Adhesion and Corrosion Resistance of Micro-Arc Oxidation/Polyurethane Composite Coating on Aluminum Alloy Surface
Abstract
1. Introduction
2. Experimental Section
2.1. Pretreatment of the Aluminum Alloy Substrate
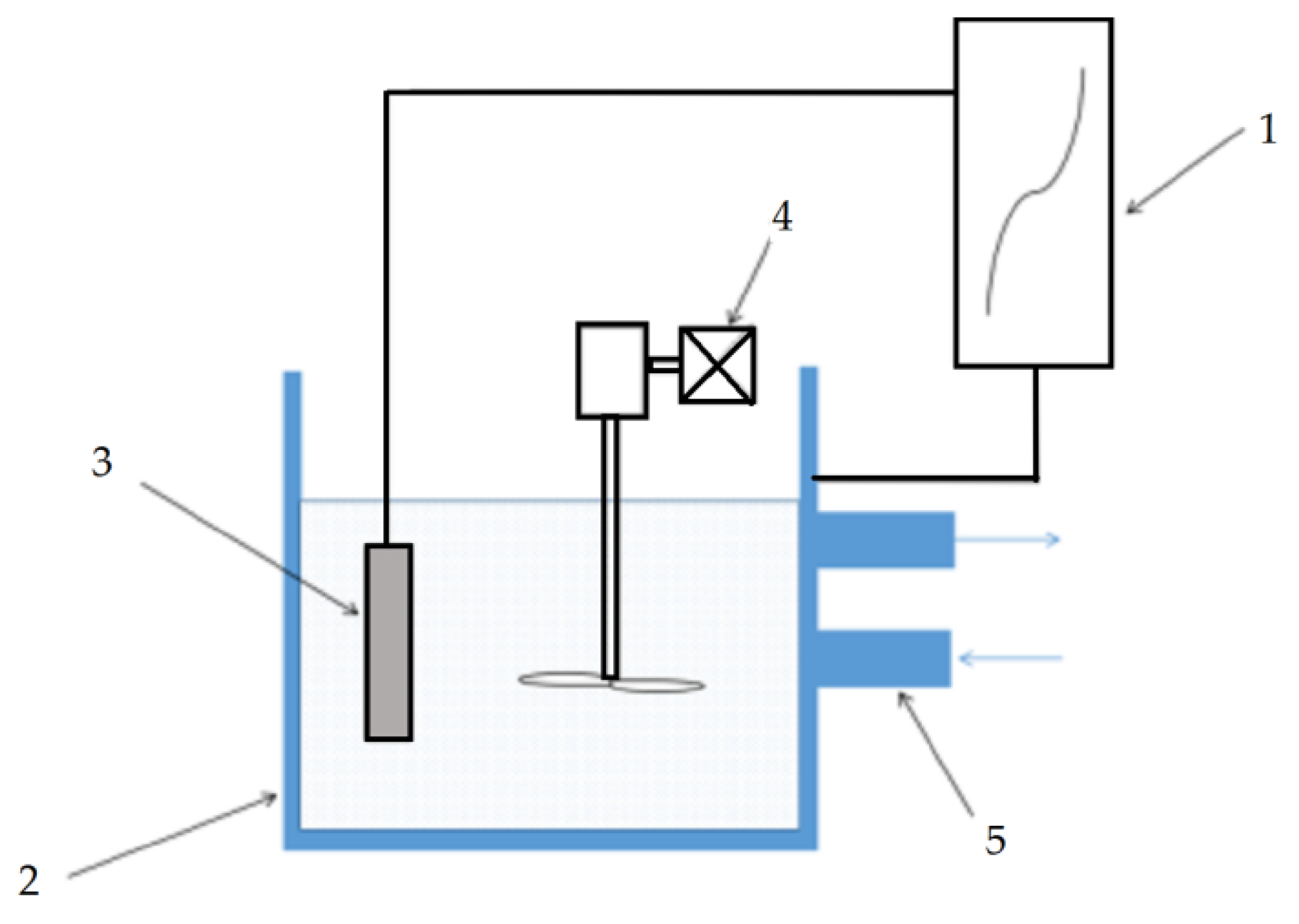
2.2. MAO Treatment
2.3. Spraying of Polyurethane Coatings
2.4. Testing and Analysis Instruments
3. Results and Discussion
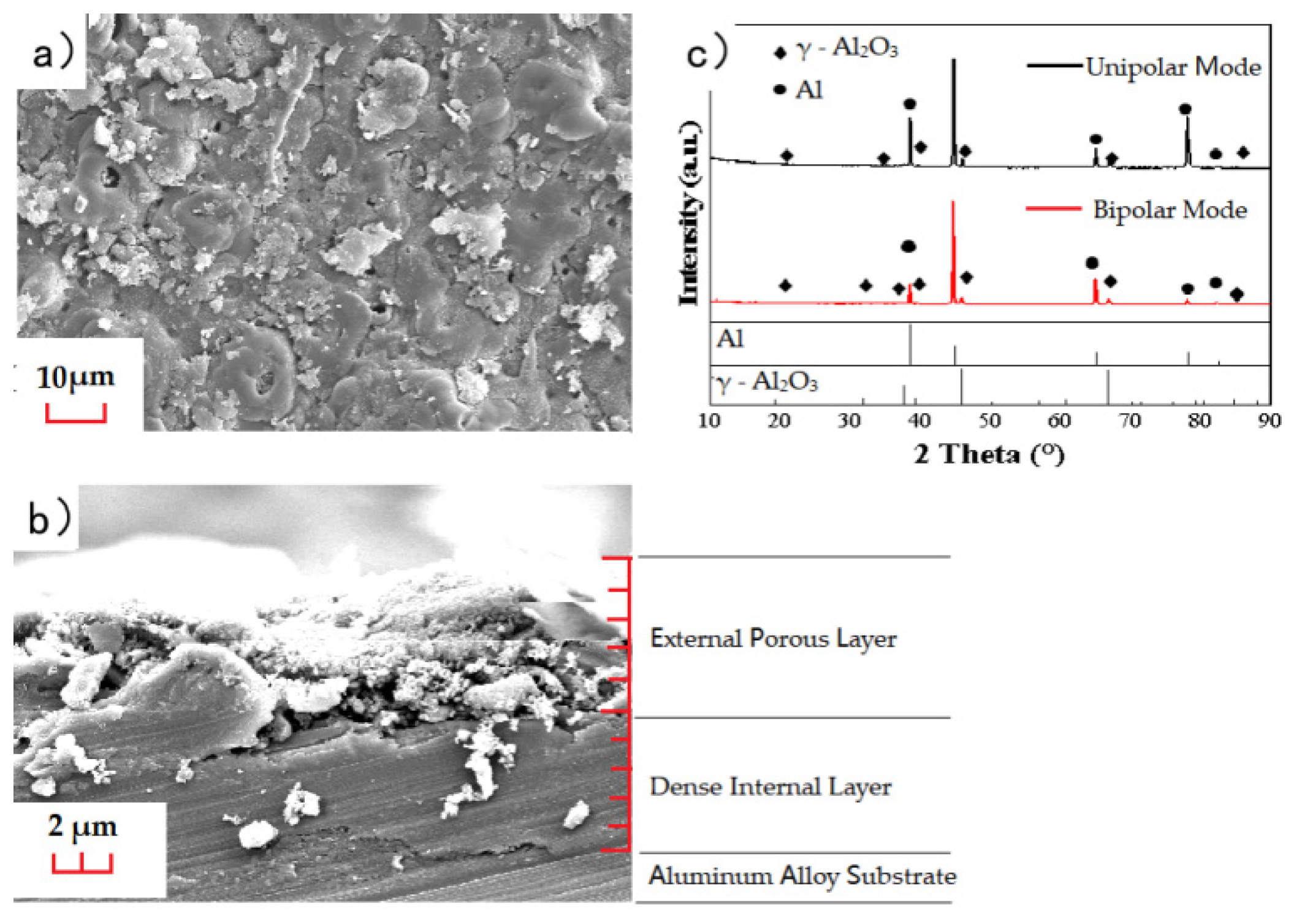
3.1. Composition and Morphology of MAO Layer
3.2. Effect of Different Concentrations of Sodium Silicate on Porosity, Thickness, and Roughness of Layers
3.3. Adhesion Strength of Composite Coatings
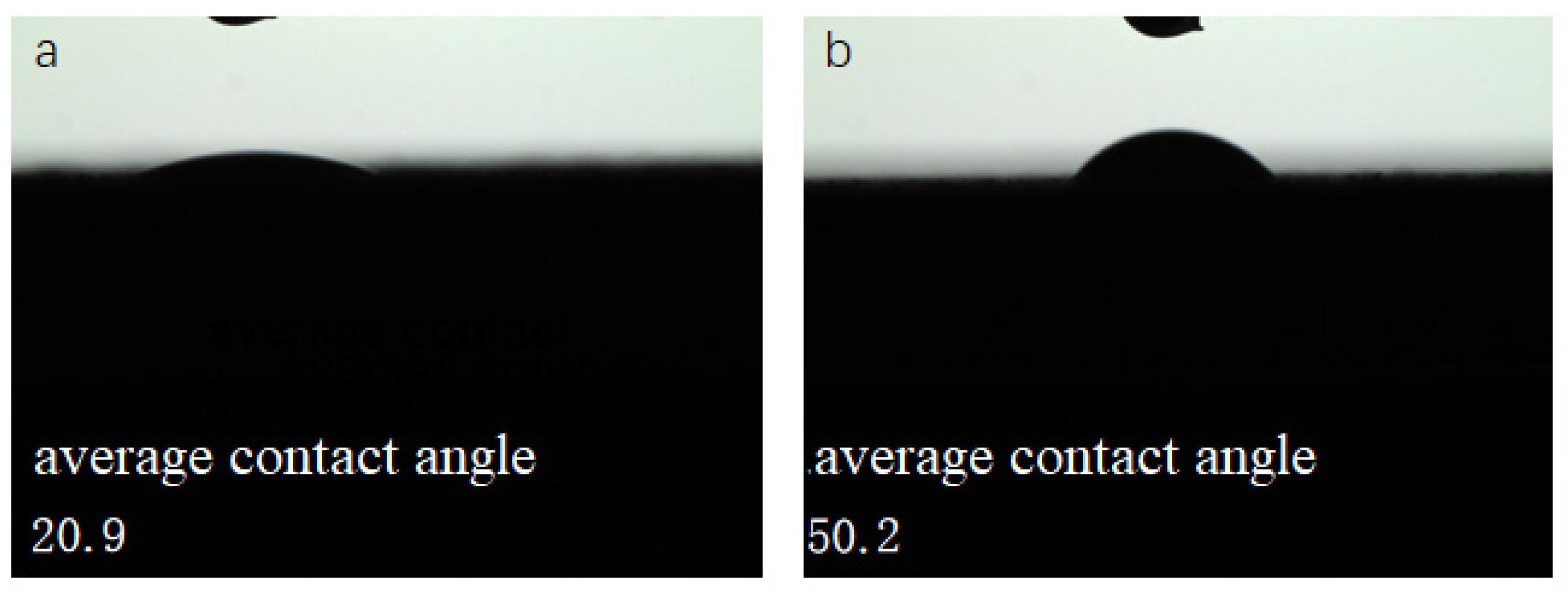
3.3.1. Effect of Silane Coupling Agent Modification on the Contact Angle of the MAO Layer
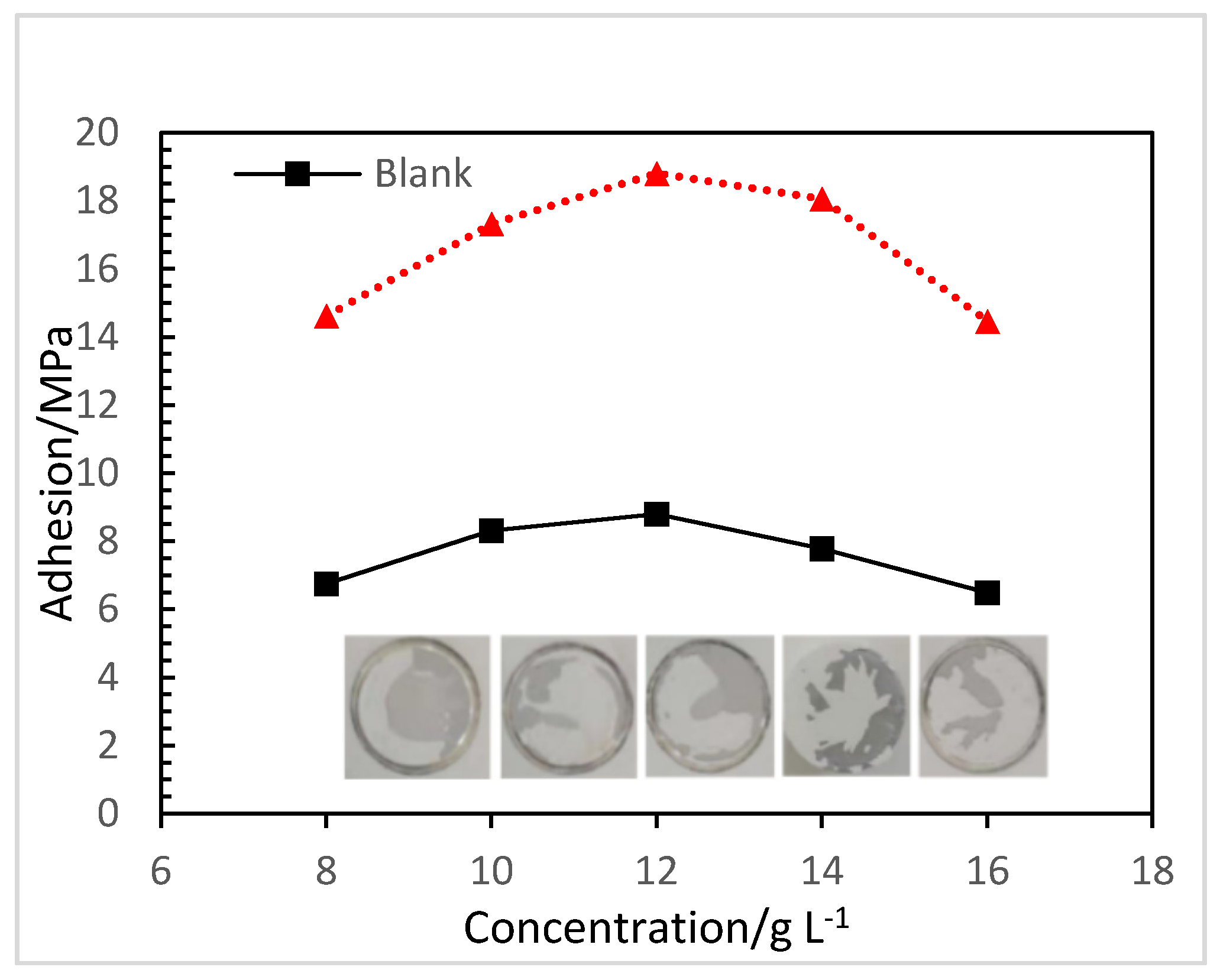
3.3.2. Adhesion Strength of Composite Coatings
3.4. Corrosion Resistance of Composite Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, C.; Zheng, W.; Ma, J.; Zhao, Y. Shear strengths of different bolt connectors on the large span of aluminium alloy honeycomb sandwich structure. Appl. Sci. 2017, 7, 450. [Google Scholar] [CrossRef]
- Sajadifar, S.V.; Scharifi, E.; Weidig, U.; Steinhoff, K.; Niendorf, T. Performance of thermo-mechanically processed AA7075 alloy at elevated temperatures—From microstructure to mechanical properties. Metals 2020, 10, 884. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Jiang, B.; Liu, C.; Gu, X.; Chen, D. A Comparative Study of Corrosion Behavior of Hard Anodized and Micro-Arc Oxidation Coatings on 7050 Aluminum Alloy. Metals 2018, 8, 165. [Google Scholar] [CrossRef]
