Hazards Resulting from the Burning Wood Impregnated with Selected Chemical Compounds
Abstract
1. Introduction
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 on the registration, evaluation, authorisation and restriction of chemicals (REACH) covered by the European Chemicals Agency, the changing requirements of 1999/45/EC and repealing Council (EEC) No 793/93 and Commission (EC) No 1488/94, as well as Council Directives 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC,
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on the classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC)) No. 1907/2006, and
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products.
2. Chemism of Wooden Materials
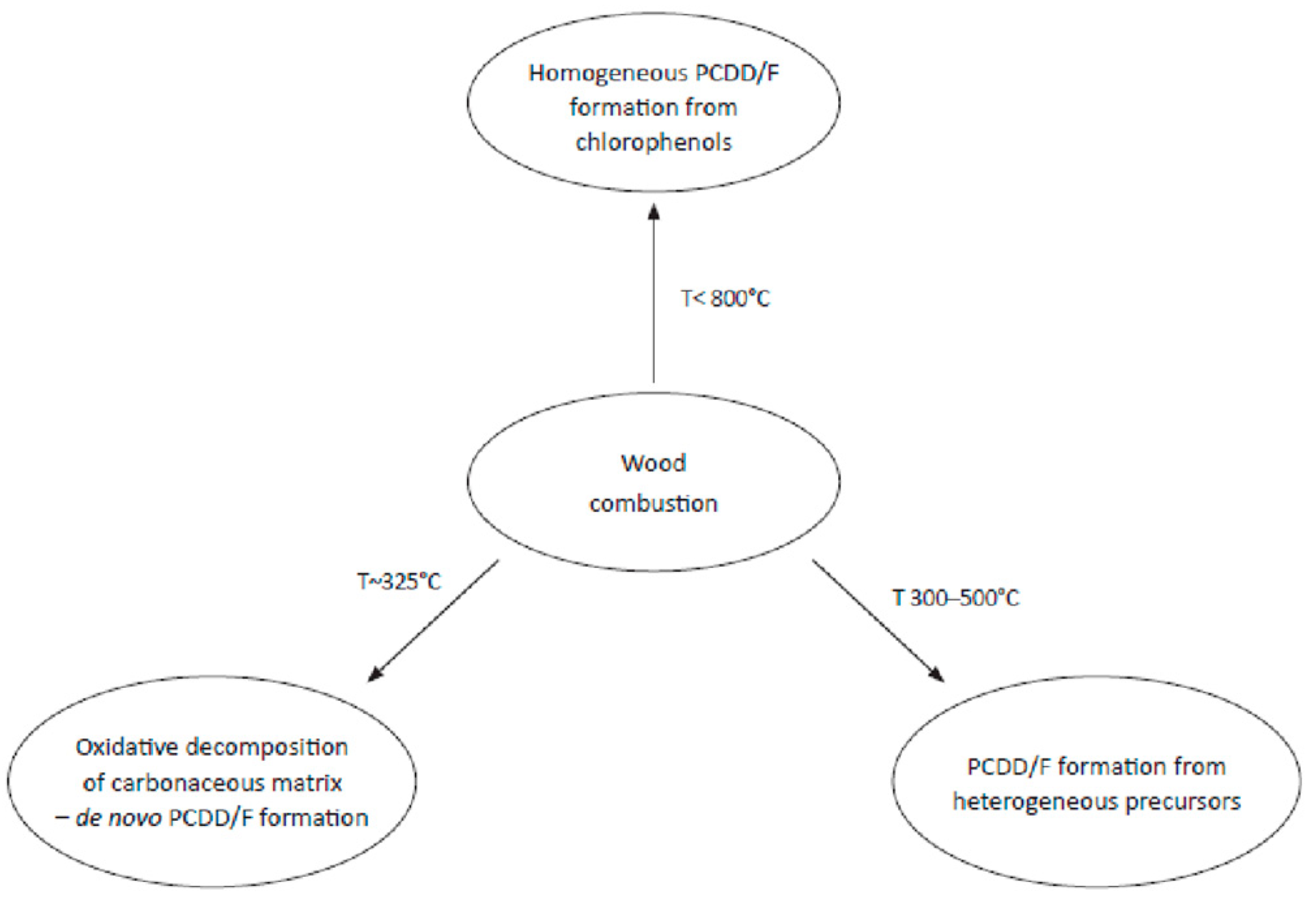
3. Flaming and Smouldering Combustion Processes
4. Emission of Pollutants and Methods of Measurement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Can, A.; Özlüsoylu, İ.; Grzeskowıak, W.; Sözen, E. Improvement of Fire Performance of Impregnated Wood with Copper Based Chemicals. Implementation of Wood Science in Woodworking Sector. In Proceedings of the 28th International Conference on Wood Science and Technology 2017, Zagreb, Croatia, 7–9 December 2017. [Google Scholar]
- Baysal, E.; Deveci, I.; Turkoglu, T.; Toker, H. Thermal analysis of oriental beech sawdust treated with some commercial wood preservatives. Maderas. Ciencia y Tecnología 2017, 19, 329–338. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Morén, T. Moisture properties of heat-treated Scots pine and Norway spruce sapwood impregnated with wood preservatives. Wood Fiber Sci. 2012, 44, 85–93. [Google Scholar]
- Ajuong, E.; Pinion, L.C. Corrosion and degradation of engineering materials. In Shreir’s Corrosion; Richardson, T.J.A., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2010; pp. 2439–2446. [Google Scholar]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood modification technologies—A review. iForest 2017, 10, 895–908. [Google Scholar] [CrossRef]
