 | 63 | No | None |
| 8 | F | IVS1 (−13T > G) | IVS9 G > C) | 26 | 15 | 67 | No | None |
| 9 | F | IVS1 (−13T > G) | c307 T > G | 50 | 23 | 119 | Yes | Rolling walker |
| 10 | M | IVS1 (−13T > G) | c.832delC | 46 | 6 | 17 | Yes | None |
| 11 | F | IVS1 (−13T > G) | c.2481 + 102_2646 + 31del | 54 | 19 | 48 | No | None |
| 12 | F | IVS1 (−13T > G) | c.2481 + 102_2646 + 31del | 57 | 18 | 44 | No | Rolling walker |
| 13 | M | IVS1 (−13T > G) | c.525delT | 19 | 19 | 132 | No | None |
| 14 | F | IVS1 (−13T > G) | c.525delT | 66 | 7 | 33 | Yes | Rolling walker |
| 15 | M | IVS1 (−13T > G) | c.2136-7delGT | 50 | 40 | 116 | Yes | None |
| 16 | F | IVS1 (−13T > G) | c.1019T > C | 70 | 20 | 0 | No | Rolling walker |
| Subject ID Number | Stride Length (m) | Walking Speed (m/s) | Cadence (steps/min) | Stance Phase (%) | Single Support (%) | Maximum Foot Height (m) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | Value | RR | Value | RR | Value | RR | Value | RR | Value | RR | Value | |
| 1 | 0.98–1.34 | 1.06 | 1.18–1.60 | 0.72 * | 96–110 | 80 * | 57–64 | 65 * | 36–42 | 32 * | 0.10–0.24 | 0.18 |
| 2 | 1.25–1.53 | 1.41 | 1.15–1.59 | 1.30 | 108–124 | 108 | 57–63 | 57 | 37–43 | 40 | 0.09–0.24 | 0.17 |
| 3 | 1.11–1.45 | 1.18 | 0.80–1.43 | 0.98 | 102–116 | 98 * | 57–64 | 61 | 36–42 | 36 | 0.10–0.24 | 0.15 |
| 4 | 0.78–1.16 | 0.70 * | 1.21–1.61 | 0.34 * | 89–100 | 55 * | 58–65 | 79 * | 35–41 | 22 * | 0.09–0.24 | 0.16 |
| 5 | 1.13–1.47 | 1.11 * | 1.15–1.59 | 1.04 * | 103–117 | 110 | 57–63 | 65 * | 36–42 | 37 | 0.10–0.24 | 0.17 |
| 6 | 1.23–1.53 | 1.41 | 1.21–1.61 | 1.19 * | 105–121 | 99 * | 57–63 | 57 | 36–43 | 42 | 0.11–0.24 | 0.14 |
| 7 | 1.26–1.58 | 1.42 | 1.16–1.59 | 1.29 | 108–124 | 104 * | 57–63 | 64 * | 37–43 | 38 | 0.10–0.25 | 0.20 |
| 8 | 1.28–1.61 | 1.60 | 1.14–1.55 | 1.34 | 107–125 | 99 * | 56–63 | 59 | 37–43 | 40 | 0.10–0.25 | 0.18 |
| 9 | 0.85–1.20 | 0.91 | 1.22–1.61 | 0.49 * | 92–104 | 63 * | 58–65 | 64 | 35–41 | 32 * | 0.09–0.24 | 0.12 |
| 10 | 1.32–1.64 | 1.51 | 1.22–1.61 | 1.41 | 109–127 | 112 | 56–62 | 59 | 37–43 | 40 | 0.10–0.25 | 0.20 |
| 11 | 1.20–1.50 | 1.41 | 1.20–1.61 | 1.18 * | 106–121 | 100 * | 57–63 | 60 | 37–43 | 37 | 0.09–0.24 | 0.13 |
| 12 | 0.70–1.09 | 0.38 * | 1.19–1.61 | 0.19 * | 86–97 | 57 * | 58–66 | 82 * | 35–41 | 18 * | 0.09–0.24 | 0.07 * |
| 13 | 1.41–1.72 | 1.61 | 1.09–1.50 | 1.58 * | 111–131 | 117 | 56–62 | 58 | 37–44 | 42 | 0.10–0.25 | 0.20 |
| 14 | 0.82–1.10 | 0.67 * | 1.10–1.56 | 0.45 * | 92–104 | 79 * | 58–65 | 66 * | 36–41 | 29 * | 0.08–0.21 | 0.10 |
| 15 | 1.02–1.38 | 1.06 | 1.04–1.54 | 0.80 * | 97–112 | 91 * | 57–64 | 63 | 36–42 | 32 * | 0.10–0.25 | 0.13 |
| 16 | 0.73–1.10 | 0.40 * | 1.04–1.54 | 0.25 * | 88–98 | 74 * | 58–65 | 76 * | 35–41 | 27 * | 0.09–0.23 | 0.09 |
| ∑ * (n/%) | 5/31% | 11/69% | 12/75% | 7/44% | 7/44% | 1/6% | ||||||