- Langebeck, A.; Bohlen, A.; Rentsch, R.; Vollertsen, F. Mechanical properties of high strength aluminum alloy EN AW-7075 additively manufactured by directed energy deposition. Metals 2020, 10, 579. [Google Scholar] [CrossRef]
- Cabrini, M.; Bocchi, S.; D’Urso, G.; Giardini, C.; Lorenzi, S.; Testa, C.; Pastore, T. Stress corrosion cracking of friction stir-welded AA-2024 T3 alloy. Materials 2020, 13, 2610. [Google Scholar] [CrossRef]
- Matuszak, J.; Kłonica, M.; Zagórski, I. Measurements of forces and selected surface layer properties of AW-7075 aluminum alloy used in the aviation industry after abrasive machining. Materials 2019, 12, 3707. [Google Scholar] [CrossRef]
- Cabrini, M.; Bocchi, S.; D’Urso, G.; Giardini, C.; Lorenzi, S.; Testa, C.; Pastore, T. Effect of load on the corrosion behavior of friction stir welded AA 7075-T6 aluminum alloy. Materials 2020, 13, 2600. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Jiang, Z.-H.; Wang, Z. Self-assembly of amphiphilic linear-dendritic carbosilane block surfactant for waterborne polyurethane coating. Polymers 2020, 12, 1318. [Google Scholar] [CrossRef]
