Removal of Lead from Water Solution by Reusable Magnetic Adsorbent Incorporating Selective Lead-Binding Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption on Bare Magnetic Bead
2.3. Construction of Peptide-Linked Magnetic Bead
2.4. Adsorption on Peptide-Linked Magnetic Bead
2.5. Analysis
3. Results
3.1. Construction of Peptide-Linked Magnetic Bead
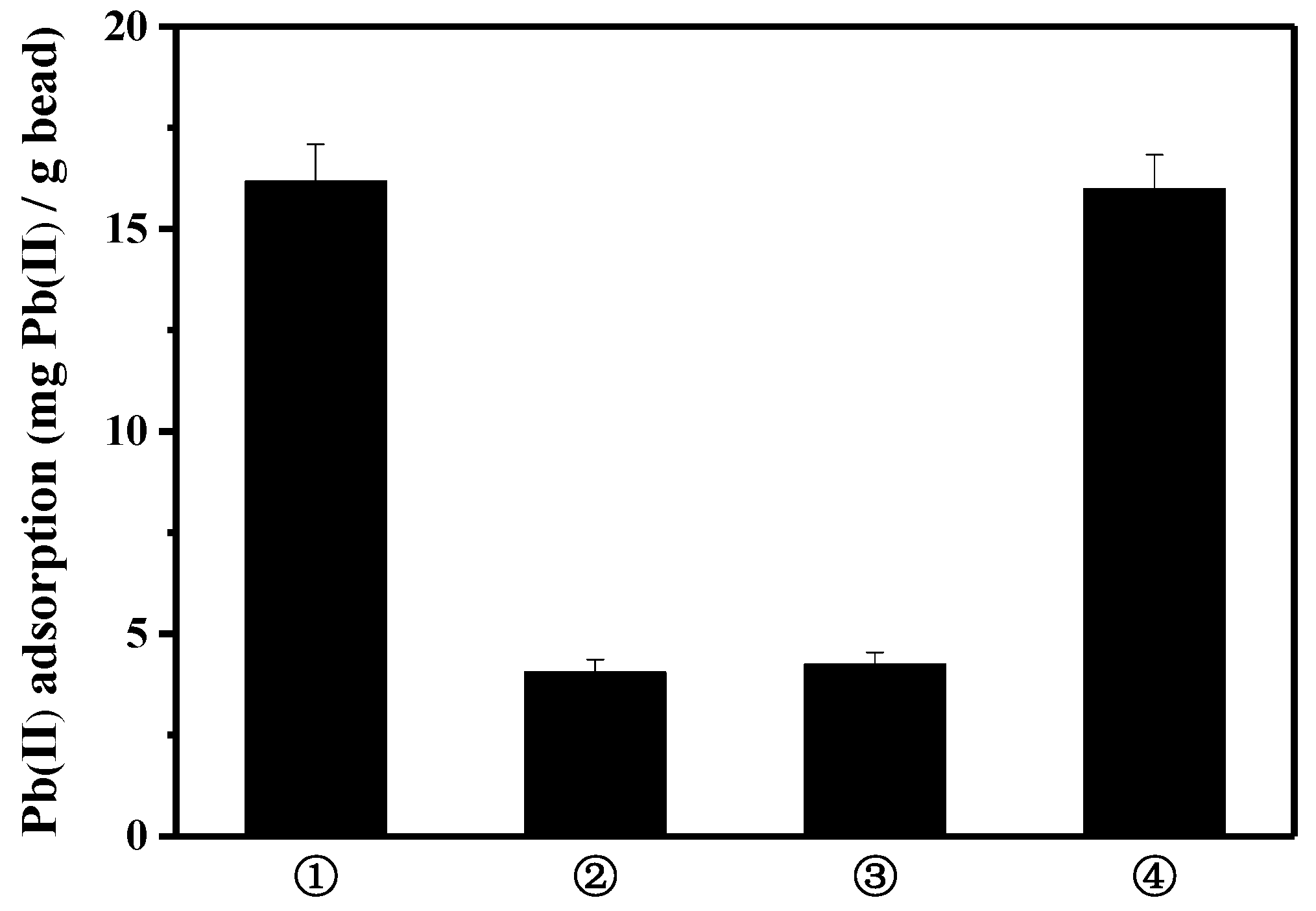
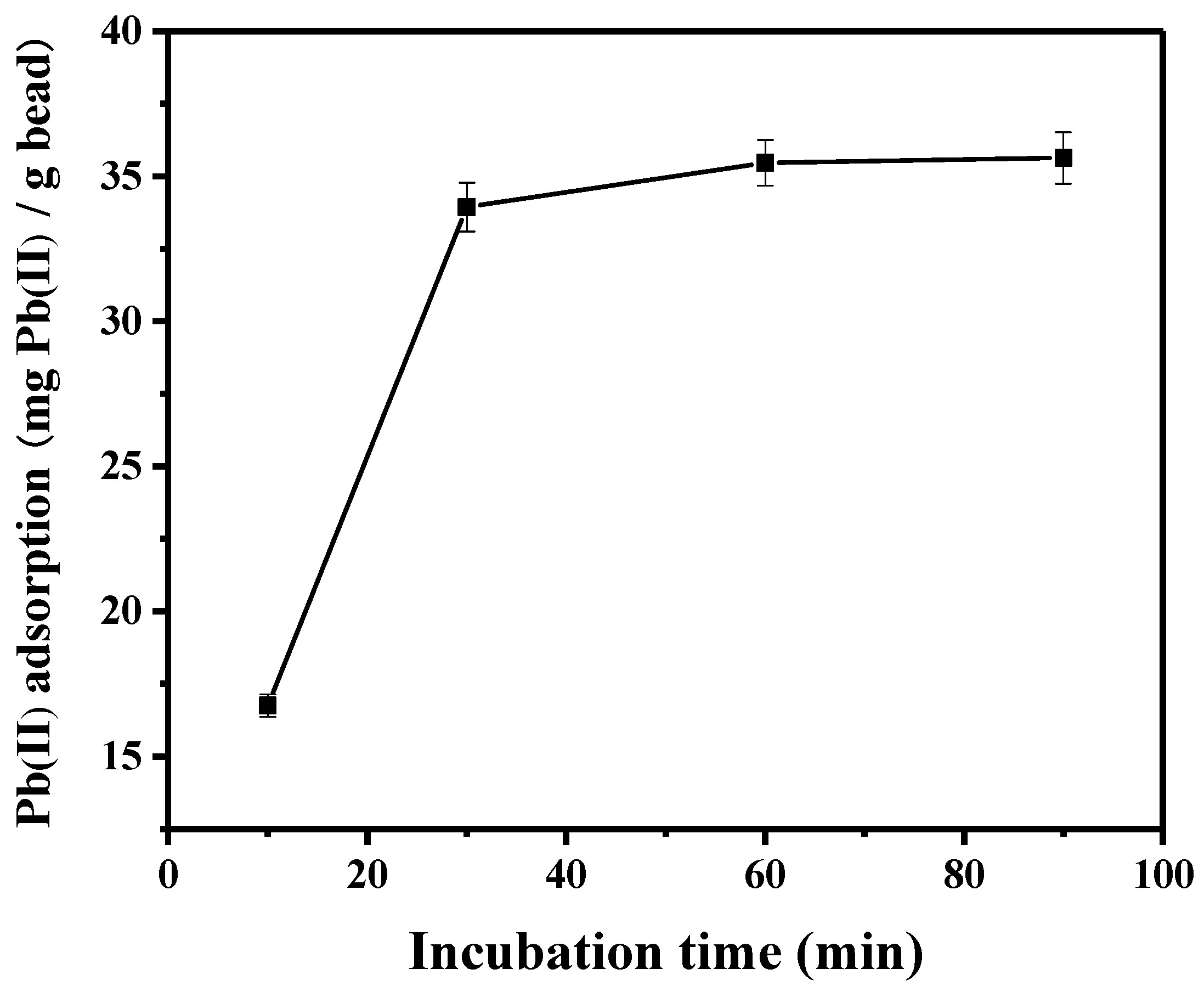
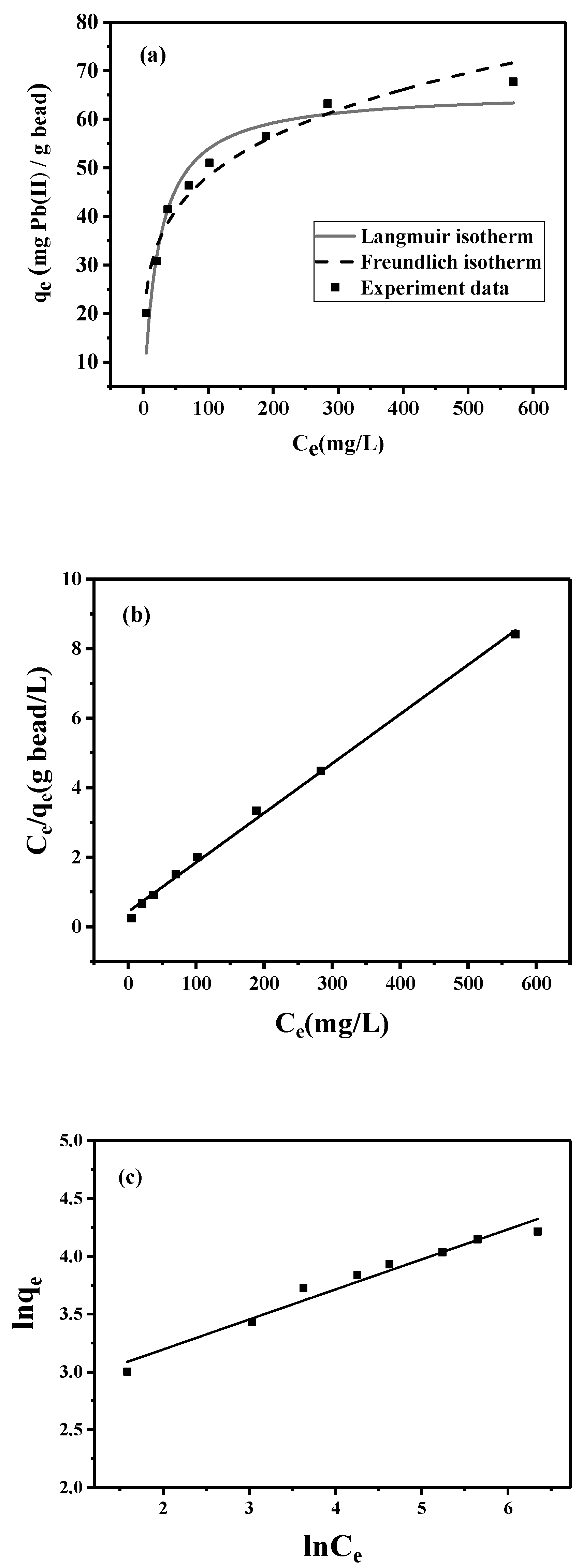
3.2. Lead Adsorption Using Peptide-Linked Magnetic Bead
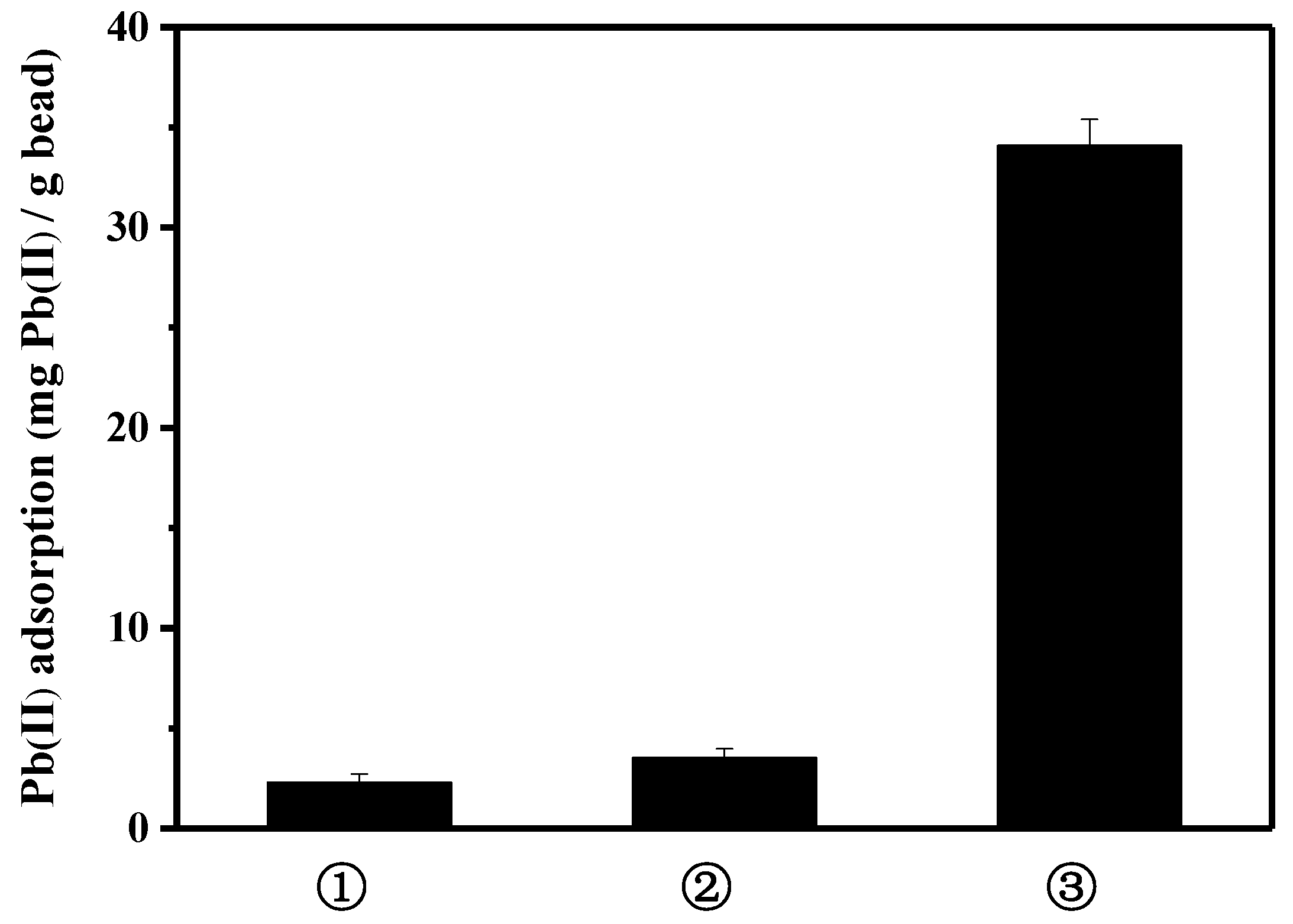
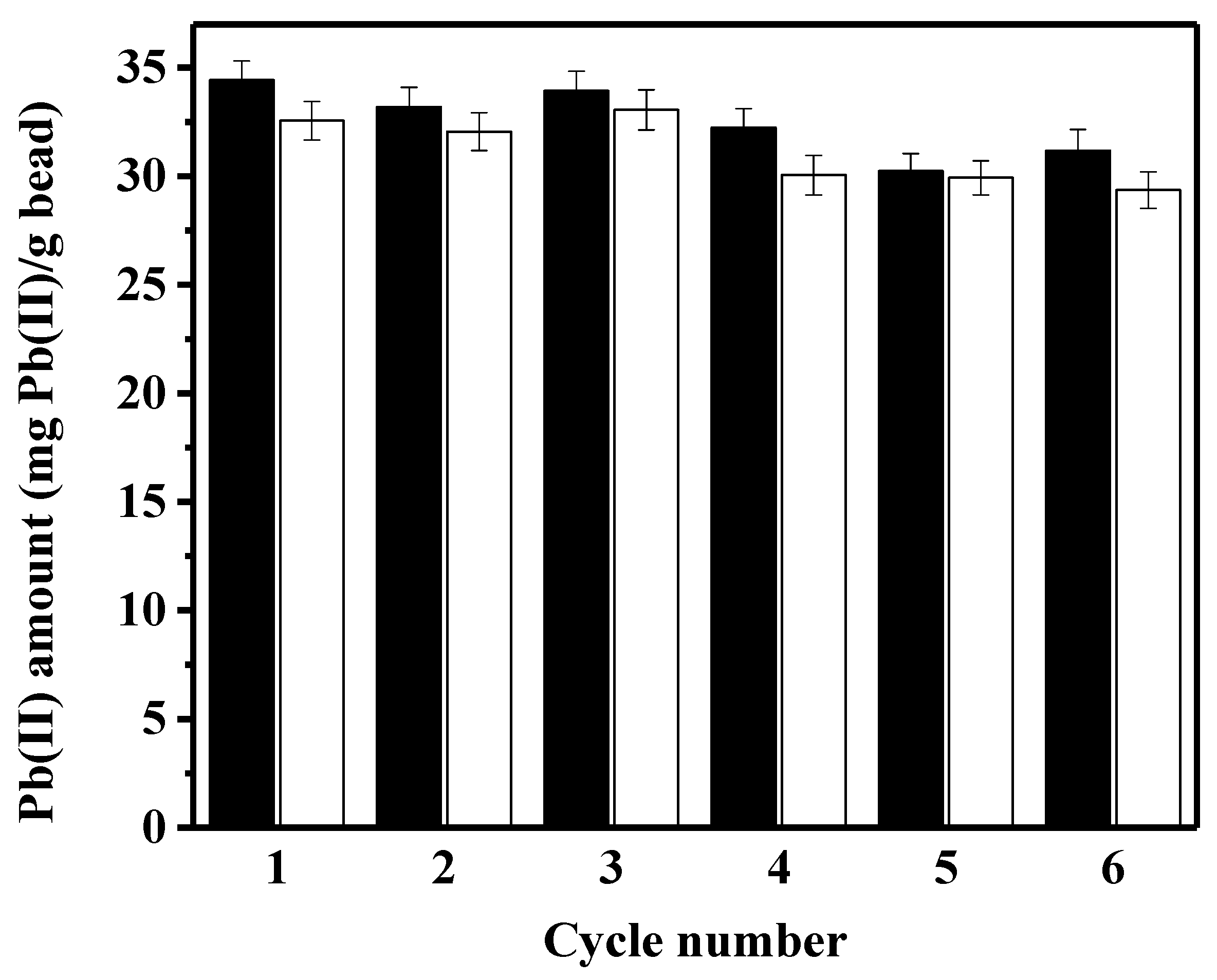
3.3. Reusability of Peptide-Linked Bead
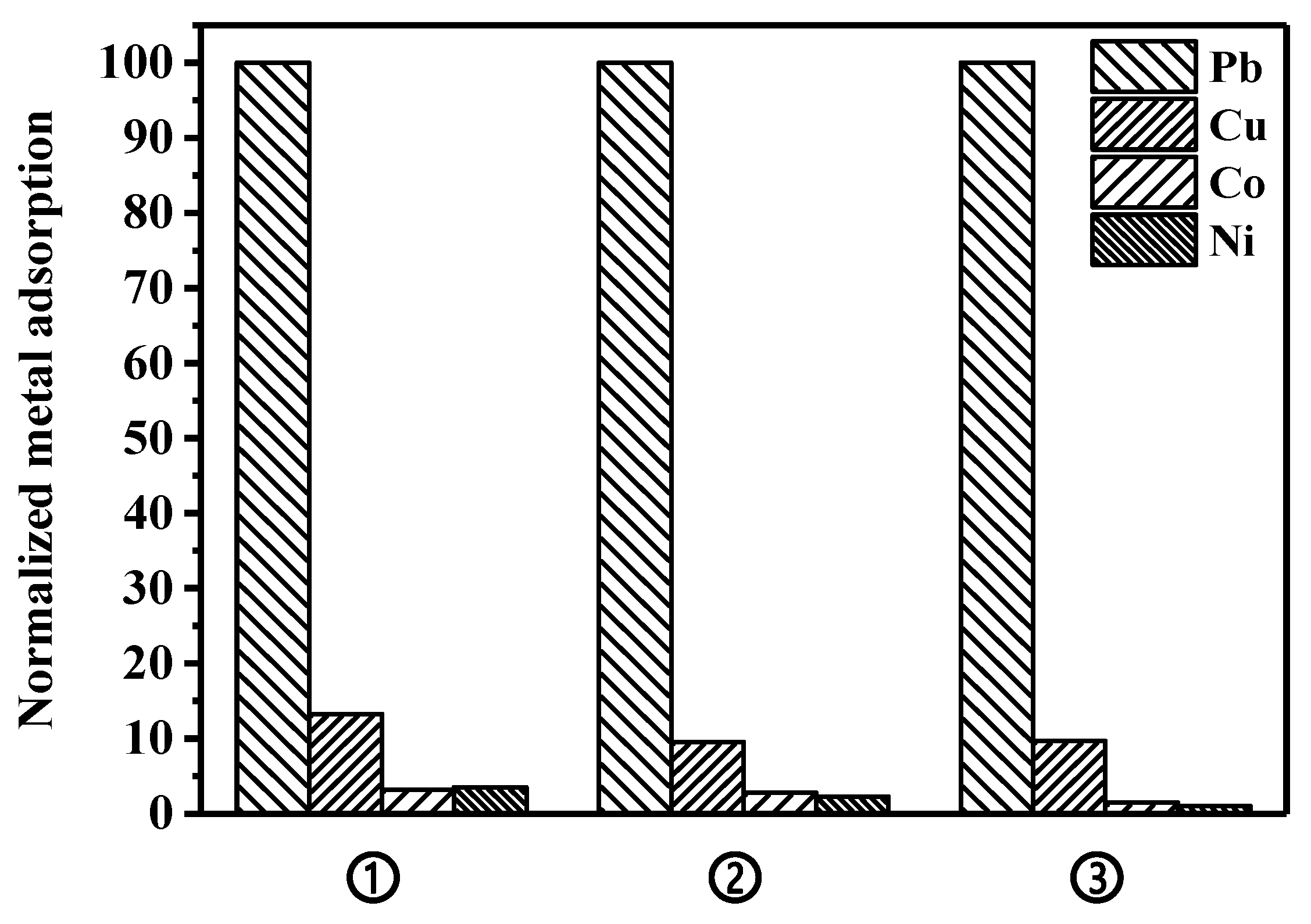
3.4. Selective Lead Adsorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azarudeen, R.S.; Riswan Ahamed, M.A.; Subha, R.; Burkanudeen, A.R. Heavy and toxic metal ion removal by a novel polymeric ion-exchanger: Synthesis, characterization, kinetics and equilibrium studies. J. Chem. Technol. Biotechnol. 2015, 90, 2170–2179. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2018, 17, 729–754. [Google Scholar] [CrossRef]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef]
