Abstract
Sterilization methods for individuals and facilities are extremely important to enable human beings to continue the basic tasks of life and to enable safe and continuous interaction of citizens in society when outbreaks of viral pandemics such as the coronavirus. Sterilization methods, their availability in gatherings, and the efficiency of their work are among the important means to contain the spread of viruses and epidemics and enable societies to practice their activities almost naturally. Despite the effective solutions given by traditional methods of surface disinfection, modern nanotechnology has proven to be an emergent innovation to protect against viruses. On this note, recent scientific breakthroughs have highlighted the ability of nanospray technology to attach to air atoms in terms of size and time-period of existence as a sterilizer for renewed air in large areas for human gatherings. Despite the ability of this method to control the outbreak of infections, the mutation of bactericidal mechanisms presents a great issue for scientists. In recent years, science has explored a more performant approach and techniques based on a surface-resistance concept. The most emergent is the self-defensive antimicrobial known as the self-disinfection surface. It consists of the creation of a bacteria cell wall to resist the adhesion of bacteria or to kill bacteria by chemical or physical changes. Besides, plasma-mediated virus inactivation was shown as a clean, effective, and human healthy solution for surface disinfection. The purpose of this article is to deepen the discussion on the threat of traditional methods of surface disinfection and to assess the state of the art and potential solutions using emergent nanotechnology.
1. Introduction
The topic of viruses is well discussed and documented by scientists from different perspectives such as biologic evolution, health effect, and reactivity with surface and space. The most objective was to understand how to control their microbial viability. COVID-19 is a disease caused by a new kind of coronavirus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) manifested in Wuhan, Hubei, China at the end of 2019 [1]. This novel coronavirus can cause fever, respiratory failure, septic shock, and even death. Researchers believe that SARS-CoV-2 has zoonotic origin as bat coronaviruses, pangolin coronaviruses, and SARS-CoV previously discovered [2,3]. After being transmitted to humans, this new coronavirus spreads mainly from person to person in several ways. One of the common ways of infection is contacting surfaces containing droplets from a person who is infected with the coronavirus by coughing, talking, or sneezing [4,5]. Research is still ongoing on how long the virus spends on surfaces before it loses its effectiveness. However, it was revealed that it depends on the material from which the surface is made from hours to several days [6,7]. According to the World Health Organization, cleaning and disinfecting are two effective ways to stop this virus and eliminate it from surfaces. Disinfection can be carried out either with traditional methods like liquid solutions such as sodium hypochlorite (bleach/chlorine) and alcohol at 70–90%, or ultraviolet (UV) radiation which has the capacity to destroy the DNA of the virus. However, these techniques were widely criticized by the Environmental Protection Agency opening the horizon to other innovative methods and strategies.
This paper gives a retrospective study of the standard cleaning and disinfection techniques in relation to the surface type and properties and discusses the possible use of nanotechnology to resolve several issues of the traditional methods in front of COVID virus privation.
2. Traditional Methods of Surface Decontamination: Influence of Surface Size and Type
The most important factor of surface decontamination is the surface type and properties and the substrate quality. Surface type is related to the goods material properties ((i) source (nature such as fruits and legume, hard or soft (such as a polymer)), (ii) permeability, (iii) stiffness, (iv) hydrophilicity, etc.). Besides, surface size related to the dimension of equipment and facilities is the second parameter which conditions the choice of the most suitable method. In the following, we reported the most popular methods of disinfection such as fogging, fumigation, wide-area or electrostatic spraying, and ultraviolet light techniques.
2.1. Fogging Method
The process consists of the creation of an envelope to induce a faster loss of reactivity using microbial agents. There are different fogging disinfection systems. The difference exists in the used chemical product: Sterilox hypochlorous acid, tartaric acid solution, liquid peracetic acid, alkyl amine/peracetic acid, the 7.5% of the hydrogen peroxide, and 0.2% of the chlorine dioxide solutions. This disinfection method was used in different applications, especially in the case of the human norovirus.