- Bai, T.; Lv, L.; Du, W.; Fang, W.; Wang, Y. Improving the tribological and anticorrosion performance of waterborne polyurethane coating by the synergistic effect between modified graphene oxide and polytetrafluoroethylene. Nanomaterials 2020, 10, 137. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/epoxy coating as multifunctional material for aircraft structures. Aerospace 2015, 2, 423–434. [Google Scholar] [CrossRef]
- Wang, N.; Yin, X.; Zhang, J.; Gao, H.; Diao, X.; Yao, H. Preparation and anti-corrosive properties of waterborne epoxy composite coating containing graphene oxide grafted with sodium tripolyphosphate. Coatings 2020, 10, 307. [Google Scholar] [CrossRef]
- Xin, S.G.; Song, L.X.; Zhao, R.G.; Hu, X.F. Composition and thermal properties of the coating containing mullite and alumina and alumina. Mater. Chem. Phys. 2006, 97, 132–136. [Google Scholar]
- Dunleavy, C.; Curran, J.; Clyne, T. Time dependent statistics of plasma discharge parameters during bulk AC plasma electrolytic oxidation of aluminium. Appl. Surf. Sci. 2013, 268, 397–409. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Naderi, R. Ceria-embedded MAO process as pretreatment for corrosion protection of epoxy films applied on AZ31-magnesium alloy. J. Alloys Compd. 2019, 785, 669–683. [Google Scholar]
- Rad, R.H.; Toorani, M.; Zarei, H. Corrosion behavior and adhesion strength of PEO/Epoxy duplex coating applied on aluminum alloy. Anti-Corros. Methods Mater. 2019, 66, 138–147. [Google Scholar]