| Subject ID | 13 | 10 | 8 | 11 | 6 | 7 | 2 | 3 | 1 | 16 | 15 | 5 | 9 | 14 | 4 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobile gait parameters using RehaGait | ||||||||||||||||
| Stride length (m) | - | - | - | - | - | - | - | - | - | X | - | - | - | X | X | X |
| Walking speed (m/s) | - | - | - | X | X | - | - | - | X | X | X | X | X | X | X | X |
| Cadence (steps/min) | - | - | X | X | X | X | X | X | X | X | X | - | X | X | X | X |
| Stance (%) | - | - | - | - | - | X | - | - | X | X | - | X | - | X | X | X |
| Single support (%) | - | - | - | - | - | - | - | - | X | X | X | - | X | X | X | X |
| Double support (%) | - | - | - | - | - | X | - | - | X | X | X | X | X | X | X | X |
| Maximum foot high (m) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | X |
| Treadmill gait parameters using FDM-T system | ||||||||||||||||
| Foot rotation (°) | X | X | X | X | - | - | X | X | X | X | X | NA | - | - | NA | X |
| Step width (m) | - | X | X | - | - | X | X | - | - | - | X | NA | - | - | NA | - |
| Initial stance (%) | - | X | X | X | X | X | - | - | - | - | - | NA | X | - | NA | X |
| Mid stance (%) | X | X | X | - | X | X | X | - | X | - | - | NA | - | X | NA | X |
| Terminal stance (%) | X | X | X | - | X | X | X | - | X | - | - | NA | - | - | NA | X |
| Lateral displacement of Gait line (m) | X | - | - | - | X | X | X | X | X | X | X | NA | X | X | NA | - |
| Clinical data | ||||||||||||||||
| Muscle strength hip | - | X | X | X | X | X | X | X | X | - | X | X | X | X | X | X |
| Muscle strength knee | - | - | X | X | X | X | X | X | X | X | X | X | X | X | - | X |
| Muscle strength ankle | - | - | - | X | X | X | - | X | - | - | - | - | - | - | - | - |
| Polyneuropathy | - | - | - | X | - | X | - | X | X | - | - | X | - | X | - | - |
| 6MWT (m) | 800 | 590 | 470 | 445 | 420 | 370 | 323 | 318 | 315 | 265 | 254 | 200 | 125 | 114 | 105 | NA |
| Time 10-m fast walk (s) | 5 | 5.3 | 6.2 | 6.7 | 9.6 | 10.6 | 9.4 | 12 | 12.7 | 24 | 17 | 12.7 | 17.4 | 29 | 28 | 25 |
| Para-Meter | F1 | F2–4 | F5–6 | F7–8 | ST | Synch | Heel (%) | Left (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | RR | Value | RR | Value | RR | Value | RR | Value | RR | Value | RR | Value | RR | Value | RR | Value |
| 1 | 10.7–25.8 | 20.0 | 6.25–14.8 | 15.2 * | 2.92–6.81 | 6.62 | 0.51–1.08 | 1.33 * | 13.8–29.3 | 36.6 * | 379–807 | 720 | 36.5–67.4 | 49.0 | 46.0–55.1 | 46.7 |
| 2 | 9.48–21.0 | 22.7 * | 6.86–13.0 | 10.0 | 2.94–6.96 | 4.29 | 0.53–1.29 | 0.77 | 13.4–32.8 | 24.6 | 426–762 | 664 | 36.8–57.4 | 35.4 * | 43.6–55.3 | 58.0 * |
| 3 | 12.2–22.9 | 16.1 | 7.98–15.1 | 11.3 | 3.08–8.32 | 4.59 | 0.54–2.56 | 0.86 | 15.1–40.1 | 25.5 | 341–776 | 161 * | 39.1–57.0 | 25.4 * | 42.6–56.4 | 52.6 |
| 4 | 10.6–22.7 | 13.1 | 6.98–12.4 | 8.9 | 2.75–6.73 | 5.91 | 0.48–1.17 | 1.02 | 13.5–30.4 | 31.0 * | 528–840 | 654 | 42.2–64.8 | 46.8 | 44.3–54.3 | 49.0 |