The study done by Stein et al. [8] shows the efficacy of the fogging system using a tartaric acid solution in the pigs’ health environmental and hygiene parameters but this study should be limited to the low concentration of the tartaric acid because of its irritative effect. Krishnan et al. [9] studied the microbial activity of a dry fogging system using commercial products, whereas Krishnan et al. [10] used liquid peracetic acid (PAA). Both teams demonstrated good compatibility with electronic sensors and facilities used in laboratories, particularly hardware of computers. However, the fogging method was presented as a potential issue to other materials like steel surface.
In other fields and applications, surfaces are huger and more extended such as hospital-acquired infections. These locations are fertile zones for virus development. As a consequence, increased transmission of pathogens is noticed, from patient to patient or patient to hospital staff [11]. In fact, surfaces are more extended, spaces are more closed, and the degree risk is higher than other surfaces [12,13]. A description of hospital space disinfection with a specification of the exposure time and type of disinfection is well documented [14]. Particular attention was given to the advantages and disadvantages of the used chemical sterilant in relation to the particularity of such types of surfaces [15]. The most important was the continued evaluation of the used protocol and the strategy of fogging. In fact, Tanner et al. [16] reported that the use of hydrogen peroxide vapor in clinical surfaces provided better results compared to the steam vapor system. Ali et al. [17] clarified that the use of airborne hydrogen peroxide gives excellent results in surgical wards, single isolation rooms, and bathrooms. However, this technique is obliged to the removal of patients and has a high acquisition cost and increased room turnover time [18].
2.2. Fumigation
Fumigation is the operation of introducing a gas or a substance giving rise to a gas in the atmosphere of a partially or completely closed enclosure with a view to destroying so-called “harmful” living organisms. It was formerly the combustion of plants producing vapors charged with the active ingredients of the plant. For example, one could also produce water vapor charged with these active ingredients by boiling eucalyptus leaves in a room that we wanted to disinfect. Fumigation has been mentioned in various ancient texts; it was applied to disinfect the environments using different herbs [19]. As well as fumigation, mineral and animal products have been specified in the central nervous system (CNS) treatment fields and bacterial infections [20,21,22]. Zhang et al. [21] demonstrated that the combination of traditional Chinese fumigation with western medicine has a positive effect on the symptoms of diabetic peripheral neuropathy. The use of fumigation treatment in the case of vitiligo is very diffuse in Southeast Asia. However, researchers have not approved its efficacy until now. Bhatwalkar et al. [23] studied fumigation using turmeric powder, garlic peels, and other plants. This study demonstrated the efficiency of this traditional method of disinfection. Nautiyal et al. [24] and Bisht et al. [25] studied the influence of fumigation using different herbs on the reduction of airborne bacteria. Recently, several authors demonstrated the application of the grain fumigation method using plant oils to avoid insect pests [22].
2.3. Wide-Area or Electrostatic Spraying Techniques
Most surface areas are uncharged or negative. The surface disinfection using electrostatic application consists of using a disinfectant registered with the Environmental Protection Agency on the surfaces with electrostatic spraying according to Coulomb’s law [26,27]. Joshua et al. [28] demonstrated that electrostatic spraying can minimize preventable infections in large surfaces in hospital environments, improving patient experience and increasing hospital revenues. However, in order to supply more advantages over the conventional systems, the wide-area technology should be applied correctly [27]. There are many elements that can affect its efficiency: the charge/mass ratio, spraying distance, and liquid deposition efficiency target [26]. Therefore, Sasaki et al. [29] showed in their study the evolution of the factor affecting the electrostatic spraying disinfection method. Cadnum et al. [30] studied the efficiency of electrostatic spraying using a dilute sodium hypochlorite solution against the coronavirus disease pandemic. This research showed great results in the decontamination of big open areas such as airports, waiting areas, classrooms, gyms, and portable equipment.
2.4. Ultraviolet Light
The World Health Organization approved that ultraviolet (UV) is effective as a no-touch technology in the case of healthcare settings [31]. Furthermore, Wilson et al. [32] studied the ultraviolet lamps sterilization method applied to biological safety cabinets (BSC). Boyce [33] showed the importance of ultraviolet light technology in the field of disinfection to reduce contamination compared with manual techniques, especially in the medical field and hospital environment. However, this method is effective from the point of view of sterilization results and limited to closed places without the presence of people because, as known, UV has a negative effect on the human skin and eyes.