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products Text with EEA Relevance. The Biocidal Products Directive (BPD 98/08/EC). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:167:0001:0123:EN:PDF (accessed on 1 September 2020).
- Mačiulaitis, R.; Jefimovas, A.; Zdanevičius, P. Research of natural wood combustion and charring processes. J. Civ. Eng. Manag. 2012, 18, 631–641. [Google Scholar] [CrossRef]
- Franco, L.S.; Shanahan, D.F.; Fuller, R.A. A review of the benefits of nature experiences: More than meets the eye. Int. J. Environ. Res. Public Health 2017, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Altax Impregnation for Construction Wood, Safety Data Sheet. Available online: https://www.altax.pl/wp-content/uploads/2018/03/Altax-impregnat-do-drewna-konstrukcyjnego-08.05.2017.pdf (accessed on 12 May 2020).
- Altax Wood Oil, Safety Data Sheet. Available online: https://www.altax.pl/wp-content/uploads/2018/03/Altax-olej-do-drewna-05.12.2016.pdf (accessed on 12 May 2020). (In Polish).
- IMPRAPOL PQ40—Wood Impregnation, Safety Data Sheet. Available online: https://tartak.pl/wp-content/uploads/2015/02/IMPRAPOL__PQ40_karta_charakterystyki.pdf (accessed on 12 May 2020). (In Polish).
- PENETRIN—Solvent-Based Wood Impregnation, Safety Data Sheet. Available online: http://cdn11.pb.smcloud.net/t/files/4b/93/be/b9ef92ac90/gruntujacy-impregnat-do-drewna-altax-penetrin-kch.pdf (accessed on 12 May 2020). (In Polish).
- Cabinet & Wood Cleaner—3063. SAFETY DATA SHEET Confirms to OSHA Hazard Communication Standard (CFR 29 1910.1200) HazCom 2012 Revision on 27 January 2015. Available online: https://amarillobolt.com/files/sds/3063_sds.pdf (accessed on 1 September 2020).