- Manawi, Y.; McKay, G.; Ismail, N.; Kayvani Fard, A.; Kochkodan, V.; Atieh, M.A. Enhancing lead removal from water by complex-assisted filtration with acacia gum. Chem. Eng. 2018, 352, 828–836. [Google Scholar] [CrossRef]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2914. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; Ulson de Souza, S.M.A.G.; Ulson de Souza, A.A. Study of lead (II) adsorption onto activated carbon originating from cow bone. J. Clean. Prod. 2014, 65, 342–349. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of Heavy Metal Ions from Water by Magnetic Cellulose-Based Beads with Embedded Chemically Modified Magnetite Nanoparticles and Activated Carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Chen, C.L.; Rosi, N.L. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 1924–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Z.; Huang, W.; Zhao, Y.; Dong, S.; Zeng, M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods 2013, 5, 689–697. [Google Scholar] [CrossRef]
- Shen, W.Z.; Cetinel, S.; Sharma, K.; Borujeny, E.R.; Montemagno, C. Peptide-functionalized iron oxide magnetic nanoparticle for gold mining. J. Nanopart. Res. 2017, 19, 74. [Google Scholar] [CrossRef]
- Braun, R.; Bachmann, S.; Schonberger, N.; Matys, S.; Lederer, F.; Pollmann, K. Peptides as biosorbents - Promising tools for resource recovery. Res. Microbiol. 2018, 169, 649–658. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, X.X.; Yang, J.Y.; Wang, Y.T.; Chen, M.L. Screening arsenic(III)-binding peptide for colorimetric detection of arsenic(III) based on the peptide induced aggregation of gold nanoparticles. Talanta 2018, 177, 212–216. [Google Scholar] [CrossRef]
- Nian, R.; Kim, D.S.; Nguyen, T.; Tan, L.; Kim, C.W.; Yoo, I.K.; Choe, W.S. Chromatographic biopanning for the selection of peptides with high specificity to Pb2+ from phage displayed peptide library. J. Chromatogr. A 2010, 1217, 5940–5949. [Google Scholar] [CrossRef]