Table 1 summarizes the most used disinfectant solutions and applications.

Table 1.
Examples of disinfectant solutions and applications.
3. Reproach and Complaint of EPA (Environmental Protection Agency)
As reported in [34], the Environmental Protection Agency (EPA) has different reasons against fogging/misting methods of application, specifically that they may not be adequately effective. Indeed, disinfection product applications by spraying, sponging, wiping, or mopping are more efficient than fogging/misting applications, which result in much smaller particle sizes and different surface coverage characteristics. Additionally, precleaning in the presence of soil contamination and potential reaction with the absorption of the active ingredient for different surfaces with variations in the humidity and the temperature is important, which develops the distribution and efficacy of the product. The standard methods are more effective in the distribution on the surface than the area treatment by fogging.
4. Innovation in Surface Disinfection Method
As the reactivity of microorganisms varies from most resistant to less resistant, besides the reproach of the EPA, more efficient, novel, and environmentally friendly tools and techniques are required for virus inactivation. This issue was clearly shown with the appearance of the novel and specific virus SARS-CoV-2. This virus, 100–300 nm in size and comprising nucleic materials surrounded by proteins and enveloped by a rich lipid layer, can survive on different surfaces and places such as on metal, glass, wood, textiles, etc., for several hours to days [2,4,5]. It is characterized also by a virus breed mutation in relation to the environmental conditions and exposed surface [35]. Therefore, innovation in the surface disinfection method can prevent infection and the spread of disease. Modern technology specific to the disinfection of environmental surfaces in hospitals and healthcare staff is discussed.
4.1. Antimicrobial Spray Nanocoating
4.1.1. Principle
Antimicrobial spray nanocoating was recently developed to control surface reactivity and properties. Dray nanospray is one of the most innovative technologies and has many advantages in terms of its ability to produce nanoparticles with much smaller droplets and a narrow area distribution [36]. Nanospray drying offers high stability to control particle size and morphology by optimizing the process parameters and nanoparticle characteristics. Spray drying is able to produce different forms of particle shapes [37]. Nanospray drying allows volume reduction, defined particle size, changing chemical, and physical properties with chemical and biological stability and high specific surface. Additionally, nanospray drying offers easy dosage administering and handling. It was initially utilized in the pharmaceutical field. Table 2 shows examples of FDA-approved medicine using nanospray drying technology as a preparation method.

Table 2.
Examples of FDA-approved medicine using nanospray drying technology as a preparation method [38,39,40,41,42,43,44,45].
The nanospray drying process consists of six fundamental steps [45,46,47,48]. The first stage is heating the inlet air to the desired temperature not to exceed 220 °C. The second stage is droplet formation using a two-fluid nozzle or ultrasonic spray head. Before the collection of the particle using cyclone technology or electrostatic particle collector, the drying step between drying gas and sample droplets is important. Then, the finest particles were collected to protect the user and environment using an outlet filter. Finally, was drying gas delivered by aspirator or with compressed air. A piezoelectric actuator vibrates a small replaceable spray cap, containing nanometric holes, to generate droplets. The latter leads to rapid vertical movement of the spray mesh, ejecting many droplets in size accurately through the holes in the drying chamber. The size of the droplets depends on the size of the holes and the physicochemical characteristic of the disinfectant. The flow of concurrent drying gas directs the particles to the electrostatic particle collector. The electrostatic particle collector can capture nanometric particles (<1 μm) with separation efficiency. The particles are gently removed from the inner surface of the collecting electrode cylinder using the particle scraper [49,50,51]. Figure 1 shows a schematic representation of the nanospray dryer principle.
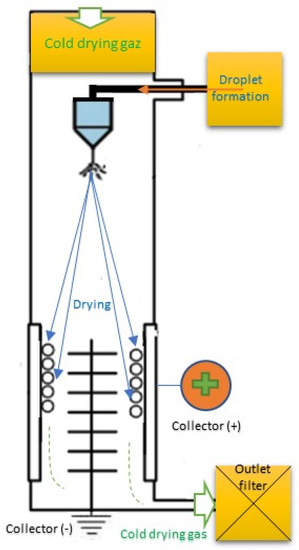
Figure 1.