- AUSPLAST. Ausplast Wood Pole preServing Compound. In Safety Data Sheet, Hazardous Substance, Material and Supply Company Identification; Ausmose Pty Ltd.: Cheltenham, Australia, 2016; p. 7. [Google Scholar]
- CREOSOTE. Safety Data Sheet, According to Regulation (EC) No 1907/2006, Annex II, as Amended. Available online: https://www.poles.se/wp-content/uploads/sites/3/2019/01/CREOSOTE_English.pdf (accessed on 12 May 2020).
- Georgia-Pacific Treated Lumber LLC, ACQ Pressure Treated Lumber, Material Safety Data Sheet, ID: GP-33Q Effective on 29 June 2009. Available online: https://msdsdigital.com/system/files/ACQ%20Pressure%20Treated%20Lumber.pdf (accessed on 1 September 2020).
- Koppers Performance Chemicals Inc. Safety Data Sheet, MCQ Treated Wood Other. 2015. Available online: https://www.yellawood.com/media/1906/227-kpc_mcq_treated_wood-sds_us-english_rev2-426.pdf (accessed on 1 September 2020).
- Akzo Nobel Coatings, Inc. 230-65XX Chemlack White NC Pigmented TC. 2018. Available online: https://chemcraft.com/en/documents/public/public-pis/us-pis/1786-us-pis-230-65xx-chemlack-white-nc-pigmented-tc-pdf/file (accessed on 1 September 2020).
- Vidaron, F.F.I.L.; Snieżka S.A. Protective and Decorative Impregnate for Wood, Technical Card. 2010. Available online: https://www.jula.pl/globalassets/catalog/productdocuments/msds/500809500810500811500812500813500814500815500816500817_pl.pdf (accessed on 1 September 2020).
- Safeguard Europe Ltd. ROXIL—10 YEAR WOOD PROTECTOR, Safety Data Sheet. 2015. Available online: https://www.mightonproducts.com/media/downloads/149/roxil-10-year-wood-protector-msds.pdf (accessed on 1 September 2020).
- Rawlins. Trade Paint & Coatings Superstore. Available online: https://www.rawlinspaints.com/home/fire-retardant-paints/timber-plasterboard/128-zeroflame-fire-retardant-treatment.html (accessed on 26 June 2020).
- TIKKURILA. Solven-Borne One Component Acid Catalysed Lacquer System, Merit Zirkon Extra 25005 2717, WC13. November 2014. Available online: https://tikkurila.com/sites/default/files/WC13_November2014_1.pdf (accessed on 1 September 2020).
- Salminen, E.; Valo, R.; Korhonen, M.; Jernlås, R. Wood Preservation with Chemicals. In Best Available Techniques (BAT), TemaNord 2014:550; Nordic Council of Ministers: Copenhagen, Denmark, 2014; p. 56. [Google Scholar]
- EN 335. Durability of Wood and Wood-Based Products—Use Classes: Definitions, Application to Solid Wood and Wood-Based Products; BSI Standards Limited: London, UK, 2013.