- Gaber, D.; Abu Haija, M.; Eskhan, A.; Banat, F. Graphene as an Efficient and Reusable Adsorbent Compared to Activated Carbons for the Removal of Phenol from Aqueous Solutions. Water Air Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Z.; Wang, L.; Meng, Q.; Yuan, Y.; Zhu, G. Constructing synergistic groups in porous aromatic frameworks for the selective removal and recovery of lead(ii) ions. J. Mater. Chem. A 2018, 6, 5202–5207. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Li, S.; Tian, Y.; Zhu, G. Porous Aromatic Framework Modified Electrospun Fiber Membrane as a Highly Efficient and Reusable Adsorbent for Pharmaceuticals and Personal Care Products Removal. ACS Appl. Mater. Interfaces 2019, 11, 16662–16673. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, Y.; Shen, H.; Hu, M. An intensive study on the magnetic effect of mercapto-functionalized nano-magnetic Fe3O4 polymers and their adsorption mechanism for the removal of Hg(II) from aqueous solution. Chem. Eng. 2012, 210, 564–574. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Eng. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Liu, F.; Jin, Y.; Liao, H.; Cai, L.; Tong, M.; Hou, Y. Facile self-assembly synthesis of titanate/Fe3O4 nanocomposites for the efficient removal of Pb2+ from aqueous systems. J. Mater. Chem. A 2013, 1, 805–813. [Google Scholar] [CrossRef]
- Liu, T.; Han, X.; Wang, Y.; Yan, L.; Du, B.; Wei, Q.; Wei, D. Magnetic chitosan/anaerobic granular sludge composite: Synthesis, characterization and application in heavy metal ions removal. J. Colloid Interface Sci. 2017, 508, 405–414. [Google Scholar] [CrossRef]
- Guo, B.; Deng, F.; Zhao, Y.; Luo, X.; Luo, S.; Au, C. Magnetic ion-imprinted and –SH functionalized polymer for selective removal of Pb(II) from aqueous samples. Appl. Surf. Sci. 2014, 292, 438–446. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Zhou, X.; Ren, J.; Zhong, C. Magnetic multi-porous bio-adsorbent modified with amino siloxane for fast removal of Pb(II) from aqueous solution. Appl. Surf. Sci. 2018, 427, 976–985. [Google Scholar] [CrossRef]