Schematic representation of the drying nanospray principle.
The sizes of the particles formed through encapsulation are nano (<1 μm) [52,53,54,55] and known as nanocapsules [56]. The smaller the particles, the better the solubility of the encapsulated liquids. The droplets obtained at the nanoscale lead to an increase in the volume distribution in the surface, which generally occurs by reducing the size of the drops, thereby increasing the particles’ efficiency [57]. The cost reduces by using nanodrop sterilization instead of other sprayers [58]. There are important input parameters identified: (i) spray mesh size, (ii) spray rate intensity, (iii) solid concentration, (iv) polymer and surfactant concentrations, (v) drying gas inlet temperature, (vi) solvent type and (vii) drying gas flow rate. On the other hand, there are important output parameters: (i) particle size, (ii) product yield, (iii) amount particles produced, (iv) particle morphology, (v) bioactive loading, (vi) controlled release profile, (vii) encapsulation efficiency and stability.
4.1.2. Possible Exploitation of Nanocoating to Protect against COVID-19
In the last decimal, many laboratories and researchers have focused on the development of antiviral nanocoatings. As innovation, they tested nanopowder technology to create a more stable layer and ensure a large surface area compared to the volume ratio: a small number of particles induces an efficient antiviral surface [59,60,61]. To visualize the morphology of the nanopowder, observations of the powder with a resolution smaller than 1 nm and high magnification should be made [62]. The morphology of the nanosprayed powder is a spherical shape with a porous surface. The pore size is in the order of nanometers distributed on the spherical surface of the nanosprayed particle powder. The nanoparticles are produced with an irregular form and spherical shape. There is a slight difference between the nanoparticle sizes [63]. To measure the size of the nanosprayed powder, there are different instruments such as dynamic and static light scattering ((DLS) and (SLS)), gravitational settling and centrifugation (GSC), laser-induced breakdown detection (LIBD), and laser particle analyzer (LPA) [58,64,65]. Using the old nanospray dryer classic instrument, the dried particle size is in the range of 0.3 µm to 5 µm [66,67,68,69]. Indeed, using the new concept mechanical technology method can create powders in the nanometric size range with stable distributions and high formulation yields. This novel spray produces and collects submicron particles from a disinfectant solution. The dried particle size is in the range of the 285 nm to 999 nm [70].
Many different types of nanoparticles have been shown to be effective at inactivating viruses, including coronaviruses such as SARS-CoV-2. For example, nanoscale zinc oxide, cuprous oxide, silver, copper iodide, polymer, titanium oxide, gold on silica, and quaternary ammonium cations (quats) have all shown promise [71,72,73,74,75]. The choice of the sprayed nanoparticle protocol and methodology is depending on the application field. For example, last semester, researchers from Hong Kong University of Science and Technology developed a nanocapsule formed with heat-sensitive polymers that contain disinfectants [76]. This material is known by multilevel antibacterial polymer MAP-1. It is prepared from polymer encapsulated chlorine dioxide [77]. The polymer releases the biocides at the sites of contamination when warmed by human contact [78]. It was used in public places such as shopping malls, schools, and buses as a surface sterilization solution against COVID-19.
Another innovative solution used a combination of nanoactive elements formed by positively charged silver nanoparticles sprayed on surfaces to create a low surface energy nanocoating [79]. The stages of the formation of these elements under ambient conditions were widely described in [80]. The mechanism of bacterial inactivation described in Figure 2 is based on the interaction of Ag nanoparticles with the inside and the outside of the cell membrane rich in sulfur containing amino acids to inhibit microorganism activities. Despite that this nanocoating was initially developed to protect against bacteria, fungi, and algae, it was tested to be effective against COVID-19 since February 2020 on many types of materials such as metals and plastics [81]. More than 99% of antiviral efficiency was noticed in 2 h of contact in different places (airports, schools, and markets). However, more tests of performance are in development to extend the use of this method for other materials.
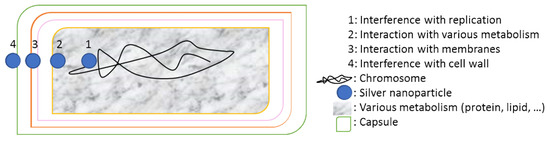
Figure 2.