- Fu, F.; Lin, L.; Xu, E. Functional pretreatments of natural raw materials. In Advanced High. Strength Natural Fibre Composites in Construction; Fan, M., Fu, F., Eds.; Woodhead Publishing Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- European Chemicals Agency. Transitional Guidance on Efficacy Assessment for Product Type 8 Wood Preservatives; © European Chemicals Agency: Helsinki, Finland, 2015; p. 38. [Google Scholar]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A mechanistic model of fast pyrolysis of hemicellulose. Energy Environ. Sci. 2018, 11, 1240–1260. [Google Scholar] [CrossRef]
- Esmailpour, A.; Majidi, R.; Taghiyari, H.R.; Ganjkhani, M.; Armaki, S.M.M.; Papadopoulos, A.N. Improving Fire Retardancy of Beech Wood by Graphene. Polymers 2020, 12, 303. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Pfriem, A. Treatments and modification to improve the reaction to fire of wood and wood based products—An overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, S.A.; Tohir, M.Z.M. Fire test and effects of fire retardant on the natural ability of timber: A review. Pertanika J. Sci. Technol. 2019, 27, 867–895. [Google Scholar]
- Croitoru, C.; Patachia, S.; Lunguleasa, A. New method of wood impregnation with lnorganic compounds using ethyl methylimidazolium chloride as carrier. J. Wood Chem. Technol. 2015, 35, 113–128. [Google Scholar] [CrossRef]
- Melber, C.; Kielhorn, J.; Mangelsdorf, I. Coal Tar Creosote (Report); United Nations Environment Programme: Nairobi, Kenya; International Labour Organization: Geneva, Switzerland; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Platten, W.; Luxton, T.; Gerke, T.; Harmon, S.; Sylvest, N.; Bradham, K.; Rogers, K. Release of Micronized Copper Particles from Pressure Treated Wood Products; EPA/600/R-14/365; U.S. Environmental Protection Agency: Washington, DC, USA, 2014. [Google Scholar]
- Aqlibous, A.; Tretsiakova-McNally, S.; Fateh, T. Waterborne intumescent coatings containing industrial and bio-fillers for fire protection of timber materials. Polymers 2020, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M. Home Structure Fires. Report: NFPA’s “Home Structure Fires”; National Fire Protection Association (NFPA): Quincy, MA, USA, 2019; p. 18. [Google Scholar]
- Rein, G. Smoldering combustion. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J., Wieczoreks, C., Eds.; Springer: New York, NY, USA, 2016; pp. 581–603. [Google Scholar]
- Santoso, M.A.; Huang, X.; Prat-Guitart, N.; Christensen, E.; Hu, Y.; Rein, G. Smouldering fires and soils, Chapter 13. In Fire Effects in Soil Properties; Pereira, P., Mataix-Solera, J., Úbeda, X., Rein, G., Cerdà, A., Eds.; VIC: Clayton, CA, USA; CSIRO: Canberra, Australia, 2019; pp. 203–216. [Google Scholar]
- Watts, A.C.; Kobziar, L.N. Smoldering Combustion and Ground Fires: Ecological Effects and Multi-Scale Significance. Fire Ecol. 2013, 9, 124–132. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. (Eds.) Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; U.S. Department of Agriculture: Washington, DC, USA; Forest Service: Cassville, MO, USA; Rocky Mountain Research Station: Fort Collins, CO, USA, 2005; 250p. [Google Scholar]
- Shrivastava, P.; Baweja, C.; Nalawade, H.; Kumar, A.V.; Ramanan, V.; Malhotra, V. An Experimental Insight into the Smoldering-Flaming Transition Phenomenon. J. Combust. 2017, 2017, 4062945. [Google Scholar] [CrossRef]
- Maloziec, D.; Koniuch, A. Reaction to Fire Test Methods and Classification Criteria. Saf. Fire Technol. 2010, 17, 63–74. [Google Scholar]
- Bartlett, A.I.; Hadden, R.M.; Bisby, L.A. A Review of Factors Affecting the Burning Behaviour of Wood for Application to Tall Timber Construction. Fire Technol. 2019, 55, 1–49. [Google Scholar] [CrossRef]
- Air Quality in Europe—2019 Report; EEA Report No. 10/2019; European Environment Agency: Copenhagen, Denmark, 2019; 104p.
- Brózda, K.; Slejdak, J. The issue of wooden and concrete railway sleepers utilization. Prod. Eng. Arch. 2015, 9, 35–37. [Google Scholar] [CrossRef]
- European Commission. Environment Waste. Available online: https://ec.europa.eu/environment/waste/framework/framework_directive.htm (accessed on 8 May 2020).
- gov.uk. Available online: https://www.gov.uk/government/publications/classifying-waste-wood-from-mixed-waste-wood-sources-rps-207/classifying-waste-wood-from-mixed-waste-wood-sources-rps-207#enforcement (accessed on 8 May 2020).
