CQDs@NiO: An Efficient Tool for CH4 Sensing
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthetic Procedure
2.2. Materials and Equipment
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Boulart, C.; Mowlem, M.C.; Connelly, D.P.; Dutasta, J.-P.; German, C.R. A novel, low cost, high performance dissolved methane sensor for aqueous environments. Opt. Express 2008, 16, 12607–12617. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Sun, P.; Yan, X.; Du, X.; Jiang, Y. Fabrication of methane gas sensor by layer by-layer self-assembly of polyaniline/PdO ultra thin films on quartz crystal microbalance. Sens Actuators B Chem. 2010, 145, 373–377. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuat. A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.H. Direct methanol synthesis from methane in a plasma-catalyst hybrid system at low temperature using metal oxide-coated glass beads. Sci. Rep. 2018, 8, 9956. [Google Scholar] [CrossRef]
- Jebreiil Khadema, S.M.; Abdia, Y.; Darbari, S.; Ostovari, F. Investigating the effect of gas adsorption on the electromechanical and electrochemical behavior of graphene/ZnO structure, suitable for highly selective and sensitive gas sensors. Curr. Appl. Phys. 2014, 14, 1498–1503. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Palleschi, G.; Flammini, R.; Bauer, E.M.; Nasillo, G.; Caponetti, E. Oxidized graphene in ionic liquids for assembling chemically modified electrodes: A structural and electrochemical characterization study. Anal. Chem. 2012, 84, 5823–5831. [Google Scholar] [CrossRef]
- Valentini, F.; Carbone, M.; Palleschi, G. Carbon nanostructured materials for applications in nano-medicine, cultural heritage, and electrochemical biosensors. Anal. Bioanal. Chem. 2013, 405, 451–465. [Google Scholar] [CrossRef]
- Pungjunun, K.; Chaiyo, S.; Praphairaksit, N.; Siangproh, W.; Ortner, A.; Kalcher, K.; Chailapakul, O.; Mehmet, E. Electrochemical detection of NOx gas based on disposable paper-based analytical device using a copper nanoparticles-modified screen-printed graphene electrode. Biosens. Bioelectron. 2019, 143, 111606. [Google Scholar] [CrossRef]
- Valentini, F.; Carbone, M.; Palleschi, G. Graphene oxide nanoribbons (GNO), reduced graphene nanoribbons (GNR), and multi-layers of oxidized graphene functionalized with ionic liquids (GO-IL) for assembly of miniaturized electrochemical devices. Anal. Bioanal. Chem. 2013, 405, 3449–3474. [Google Scholar] [CrossRef]
- Zainal, P.N.S.; Ahmad, S.A.A.; Ngee, L.H. Surface Modification of Screen-Printed Carbon Electrode (SPCE) with Calixarene-Functionalized Electrochemically Reduced Graphene Oxide (ERGO/C4) in the Electrochemical Detection of Anthracene. J. Electrochem. Soc. 2019, 166, B110. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Bauer, E.M.; Ditaranto, N.; Sabbatini, L.; Caponetti, E.; Chillura-Martino, D. Graphene and ionic liquids new gel paste electrodes for caffeic acid quantification. Sens. Actuat. B Chem. 2015, 212, 248–255. [Google Scholar] [CrossRef]
- Valentini, F.; Ciambella, E.; Boaretto, A.; Rizzitelli, G.; Carbone, M.; Conte, V.; Cataldo, F.; Russo, V.; Casari, C.S.; Chillura-Martino, D.F.; et al. Sensor Properties of Pristine and Functionalized Carbon Nanohorns. Electroanalysis 2016, 28, 2489–2499. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Kysar, J.; Brosha, E.L.; Kreller, C. Development and testing of an electrochemical methane sensor. Sens. Actuat. B 2016, 228, 162–167. [Google Scholar] [CrossRef]
- Chaiyo, S.; Jampasa, S.; Thongchue, N.; Mehmeti, E.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Wide electrochemical window of screen-printed electrode for determination of rapamycin using ionic liquid/graphene composites. Microchim. Acta 2020, 187, 245. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Tang, S.; Tian, Y.; Shuang, S.; Dong, C.; Choi, M.M.F. Electro-catalytic oxidation of methane at multi-walled carbon nanotubes-Nafion/nickel hydroxide modified nickel electrode. Sens. Actuat. B Chem. 2009, 138, 402–407. [Google Scholar] [CrossRef]