Schematic representation of the bacterial inactivation mechanisms using silver nanoparticle.
4.2. Self-Disinfection Surface
In critical areas like hospitals and healthcare facilities, surfaces are considered clean if 50% of the surfaces are disinfected and hospital staff members wash their hands for 15 s [82]. Therefore, the protocol of classical methods of sterilization is not always required [83]. Recently, researchers developed new technology able to kill and disable microbes in contact [84,85,86]. The most emergent is the self-defensive antimicrobial known as the self-disinfection surface. It consists of the creation of a bacteria cell wall to resist the adhesion of bacteria or to kill bacteria by chemical or physical changes [87,88,89,90,91]. This technique is classified in three categories: surface resisting the bacteria attachment (Figure 3a) using the photoactivation process for example [90], surface leaching antibacterial agents (Figure 3b) called intrinsically antimicrobial ability [91], and surface killing or delivery bacteria by contact using antimicrobial loading (Figure 3c), based on the coating technology [92] or incorporation process [93]. The mechanisms of surface reaction were detailed in [94] and illustrated in Figure 4. However, this technique is not standard and depends on the materials’ family, since each material has its own way of reacting according to its properties [95]. The most important functional materials are photoactive building monocrystals (CuInZn4S6) [96], both TiO2 and SiO2 nanocoatings [97,98,99], antimicrobial peptide substance [100], and membrane-active polycations [101].
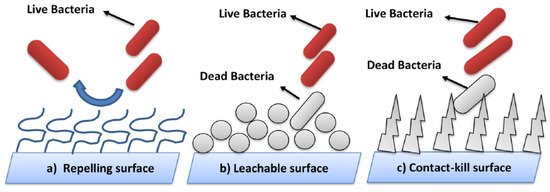
Figure 3.
(a–c) Three categories of self-disinfection surfaces [93].
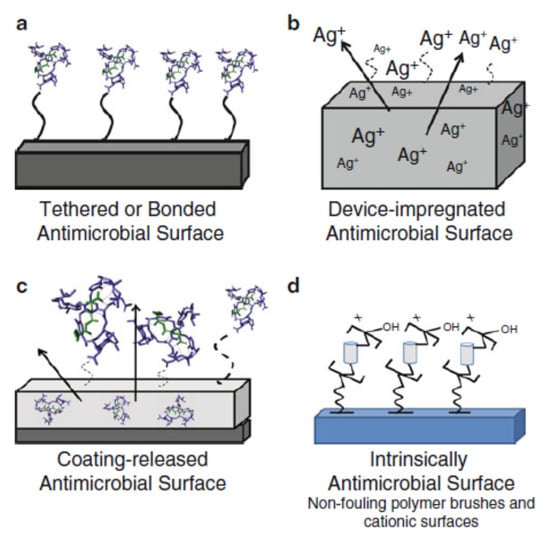
Figure 4.
Mechanisms of antimicrobial surface activation [94].
Besides, few pieces of research have successfully clarified the long-term efficacy, durability, and mechanical stability of the antiadhesive action in relation to the environmental conditions, the scalability of production, and the real cost of these settings when we talk about large areas [102,103,104,105].
4.3. Plasma-Mediated Virus Inactivation
Plasma generation is attributed to the creation of anodized gas which contains reactive chemical elements such as electrons, ions, and charged species [106]. Despite that it was discovered in 1927 as the fourth fundamental state of the matter, the microbicidal properties of plasma were really explored only in the last 15 years [107]. Several studies have investigated the effect of plasma treatment on the inactivation of both enveloped and nonenveloped viruses, bacteria, and fungi [108,109,110]. It was reported that the applicability of plasma technology in surface disinfection is potentially wide-ranging. However, nitrogen-gas plasma and UV radiation have the most effective impact on viruses and about a six-log reduction/inactivation performance for bacteria. Other techniques used innovative methods by radiofrequency plasma treatment using an Ar/O2 gas mixture [111]. They concluded that plasma can not only disinfect surfaces but also degrade toxins. The mechanisms of degradation are directly related to the used gases as well as the method used to generate plasma and the target microorganism. However, the exact mode of the plasma functionality remains largely unexplored. More innovative devices were developed for the disinfection of fruits and vegetables using atmospheric pressure plasma, others for the sterilization of medical instruments using gamma-ray treatment [112]. However, induced changes in the properties of the materials were noticed. A protocol of evaluation of each sterilization method should be developed specifically to the corresponding application with respect to the relative norms. Despite that plasma-mediated virus inactivation is a relatively young field of research, encouraging results were reported in the treatment of the viral pandemic COVID-19 using cold plasma treatment is known as a reactive oxygen and/or nitrogen species [113,114].