- Stec, A.; Hull, R. Fire Toxicity; Woodhead Publishing Ltd.: Cambridge, UK, 2010; p. 688. [Google Scholar]
- Karpovic, Z.; Sukys, R.; Gudelis, R. Toxicity research of smouldering and flaming pine timber treated with fire retardant solutions. J. Civ. Eng. Manag. 2012, 18, 600–608. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 3. C1–C29 organic compounds from fireplace combustion of wood. Environ. Sci. Technol. 2001, 35, 1716–1728. [Google Scholar] [CrossRef]
- Zou, L.Y.; Zhang, W.; Atkiston, S. The characterisation of polycyclic aromatic hydrocarbons emissions from burning of different firewood species in Australia. Environ. Pollut. 2003, 124, 283–289. [Google Scholar] [CrossRef]
- Gullett, B.K.; Touati, A.; Hays, M.D. PCDD/F, PCB, HxCBz, PAH, and PM emission factors for fireplace and woodstove combustion in the San Francisco bay region. Environ. Sci. Technol. 2003, 37, 1758–1765. [Google Scholar] [CrossRef]
- Gullett, B.K.; Touati, A. PCDD/F emissions from burning wheat and rice field residue. Atmos. Environ. 2003, 37, 4893–4899. [Google Scholar] [CrossRef]
- Launhardt, T.; Strehler, A.; Dumler-Gradl, R.; Thoma, H.; Vierle, O. PCDD/F- and PAH-emission from house heating systems. Chemosphere 1998, 37, 2013–2020. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Struschka, M.; Baumbach, G.; Hagenmaier, H.; Hein, K.R.G. PCDD/PCDF emissions from small firin systems in households. Chemosphere 2000, 40, 225–232. [Google Scholar] [CrossRef]
- Nagrodzka, M.; Maloziec, D. Impregnation of the wood by flame retardants. Saf. Fire Tech. 2011, 22, 69–75. [Google Scholar]
- Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the Protection of Workers from the Risks Related to Exposure to Carcinogens or Mutagens at Work. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004L0037-20140325&from=en (accessed on 9 January 2020).
- Humar, M.; Peek, R.D.; Jermer, J. Regulations in the European Union with Emphasis on Germany, Sweden and Slovenia in Environmental Impacts of Treated Wood; Townsend, T.G., Solo-Gabriele, H., Eds.; CRC Press: Boca Raton, FL, USA, 2006; Chapter 3; pp. 37–58. [Google Scholar] [CrossRef]
- Kercher, A.K.; Nagle, D.C. TGA modeling of the thermal decomposition of CCA treated wood. Wood Sci Technol. 2001, 35, 325–341. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Kristensen, I.V.; Pedersen, A.J.; Hansen, H.K. Handling of impregnated waste wood and characterization of ash residues after combustion of the wood. Environ. Sci. 2004, 28599632. [Google Scholar]
- Helsen, L.; Van den Bulck, E.; Cooreman, H.; Vandecasteele, C. Development of a sampling train for arsenic in pyrolysis vapours resulting from pyrolysis of arsenic containing wood waste. J. Environ. Monit. 2003, 5, 758–765. [Google Scholar] [CrossRef]
- McMahon, C.K.; Bush, P.B.; Woolson, A.E. How much arsenic is released when CCA treated wood is burned? For. Prod. J. 1986, 36, 45–50. [Google Scholar]
- Dobbs, A.J.; Grant, C. The volatilization of arsenic on burning copper-chrome-arsenic (CCA) treated wood. Holzforschung 1978, 32, 32–35. [Google Scholar] [CrossRef]
- Hirata, T.; Inoue, M.; Fujiki, Y. Pyrolysis and combustion toxicity of wood treated with CCA. Wood Sci. Technol. 1993, 27, 35–47. [Google Scholar] [CrossRef]
- Kakitani, T.; Hata, T.; Kajimoto, T.; Imamura, Y. Two possible pathways for the release of arsenic during pyrolysis of chromated copper arsenate (CCA)-treated wood. J. Hazard. Mater. 2004, 113, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Chromium interactions in CCA/CCB wood preservatives: Part I. Interactions with wood carbohydrates. Holzforschung 1990, 44, 373–380. [Google Scholar] [CrossRef]