- Liu, C.; Liu, P.; Huang, Z.; Yan, Q.; Guo, R.; Li, D.; Jiang, G.; Shen, D. The correlation between the coating structure and the corrosion behavior of the plasma electrolytic oxidation coating on aluminum. Surf. Coat. Technol. 2016, 286, 223–230. [Google Scholar]
- Terleeva, O.P.; Slonova, A.I.; Belevantsev, V.I.; Kireenko, I.B.; Ryzhikh, A.P. Correlations of electrolyte state and characteristics of microplasma coatings with quantity of transmitted electricity. Prot. Met. Phys. Chem. Surf. 2011, 47, 80–85. [Google Scholar]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar]
- Lu, X.; Feng, X.; Zuo, Y.; Zhang, P.; Zheng, C. Improvement of protection performance of Mg-rich epoxy coating on AZ91D magnesium alloy by DC anodic oxidation. Prog. Org. Coat. 2016, 104, 188–198. [Google Scholar]
- Cui, X.J.; Li, M.T.; Yang, R.S.; Yu, Z.X. Structure and properties of a duplex coating combining micro-arc oxidation and baking layer on AZ91D Mg alloy. Appl. Surf. Sci. 2016, 363, 91–100. [Google Scholar]
- Schmitt, M. Analysis of silanes and of siloxanes formation by Raman spectroscopy. RSC Adv. 2014, 4, 1907–1917. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, X.; Xu, M.; Rodrigue, D. Properties of poplar fiber/PLA composites: Comparison on the effect of maleic anhydride and KH550 modification of poplar fiber. Polymers 2020, 12, 729. [Google Scholar] [CrossRef]
- Da Ponte, G.D.; Ghosh, A.K.; Kakaroglou, A.; Van Hemelrijck, D.; Van Mele, B.; Verheyde, B. Adhesion Improvement between epoxy and stainless steel using a silane coupling agent in an atmospheric plasma process. Plasma Process. Polym. 2014, 12, 347–361. [Google Scholar] [CrossRef]