4.4. Modern Technology for Healthcare Environment and Surface
Considered as the highest exposed environment to microorganisms, healthcare workers and relative equipment and devices are suspected to be what is called high-level disinfection [115]. Therefore, modern technology was developed in recent years that investigated basic knowledge and studied cases [116]. The objective was to protect the staff and to prevent the transmission of infectious agents. Their use was associated with an improvement in the cleaning and disinfection of high touch surfaces with a specific protocol and guidelines taking into account (i) the potential of the environmental surfaces to transmit infection, (ii) mode and duration of contact, and (iii) toxicologic risk assessment [117].
Jensen described the use of ultraviolet germicidal irradiation as a disinfection method that kills airborne viruses using short-wavelength ultraviolet and by destroying its AND. Satisfying results were reported in hospitals in Italy in relation to the new COVID-19 [118]. Weber et al. explained the use of touchless cleaning robots as an innovative method that is clean and effective in decreasing microbial surface contamination [119]. However, complex surfaces may be incompletely targeted by robot technology. Therefore, traditional methods of sterilization are regularly completed to disinfect healthcare staff clothes, equipment, and devices.
5. New Framework for Prevention, Diagnostic, and Monitoring of Surface Disinfection
Based on our review work, it is clear that surface disinfection depends on several parameters and is widely used for cleaning, disinfection, and sterilization. Thanks to innovative technologies, protocols, and creativity, new methods have been developed with a continued evolution to improve performance. Figure 5 illustrates our proposed new framework with possible interaction and influences.
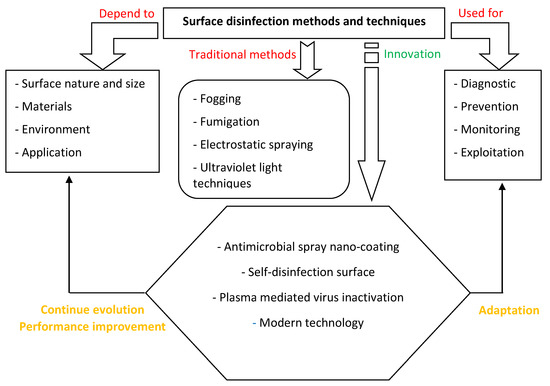
Figure 5.
New model of surface disinfection methods, techniques and possible interactions and evolution.
6. Conclusions
From this review study, the following conclusions are reported:
- -
- Virus or bacteria disinfection is widely influenced by the type, size, and properties of surfaces which are considered as the major factor contributing to the dissipation of an epidemic.
- -
- The choice of the disinfection technique is based on Multiphysics rules. Traditional techniques such as fogging, Fumigation, wide-area or electrostatic spraying, and ultraviolet light techniques are still used to conserve equipment and surface from viruses. However, many approaches specified by the Environmental Protection Agency narrow their evolution despite the progress of sterilized solution and light technology.
- -
- An antimicrobial spray nanocoating was introduced as an emergent technology to produce efficient and inhalable nanopowder pulverization for healthy surfaces and presented as possible innovative methods to protect against COVID-19.
- -
- Relatively, self-disinfection surfaces were recently developed using chemical and physical modifications of the surface to kill or eject microorganisms. As there are several points to be more standard and well-controlled, this technique is considered a step forward to the future of the controlling protocol of disinfection.
- -
- Plasma-mediated virus inactivation was in higher microorganism inactivation potential. Given the fact that this technology is clean, effective, and human friendly, it can become a promising solution when related to modern technology.