- Pizzi, A. Chromium interactions in CCA/CCB wood preservatives: Part II. with lignin. Holzforschung 1990, 44, 419–424. [Google Scholar] [CrossRef]
- Helsen, L.; Van den Bulck, E.; Van Bael, M.K.; Mullens, J. Arsenic release during pyrolysis of CCA treated wood waste: Current state of knowledge. J. Anal. Appl. Pyrol. 2003, 68–69, 613–633. [Google Scholar] [CrossRef]
- Helsen, L.; Van den Bulck, E. Review of disposal technologies for chromated copper arsenate (CCA) treated wood waste, with detailed analyses of thermochemical conversion processes. Environ. Pollut. 2005, 134, 301–314. [Google Scholar] [CrossRef]
- Helsen, L.; Van den Bulck, E.; Van den Broeck, K.; Vandecasteele, C. Low-temperature pyrolysis of CCAtreated wood waste: Chemical determination and statistical analysis of metal input and output; mass balances. Waste Manag. 1997, 17, 79–86. [Google Scholar] [CrossRef]
- Iida, K.; Pierman, J.; Tolaymat, T.; Townsend, T.; Wu, C.-Y. Control of chromated copper arsenate wood incineration air emissions and ash leaching using sorbent technology. J. Environ. Eng. 2004, 130, 184–192. [Google Scholar] [CrossRef]
- Keskin, H.; Ertürk, N.S.; Çolakoğlu, M.H.; Korkut, S. Combustion properties of Rowan wood impregnated with various chemical materials. Int. J. Phys. Sci. 2013, 8, 1022–1028. [Google Scholar] [CrossRef]
- Vargun, E.; Baysal, E.; Turkoglu, T.; Yuksel, M.; Toker, H. Thermal degradation of oriental beech wood impregnated with different inorganic salts. Woods Sci. Technol. 2019, 21. [Google Scholar] [CrossRef]
- Solo-Gabriele, H.; Kormienko, M.; Gary, K.; Townsend, T.; Stook, K.; Tolaymat, T. Alternative Chemicals and Improved Disposal-End Management Practices for CCA-Treated Wood; Report No. 00-03; Florida Center for Solid and Hazardous Waste Management: Gainesville, FL, USA, 2000. [Google Scholar]
- Ryu, J.-Y.; Mulholland, J.A. Metal-mediated chlorinated dibenzo-p-dioxin (CDD) and dibenzofuran (CDF) formation from phenols. Chemosphere 2005, 58, 977–988. [Google Scholar] [CrossRef]
- Lomnicki, S.; Dellinger, B. Formation of PCDD/F from the pyrolysis of 2-chlorophenol on the surface of dispersed copper oxide particles. Proc. Combust. Inst. 2002, 29, 2463–2468. [Google Scholar] [CrossRef]
- Huang, H.; Buekens, A. De novo synthesis of polychlorinated dibenzo-p-dioxins and dibenzofurans. Proposal of a mechanistic scheme. Sci. Total Environ. 1996, 193, 121–141. [Google Scholar] [CrossRef]
- Ryu, J.-Y.; Mulholland, J.A.; Chu, B. Chlorination of dibenzofuran and dibenzo-p-dioxin vapor by copper (ii) chloride. Chemosphere 2003, 51, 1031–1039. [Google Scholar] [CrossRef]
- Iino, F.; Tabor, D.; Imagawa, T.; Gullett, B. Experimental dechlorination isomer patterns of PCDFs, PCDDs, PCNs, and PCBs from their fully chlorinated species. Organohalogen Compd. 2001, 50, 447–450. [Google Scholar]
- Stieglitz, L.; Bautz, H.; Roth, W.; Zwick, G. Investigation of precursor reactions in the de-novo-synthesis of PCDD/ PCDF on fly ash. Chemosphere 1997, 34, 1083–1090. [Google Scholar] [CrossRef]
- Hell, K.; Stieglitz, L.; Dinjus, E. Mechanistic aspects of the denovo synthesis of PCDD/PCDF on model mixtures and MSWI fly ashes using amorphous 12C- and 13C-labeled carbon. Environ. Sci. Technol. 2001, 35, 3892–3898. [Google Scholar] [CrossRef]
- Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2012; pp. 129–149. [Google Scholar]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Tube Furnace Method for the Determination of Toxic Product Yields in Fire Effluents; BS 7990:2003; Committee FSH/16; British Standards Institution: London, UK, 2003.