| 5 | 10.4–23.4 | 26.0 * | 6.44–12.4 | 15.8 * | 3.00–6.83 | 9.36 * | 0.51–1.34 | 1.99 * | 14.7–30.9 | 57.5 * | 518–770 | 314 * | 36.9–56.6 | 36.1 * | 47.3–53.0 | 50.2 |
| 6 | 10.7–25.8 | 10.5 * | 6.25–14.8 | 10.3 | 2.92–6.81 | 4.95 | 0.51–1.08 | 0.93 | 13.8–29.3 | 29.8 * | 379–807 | 341 * | 36.5–67.4 | 18.0 * | 46.0–55.1 | 60.1 * |
| 7 | 9.48–21.0 | 15.8 | 6.86–13.0 | 9.7 | 2.94–6.96 | 2.85 * | 0.53–1.29 | 0.47 * | 13.4–32.8 | 15.8 | 426–762 | 308 * | 36.8–57.4 | 43.2 | 43.6–55.3 | 43.9 |
| 8 | 9.59–20.3 | 19.5 | 5.84–10.2 | 11.7 * | 2.40–4.64 | 4.94 * | 0.44–1.01 | 1.06 * | 11.7–21.2 | 30.2 * | 515–793 | 700 | 38.2–56.5 | 47.1 | 46.1–53.9 | 54.8 * |
| 9 | 10.6–22.7 | 25.6 * | 6.98–12.4 | 14.1 * | 2.75–6.73 | 6.75 * | 0.48–1.17 | 0.97 | 13.5–30.4 | 33.4 * | 528–840 | 704 | 42.2–64.8 | 39.1 * | 44.3–54.3 | 47.5 |
| 10 | 11.2–22.5 | 10.5 * | 6.25–10.9 | 8.9 | 2.86–4.98 | 4.90 | 0.50.–1.15 | 1.04 | 13.3–23.4 | 30.0 * | 415–787 | 435 | 38.7–54.2 | 32.9 * | 46.2–53.9 | 52.7 |
| 11 | 10.6–22.7 | 13.5 | 6.98–12.4 | 8.7 | 2.75–6.73 | 2.78 | 0.48–1.17 | 0.51 | 13.5–30.4 | 16.7 | 528–840 | 722 | 42.2–64.8 | 29.2 * | 44.3–54.3 | 50.0 |
| 12 | 10.6–22.7 | 8.5 * | 6.98–12.4 | 5.9 * | 2.75–6.73 | 3.62 | 0.48–1.17 | 0.67 | 13.5–30.4 | 21.2 | 528–840 | 500 * | 42.2–64.8 | 30.8 * | 44.3–54.3 | 52.1 |
| 13 | 12.6–24.9 | 12.6 | 7.10–12.4 | 6.5 * | 3.16–5.76 | 2.20 * | 0.58–1.19 | 0.38 * | 15.0–28.1 | 11.7 * | 229–664 | 729 * | 36.3–59.8 | 47.6 | 45.1–55.6 | 50.2 |
| 14 | 9.48–21.0 | 20.5 | 6.86–13.0 | 13.9 * | 2.94–6.96 | 8.51 * | 0.53–1.29 | 1.52 * | 13.4–32.8 | 46.8 * | 426–762 | 284 * | 36.8–57.4 | 42.4 | 43.6–55.3 | 35.9 * |
| 16 | 9.73–23.7 | 15.7 | 6.92–14.7 | 12.2 | 3.41–8.71 | 3.97 | 0.66–1.61 | 0.66 | 16.4–39.5 | 22.7 | 423–787 | 434 | 38.1–58.5 | 49.2 | 43.9–56.8 | 50.4 |
| ∑ * (n/%) | 6/40% | 7/47% | 5/33% | 6/40% | 9/60% | 7/44% | 9/53% | 4/27% | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, I.; Zierz, S.; Schulze, S.; Delank, K.-S.; Laudner, K.G.; Brill, R.; Schwesig, R. Characterization of Gait and Postural Regulation in Late-Onset Pompe Disease. Appl. Sci. 2020, 10, 7001. https://doi.org/10.3390/app10197001
Schneider I, Zierz S, Schulze S, Delank K-S, Laudner KG, Brill R, Schwesig R. Characterization of Gait and Postural Regulation in Late-Onset Pompe Disease. Applied Sciences. 2020; 10(19):7001. https://doi.org/10.3390/app10197001
Chicago/Turabian StyleSchneider, Ilka, Stephan Zierz, Stephan Schulze, Karl-Stefan Delank, Kevin G. Laudner, Richard Brill, and René Schwesig. 2020. "Characterization of Gait and Postural Regulation in Late-Onset Pompe Disease" Applied Sciences 10, no. 19: 7001. https://doi.org/10.3390/app10197001
APA StyleSchneider, I., Zierz, S., Schulze, S., Delank, K.-S., Laudner, K. G., Brill, R., & Schwesig, R. (2020). Characterization of Gait and Postural Regulation in Late-Onset Pompe Disease. Applied Sciences, 10(19), 7001. https://doi.org/10.3390/app10197001