- Kremmer, T.M.; Dumitraschkewitz, P.; Pöschmann, D.; Ebner, T.; Uggowitzer, P.J.; Kolb, G.K.H.; Pogatscher, S. Microstructural change during the interrupted quenching of the AlZnMg(Cu) alloy AA7050. Materials 2020, 13, 2554. [Google Scholar] [CrossRef]
- Polat, A.; Makaraci, M.; Usta, M. Influence of sodium silicate concentration on structural and tribological properties of microarc oxidation coatings on 2017A aluminum alloy substrate. J. Alloys Compd. 2010, 504, 519–526. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Schmitt, M.; Hempelmann, R.; Ingebrandt, S.; Munief, W.M.; Groß, K.; Grub, J.; Heib, F. Statistical contact angle analyses: ‘Slow moving’ drops on inclining flat mono-aminopropylsiloxane surfaces. J. Adhes. Sci. Technol. 2015, 29, 1796–1806. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Liu, Z.; Yao, Z.-P.; Wang, Z.; Jiang, Z.-H.; Zhao, M. Study of the effect of PGDA solvent on film formation and curing process of two-component waterborne polyurethane coatings by FTIR tracking. Coatings 2020, 10, 461. [Google Scholar] [CrossRef]
- Renie, R.W. Uhlig’s Corrosion Handbook; John Wiley & Sons Inc.: New York, NY, USA, 2000; pp. 887–904. [Google Scholar]
- Shifler, D.A.; Aylor, D.M. Testing in environments-seawater. In Corrosion Tests and Standards Application and Interpretation; Baboian, R., Ed.; ASTM International: Baltimore, MD, USA, 2005; pp. 362–379. [Google Scholar]