- ISO/TS 19700. Controlled Equivalence Ratio Method for the Determination of Hazardous Components of Fire Effluents—Steady-State Tube Furnace. In Technical Committee: ISO/TC 92/SC 3 Fire Threat to People and Environment; ICS: 13.220.01 Protection Against Fire in General; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 5659-2:2017. Plastics—Smoke Generation—Part 2: Determination of optical density by a single-chamber test. In Technical Committee: ISO/TC 61/SC 4 Burning Behaviour; ICS: 13.220.40 Ignitability and Burning Behaviour of Materials and Products. 83.080.01 Plastics; ISO: Geneva, Switzerland, 2017. [Google Scholar]

| Name of the Chemical | Chemical Composition | Application | Ref. |
|---|---|---|---|
| Altax impregnation for structural wood | (2-methoxymethylethoxy) propanol,(RS)-1-[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-ylmethyl]-1H-1,2,4-triazole,3-Iodo-2-propynyl butyl carbamate, zirconium 2-ethylhexanoate, permethrin (PN) | Protects wood against biocorrosion: mold, house fungi, blue stain, and insects feeding on wood; outside and inside | [9] |
| Altax wood oil | Naphtha (petroleum), hydrotreated heavy; low-boiling oil fraction treated with hydrogen; 2-butanone oxime; zirconium 2-ethylhexanoate | Protects wooden furniture, floors, terraces, platforms, bridges against weather conditions | [10] |
| IMPRAPOL PQ40 | Cu(OH)2:CuCO3 (1:1), ethanolamine, alkyl dimethyl benzylammonium chloride, boric acid | For industrial protection of wood against mold, house fungi, technical insects, wood pests, atmospheric factors, hazard classes I, II, III and IV (ground contact); the preparation is solidified in the wood | [11] |
| PENETRIN | Hydrocarbons, C9-C11, n-alkanes, cyclic isoalkanes, <2% aromatic hydrocarbons, hydrocarbons, C15-C20, n-alkanes, isoalkanes, cycloalkanes, <0.03% aromatics, (1RS)-cis, trans-3—3-phenoxybenzyl((2,2-dichlorovinyl)-2,2-dimethylcyclopropanecar-boxylate,(RS)-1-[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-ylmethyl]—1H-1,2,4-triazole,3-iodo-2-propynylbutyl carbamate,2-butanone oxime,1-methoxypropan-2-ol | Solvent wood impregnation, protection against fungi and insect larvae; for terraces, facades, log houses, gazebos, roof, and floor constructions | [12] |
| Cabinet & Wood Cleaner -3063 | Petroleum distillates, hydrotreated light, butane, propane | Liquid for removing dust, grease, dirt from wardrobes, furniture, doors. and other wooden surfaces | [13] |
| AUSPLAST | Sodium fluoride, paraffin, inert filler—calcium sulphate, thixatropes | Wood preservative to protect timber structures from wood decay. | [14] |
| CREOSOTE | Creosote | Biocide, wood impregnation, wood preservation (for outdoor use) | [15] |
| ACQ Pressure-Treated Lumber | Monoethanolamine, copper complex expressed as copper oxides, didecyl dimethyl ammonium carbonate/bicarbonate | Compound to protect wood from decay | [16] |
| MCQ Treated Wood—Other | Copper carbonate, expressed as copper oxide, didecyl dimethyl ammonium carbonate, and didecyl dimethyl ammonium bicarbonate | Preservative-Treated Wood for various exterior applications, including above ground, ground contact, and fresh water exposure | [17] |