- Lee, I.; Akbar, S.A.; Dutta, P.K. High temperature potentiometric carbon dioxide sensor with minimal interference to humidity. Sens. Actuat. B 2009, 142, 337–341. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, Z.; Wei, Z.; Xu, L.; Gui, Y.; Chen, W. Hydrothermal Synthesis of Hierarchical Ultrathin NiO Nanoflakes for High-Performance CH4 Sensing. Front. Chem 2018, 6, 194. [Google Scholar] [CrossRef]
- Carbone, M.; Bauer, E.M.; Micheli, L.; Missori, M. NiO morphology dependent optical and electrochemical properties. Colloid. Surf. A 2017, 532, 178–182. [Google Scholar] [CrossRef]
- Carbone, M.; Micheli, L.; Nesticò, A.; Bellucci, N.; Palleschi, G. Enhanced performances of sensors based on screen printed electrodes modified with nanosized NiO particles. Electrochim. Acta 2017, 246, 580–587. [Google Scholar] [CrossRef]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef]
- Carbone, M. Cu-Zn-Co nanosized mixed oxides prepared from hydroxycarbonate precursors. J. Alloys Compd. 2016, 688, 202–209. [Google Scholar] [CrossRef]
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial power of Cu/Zn mixed oxide nanoparticles to Escherichia coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102. [Google Scholar]
- Carbone, M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, W.; Wang, Z. Assembly of 2D nanosheets into 3Dflower-like NiO: Synthesis and the influence of petal thickness on gas-sensing properties. Ceram. Int. 2016, 42, 4567–4573. [Google Scholar] [CrossRef]
- Gagaoudakis, E.; Michail, G.; Kampylafka, V.; Tsagaraki, K.; Aperathitis, E.; Moschovis, K.; Binas, V.; Kiriakidis, G. Room Temperature p-Type NiO Nanostructure Thin Film Sensor for Hydrogen and Methane Detection. Sens. Lett. 2017, 15, 1–5. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, J.; Zhang, Z.; Xie, E. Enhanced ethanol sensing performance of porous ultrathin NiO nanosheets with neck-connected networks. RCS Adv. 2013, 3, 4018–4023. [Google Scholar] [CrossRef]
- Carbone, M.; Tagliatesta, P. NiO grained-flowers and nanoparticles for ethanol sensing. Materials 2020, 13, 1880. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R. Characterization of nickel oxide decorated-reduced graphene oxide nanocomposite and its sensing properties toward methane gas detection. J. Mater. Sci. Mater. Electron. 2016, 27, 723–3730. [Google Scholar] [CrossRef]
- Nakate, U.T.; Lee, G.H.; Ahmad, R.; Patil, P.; Bhopate, P.; Hahn, Y.B.; Yu, Y.T.; Suh, E.-K. Hydrothermal synthesis of p-type nanocrystalline NiO nanoplates for high response and low concentration hydrogen gas sensor application. Ceram. Int. 2018, 44, 15721–15729. [Google Scholar] [CrossRef]
- Wei, Z.; Zhou, Q.; Wang, J.; Gui, Y.; Zeng, W. A novel porous NiO nanosheet and its H2 sensing performance. Mater. Lett. 2019, 245, 166–169. [Google Scholar] [CrossRef]
- Wu, C.-H.; Zhu, Z.; Chang, H.-M.; Jiang, Z.-X.; Hsieh, C.Y.; Wu, R.-J. Pt@NiO core–shell nanostructure for a hydrogen gas sensor. J. Alloys Compd. 2020, 814, 151815. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Stanoiu, A.; Taylor, A.; Guldin, S.; Covington, J.A.; Carmalt, C.J.; Blackman, C.S. Humidity-Tolerant Ultrathin NiO Gas-Sensing Films. ACS Sens. 2020, 5, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, J.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.; Yang, J.; Sun, Y. Acetone sensors with high stability to humidity changes based on Ru-doped NiO flower-like microspheres. Sens. Actuat. B 2020, 313, 127965. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Y.; Tian, X.; Liang, Q.; Liu, X.; Sun, Y. P-p heterojunction composite of NiFe2O4 nanoparticles-decorated NiO nanosheets for acetone gas detection. Mater. Lett. 2020, 270, 127728. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Z.; Liu, Y.; Gao, J.; Wang, X.; Suo, H.; Yang, X.; Zhao, C.; Liu, F. Gas sensor based on Ni foam: SnO2-decorated NiO for Toluene detection. Sens. Actuat. B 2020, 318, 128167. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, X.; Fan, Y.; Sun, Y. A formaldehyde gas sensor with improved gas response and sub-ppm level detection limit based on NiO/NiFe2O4 composite nanotetrahedrons. Sens. Actuat. B 2020, 309, 127719. [Google Scholar] [CrossRef]