- Fischer, M.J. Amine coupling through EDC/NHS: A practical approach. Methods Mol. Biol. 2010, 627, 55–73. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef]
- Huang, M.R.; Peng, Q.Y.; Li, X.G. Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chemistry 2006, 12, 4341–4350. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of lead(II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8. [Google Scholar] [CrossRef]
- Xu, Y.; Yoo, I.K.; Lee, H.; Ryu, K. Adsorptive removal of heavy metal ions in water using poly(m-phenylenediamine) synthesized by laccase. Chem. Pap. 2019, 73, 1705–1711. [Google Scholar] [CrossRef]
- Pal, P.; Syed, S.S.; Banat, F. Gelatin-bentonite composite as reusable adsorbent for the removal of lead from aqueous solutions: Kinetic and equilibrium studies. J. Water Process. Eng. 2017, 20, 40–50. [Google Scholar] [CrossRef]
- Tang, C.; Shu, Y.; Zhang, R.; Li, X.; Song, J.; Li, B.; Zhang, Y.; Ou, D. Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC. Adv. 2017, 7, 16092–16103. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lee, H.R.; Hong, S.H.; Jang, J.R.; Choe, W.S.; Yoo, I.K. Selective lead adsorption by recombinant Escherichia coli displaying a lead-binding peptide. Appl. Biochem. Biotechnol. 2013, 169, 1188–1196. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yoo, I.-K. Removal of Lead from Water Solution by Reusable Magnetic Adsorbent Incorporating Selective Lead-Binding Peptide. Appl. Sci. 2020, 10, 6418. https://doi.org/10.3390/app10186418
Xu Y, Yoo I-K. Removal of Lead from Water Solution by Reusable Magnetic Adsorbent Incorporating Selective Lead-Binding Peptide. Applied Sciences. 2020; 10(18):6418. https://doi.org/10.3390/app10186418
Chicago/Turabian StyleXu, Yue, and Ik-Keun Yoo. 2020. "Removal of Lead from Water Solution by Reusable Magnetic Adsorbent Incorporating Selective Lead-Binding Peptide" Applied Sciences 10, no. 18: 6418. https://doi.org/10.3390/app10186418
APA StyleXu, Y., & Yoo, I.-K. (2020). Removal of Lead from Water Solution by Reusable Magnetic Adsorbent Incorporating Selective Lead-Binding Peptide. Applied Sciences, 10(18), 6418. https://doi.org/10.3390/app10186418