- Zhang, X.G. Corrosion forms. In Corrosion and Electrochemistry of Zinc; Springer Science & Business Media: New York, NY, USA, 1996; pp. 183–240. [Google Scholar]



| Chemical Composition | Zn | Mg | Cu | Zr | Ti | Mn | Cr | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass fraction/% | 6.42 | 2.25 | 2.02 | 0.13 | 0.03 | 0.10 | 0.04 | 0.11 | 0.07 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Xu, H.; Yao, Z.; Li, C.; Jiang, Z. Adhesion and Corrosion Resistance of Micro-Arc Oxidation/Polyurethane Composite Coating on Aluminum Alloy Surface. Appl. Sci. 2020, 10, 6779. https://doi.org/10.3390/app10196779
Wang R, Xu H, Yao Z, Li C, Jiang Z. Adhesion and Corrosion Resistance of Micro-Arc Oxidation/Polyurethane Composite Coating on Aluminum Alloy Surface. Applied Sciences. 2020; 10(19):6779. https://doi.org/10.3390/app10196779
Chicago/Turabian StyleWang, Ruitao, Hong Xu, Zhongping Yao, Chunxiang Li, and Zhaohua Jiang. 2020. "Adhesion and Corrosion Resistance of Micro-Arc Oxidation/Polyurethane Composite Coating on Aluminum Alloy Surface" Applied Sciences 10, no. 19: 6779. https://doi.org/10.3390/app10196779
APA StyleWang, R., Xu, H., Yao, Z., Li, C., & Jiang, Z. (2020). Adhesion and Corrosion Resistance of Micro-Arc Oxidation/Polyurethane Composite Coating on Aluminum Alloy Surface. Applied Sciences, 10(19), 6779. https://doi.org/10.3390/app10196779