- Bai, S.; Han, J.; Han, N.; Zhang, K.; Sun, J.; Sun, L.; Luo, R.; Li, D.; Chen, A. An α-Fe2O3/NiO p-n hierarchical heterojunction for the sensitive detection of triethylamine. Inorg. Chem. Front. 2020, 7, 1532–1539. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, S.; Huang, J.; Wang, C.; Zhao, R.; Qu, F.; Wang, P.; Yang, M. Highly selective and sensitive xylene sensors based on Nb-doped NiO nanosheets. Sens. Actuat. B 2020, 308, 127520. [Google Scholar] [CrossRef]
- Liu, T.; Xu, L.; Wang, X.; Li, Q.; Cui, Q.; Suo, H.; Zhao, C. A simple dip-coating method of SnO2-NiO composite thin film on a ceramic tube substrate for methanol sensing. Crystals 2019, 9, 621. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.; Ren, C.; Chen, X.; Hu, J.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; et al. Controllable synthesis of crescent-shaped porous NiO nanoplates for conductometric ethanol gas sensors. Sens. Actuat. B 2019, 296, 126642. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, X.; Zhou, X.; Sun, P.; Hu, X.; Shimano, K.; Lu, G.; Yamazo, N. Hierarchical α-Fe2O3/NiO Composites with a Hollow Structure for a Gas Sensor. ACS Appl. Mater. Interfaces 2014, 6, 12031–12037. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, Y.; Ran, Y.; Yang, Y.; Zhao, R.; Su, L.; Kong, Y.; Ma, D.; Chen, Y.; Wang, Y. Construction of novel Pd-SnO2 composite nanoporous structure as a high-response sensor for methane gas. J. Alloys Compd. 2020, 826, 154063. [Google Scholar] [CrossRef]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuat. B 2019, 296, 126710. [Google Scholar] [CrossRef]
- Navazani, S.; Shokufar, A.; Hassanisadi, M.; Askrieh, M.; Di Carlo, A.; Agresti, A. Facile synthesis of a SnO2@rGO nanohybrid and optimization of its methane-sensing parameters. Talanta 2018, 181, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Y.; Sun, G.; Zhang, B.; Wang, Y.; Cao, J.; Zhang, Z. Enhanced methane sensing properties of porous NiO nanaosheets by decorating with SnO2. Sens. Actuat. B 2019, 288, 373–382. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and grapheme oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Z.; Gong, A.; Zhang, Y.; Li, Q. Synthesis of highly photoluminescent carbon dots via citric acid and Tris for iron(III) ions sensors and bioimaging. Talanta 2015, 143, 107–113. [Google Scholar] [CrossRef]
- Vaz, R.; Bettini, J.; Júnior, J.G.F.; Lima, E.D.S.; Botero, W.G.; Santos, J.C.C.; Schiavona, M.A.J. High luminescent carbon dots as an eco-friendly fluorescence sensor for Cr(VI) determination in water and soil samples. Photoch. Photobiol. A 2017, 346, 502–511. [Google Scholar] [CrossRef]
- Mishra, R.K.; Upadhyay, S.B.; Kushwaha, A.; Kim, T.-H.; Murali, G.; Verma, R.; Srivastava, M.; Singh, J.; Sahay, P.P.; Lee, S.H. SnO2 quantum dots decorated on RGO: A superior sensitive, selective and reproducible performance for a H2 and LPG sensor. Nanoscale 2015, 7, 11971–11979. [Google Scholar] [CrossRef]
- Bhattacharjee, L.; Manoharan, R.; Mohanta, K.; Bhattacharjee, R.R. Conducting carbon quantum dots—A nascent nanomaterial. J. Mater. Chem. A 2015, 3, 1580–1586. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Applications. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, R.; Carbone, M.; Panero, S.; Sadun, C. Conductivity and structure of poly(ethylene glycol) complexes using energy dispersive X-ray diffraction. J. Phys. Chem. B 1999, 103, 10348–10355. [Google Scholar] [CrossRef]
- JCPDS 22-1189.