| Topcoat | Nitrocellulose | Topcoat can be used for many interior wood applications as a high-quality nitrocellulose lacquer. This product may be used on all types of wood | [18] |
| Vidaron | Naphtha treated with hydrogen (petroleum), xylene, tebuconazole, tolilofluanid, permethrin | Protect them from the harmful effects of the agent in biological and atmospheric conditions, and the damaging effects of moisture. Element used for painting external and internal carpentry, rafters, battens, roof trusses, wooden claddings of buildings, arbors, fences, etc. | [19] |
| ROXIL—10-YEAR WOOD PROTECTOR | A mixture of 5-chloro-2-methyl-2h-isothiazol-3-one [EC No. 247-500-7] and 2-methyl-2h-isothiazol-3-o | Waterproofing liquid for wood;reduced organic growth; reduction of water uptake; improved dimensional stability | [20] |
| Zeroflame Fire-Retardant Treatment | Ferric(III) phosphate; citric acid; polyoxyethylene (21) stearyl alcohol; water | Achieves Euroclass B (BS Class 0) fire propagation and the spread of flame-fire protection on solid timbers; for internal and external use | [21] |
| MERIT ZIRCON EXTRA 25 | Butyl acetate, ethanol, nitrocellulose, urea formaldehyde resin, melamine formaldehyde resin, isobutanol, isopropanol | Lacquer for both sealing and top lacquering; the lacquer may be used for furniture, doors, and other wooden surfaces | [22] |
| Reaction Type | Catalyst | Reference |
|---|---|---|
| Heterogeneous condensation of 3-chlorophenol, 3,4-dichlorophenol, and 2,4,6-trichlorophenol | CuCl2 | [74] |
| PCDD formation from 2-chlorophenol according to the Eley-Rideal mechanism, and PCDD/F formation according to the Langmuir-Hinshelwood mechanism | Copper oxide | [75] |
| Pyrolysis chlorophenols with PCDD and PCDF formation | CuO dispersed on silica surfaces | [75] |
| Reaction of carbon, oxygen, and chlorine sources | Copper or iron ions | [76] |
| Chlorination of dibenzofuran and dibenzo-p-dioxin vapour at temperatures between 200 and 400 °C | CuCl2 | [77] |
| Dechlorination of higher homologues on heating under oxygen-deficient conditions | CuO | [78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabajczyk, A.; Zielecka, M.; Małozięć, D. Hazards Resulting from the Burning Wood Impregnated with Selected Chemical Compounds. Appl. Sci. 2020, 10, 6093. https://doi.org/10.3390/app10176093
Rabajczyk A, Zielecka M, Małozięć D. Hazards Resulting from the Burning Wood Impregnated with Selected Chemical Compounds. Applied Sciences. 2020; 10(17):6093. https://doi.org/10.3390/app10176093
Chicago/Turabian StyleRabajczyk, Anna, Maria Zielecka, and Daniel Małozięć. 2020. "Hazards Resulting from the Burning Wood Impregnated with Selected Chemical Compounds" Applied Sciences 10, no. 17: 6093. https://doi.org/10.3390/app10176093
APA StyleRabajczyk, A., Zielecka, M., & Małozięć, D. (2020). Hazards Resulting from the Burning Wood Impregnated with Selected Chemical Compounds. Applied Sciences, 10(17), 6093. https://doi.org/10.3390/app10176093