- Azim-Araghi, M.E.; Krier, A. Thin film (ClAlPc) phthalocyanine gas sensors. In Selected Topics in Advanced Solid State and Fibre Optic Sensors; Vaezi-Nejad, S.M., Ed.; The Institute of Engineering and Technology (IET): London, UK, 2000. [Google Scholar]
- Wales, D.J.; Parker, R.M.; Gates, J.C.; Grossel, M.C.; Smith, P.G.R. An investigation into relative humidity measurement using analuminosilicate sol–gel thin film as the active layer in an integrated optical Bragg grating refractometer. Sens. Actuat B 2013, 188, 857–866. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuat B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kim, H.-J.; Choi, K.-I.; Kim, K.-M.; Na, C.W.; Lee, J.-H. Highly sensitive C2H5OH sensors using Fe-doped NiO hollow spheres. Sens. Actuat B 2012, 171, 1029–1037. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuat. B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuat. B 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Zhang, J.; Peng, Y.; Xie, E. Enhanced Gas Sensing Performance of Electrospun Pt-functionalized NiO Nanotubes, with Chemical and Electronic Sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef]
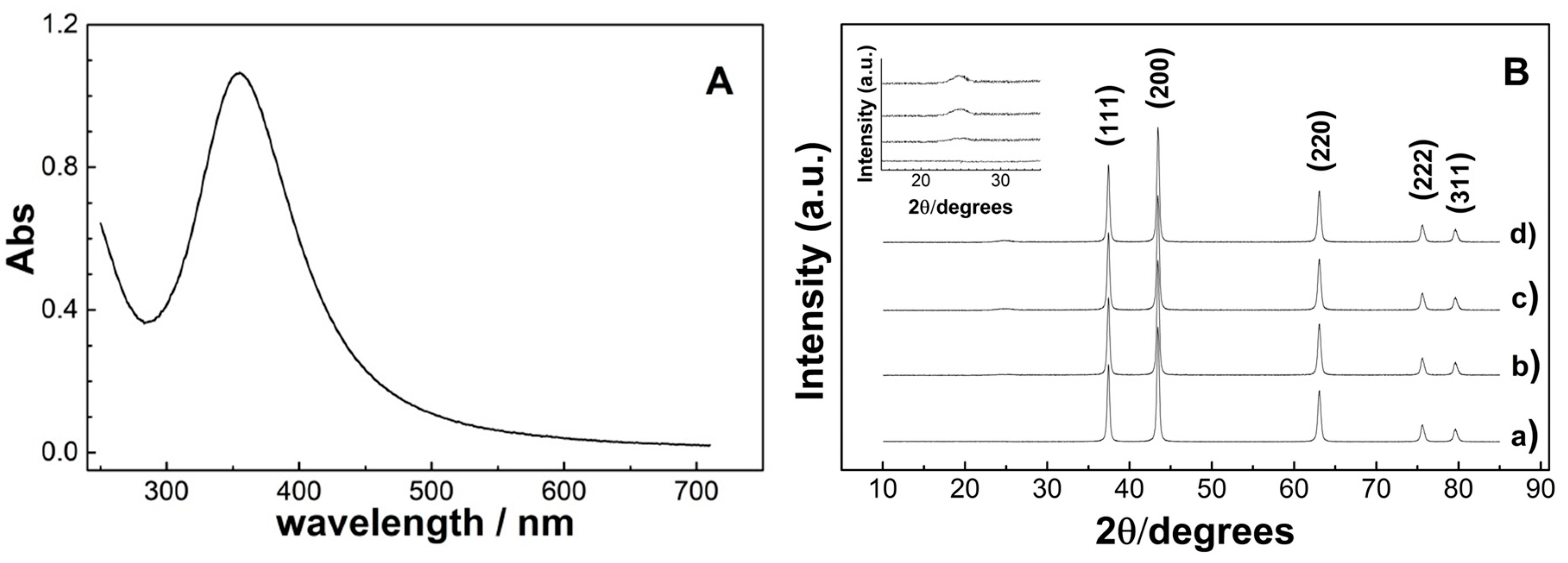
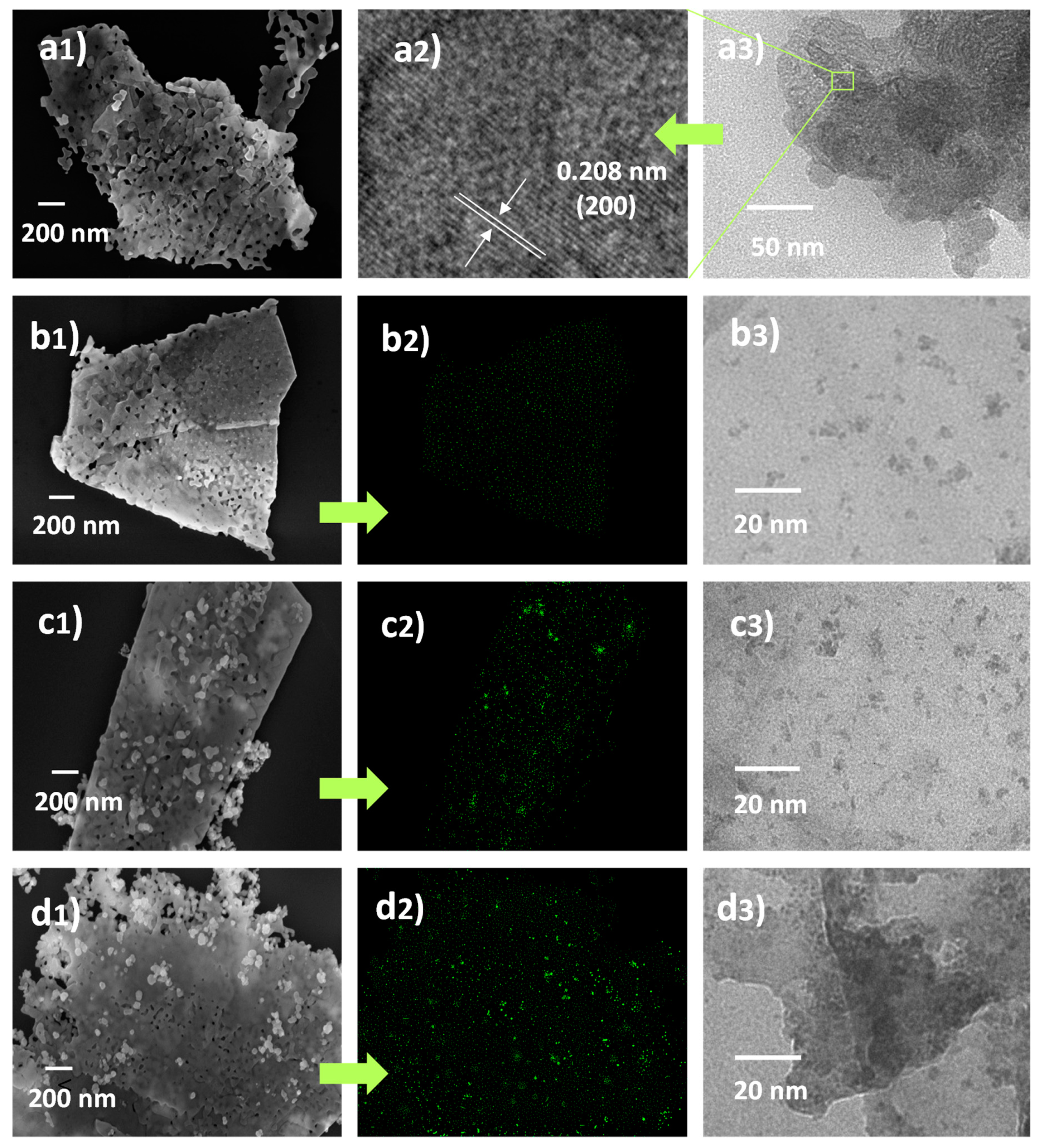
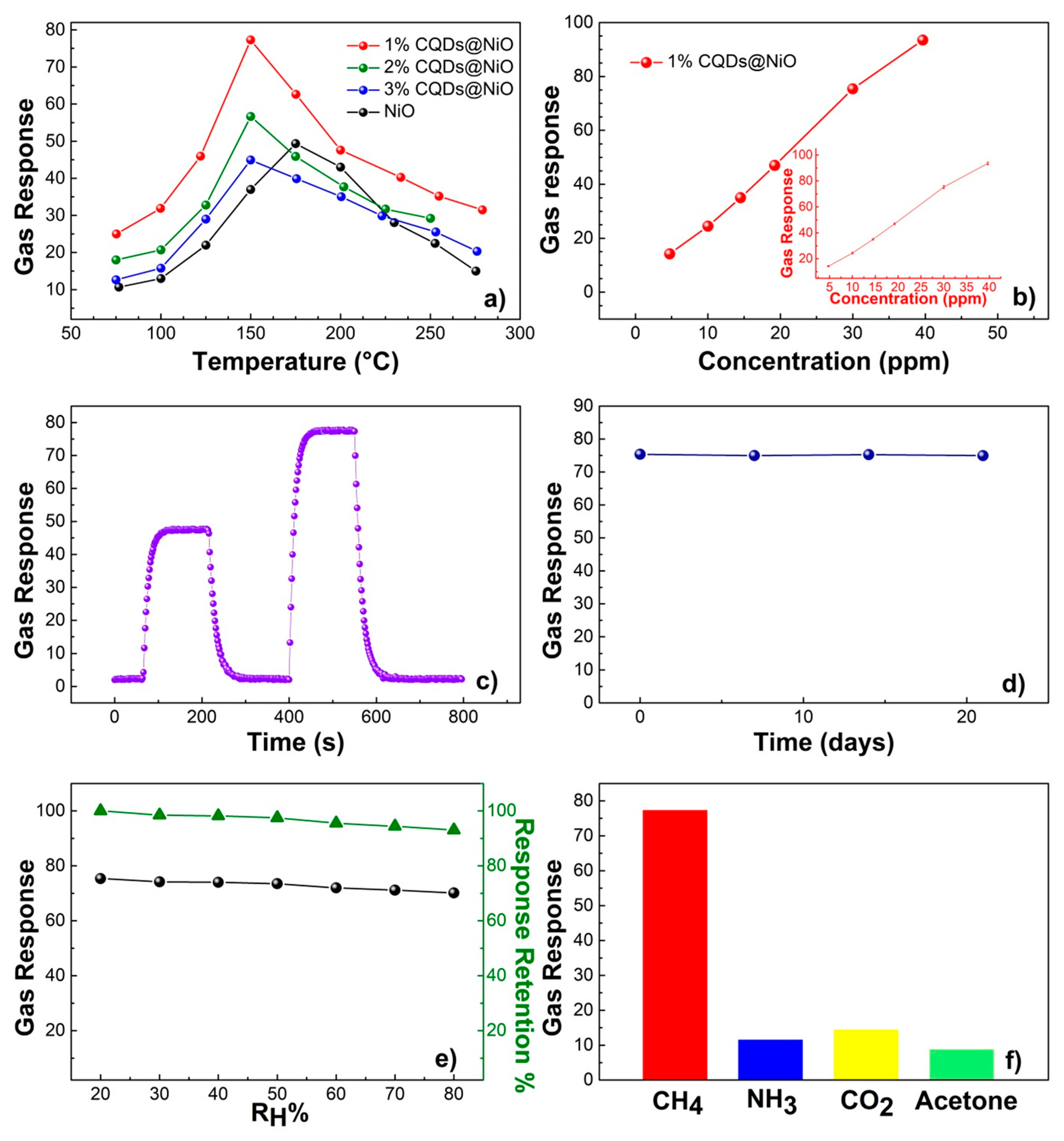
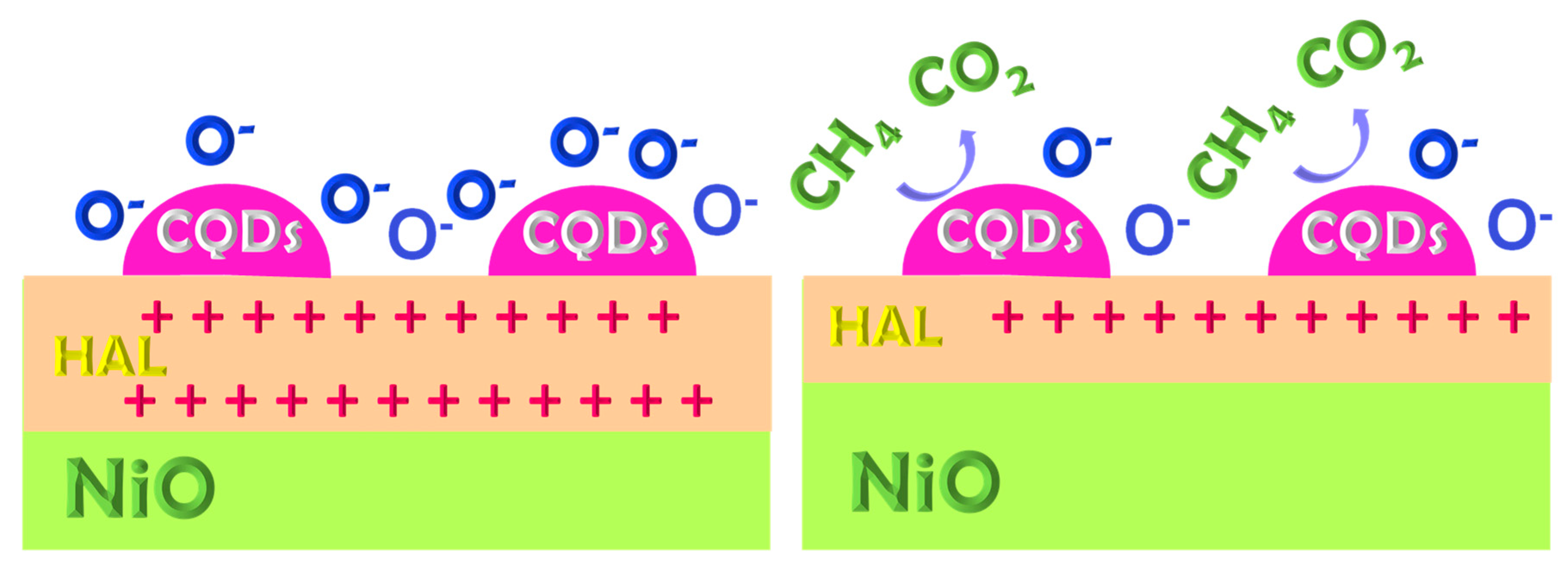
| Sample | Surface Area (m2·g−1) | Average Pore Size (nm) | Total Pore Volume (cm3·g−1) |
|---|---|---|---|
| NiO | 186.7 | 18.6 | 0.53 |
| 1%CQDs@NiO | 196.3 | 18.3 | 0.56 |
| 2%CQDs@NiO | 156.2 | 15.4 | 0.43 |
| 3%CQDs@NiO | 146.1 | 12.5 | 0.32 |
| Sensing Element | Methane (ppm) | Temp (°C) | Gas Response Rg/Ra | Gas Response Ra/Rg | Response/Recovery Time (s) | Reference |
|---|---|---|---|---|---|---|
| 1%CQDs@NiO | 30 | 150 | 77.3 | 10/14 | This work | |
| UT-NiO | 30 | 200 | ~50 | 15/20 | [17] | |
| rGO-NiO | 1000 | 250 | 15 | 6–18/16–20 | [28] | |
| rGO-SnO2 | 1000 | 150 | 47.6 | 61/330 | [44] | |
| Pd-SnO2 | 100 | 340 | 4.38 | 3/5 | [42] | |
| 2.5-Pt/SnO2 | 500 | 100 | 1.98 | -- | [43] | |
| SnO2-NiO | 500 | 330 | 15.2 | 28/44 | [45] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251. https://doi.org/10.3390/app10186251
Carbone M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Applied Sciences. 2020; 10(18):6251. https://doi.org/10.3390/app10186251
Chicago/Turabian StyleCarbone, Marilena. 2020. "CQDs@NiO: An Efficient Tool for CH4 Sensing" Applied Sciences 10, no. 18: 6251. https://doi.org/10.3390/app10186251
APA StyleCarbone, M. (2020). CQDs@NiO: An Efficient Tool for CH4 Sensing. Applied Sciences, 10(18), 6251. https://doi.org/10.3390/app10186251