- -
- A new framework for prevention, diagnostic, and monitoring of surface disinfection was proposed.
Author Contributions
Conceptualization, M.K. (Mohamed Kchaou) and K.A.; methodology, M.K. (Mohamed Kchaou); validation, M.K. (Mohamed Kchaou), M.K. (Mosaad Khadr) F.H., and M.A.; formal analysis, M.K. (Mohamed Kchaou); investigation, M.K. (Mosaad Khadr); resources, M.K. (Mohamed Kchaou) and F.H.; writing—original draft preparation, M.K. (Mohamed Kchaou), F.H., and K.A.; supervision, K.A.; project administration, M.A.; funding acquisition, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Deanship of Science Research at the University of Bisa, Saudi Arabia, grant number “UB-COVID-10-1441” and “The APC was funded by the project indicated above”.
Acknowledgments
The authors extend their appreciations to the Deanship of Science Research at University of Bisa, Saudi Arabia, for funding this work throw COVID-19 initiative Project under Grant number (UB-COVID-10-1441)
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novelcoronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Mystery deepens over animal source of coronavirus. Nature 2020, 579, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351.e2. [Google Scholar] [CrossRef] [PubMed]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface modification approaches for prevention of implant associated infections. Colloids Surf. B Bio-Interfaces 2020, 193, 111116. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, J.; Guo, Y.; Liang, L.; Jin, Y.; Qian, S.; Miao, R.; Hu, L.; Lu, F. Reversible grafting of antibiotics onto contact lens mediated by labile chemical bonds for smart prevention and treatment of corneal bacterial infections. J. Mater. Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef]
- Stein, H.; Schulz, J.; Kemper, N.; Tichy, A.; Krauss, I.; Knecht, C.; Hennig-Pauka, I. Fogging low concentrated organic acid in a fattening pig unit—Effect on animal health and microclimate. Ann. Agric. Environ. Med. 2016, 23, 581–586. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; la Bruna, A.; Luca, G.R.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef]
- Krishnan, J.; Fey, G.; Stansfield, C.; Landry, L.; Nguy, H.; Klassen, S.; Robertson, C. Evaluation of a Dry Fogging System for Laboratory Decontamination. Appl. Biosaf. 2012, 17, 132–141. [Google Scholar] [CrossRef]
- Pereira, S.S.P.; de Oliveira, H.M.; Turrini, R.N.T.; Lacerd, R.A.A. Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention: A systematic review. Rev. Esc. Enferm. USP 2015, 49, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Saccucci, M.; Bruni, E.; Uccelletti, D.; Bregnocchi, A.; Sarto, M.S.; Bossù, M.; di Carlo, G.; Polimeni, A. Surface Disinfections: Present and Future. J. Nanomater. 2018, 2018, 8950143. [Google Scholar] [CrossRef]
- Bore, E.; Langsrud, S. Characterization of micro-organisms isolated from dairy industry after cleaning and fogging disinfection with alkyl amine and peracetic acid. J. Appl. Microbiol. 2005, 98, 96–105. [Google Scholar] [CrossRef]
- Park, G.W.; Boston, D.M.; Kase, J.A.; Sampson, M.N.; Sobsey, M.D. Evaluation of Liquid- and Fog-Based Application of Sterilox Hypochlorous Acid Solution for Surface Inactivation of Human Norovirus. Appl. Environ. Microbiol. 2007, 73, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, W.; Zeballos, J.; Angenscheidt, M. Efficacy and safety in environmental decontamination with fogging with superoxidized water XTERIDES® (SW) against Bacillus atrophaeus spores (BA) and Methicillin resistant staphylococcus epidermidis (MRSE). Int. J. Infect. Dis. 2012, 16, e382. [Google Scholar] [CrossRef][Green Version]
- Tanner, B.D. Reduction in Infection Risk Through Treatment of Microbially Contaminated Surfaces with a Novel, Portable, Saturated Steam Vapor Disinfection System. Am. J. Infect. Control 2009, 37, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Muzslay, M.; Bruce, M.; Jeanes, A.; Moore, G.; Wilson, A.P.R. Efficacy of Two Hydrogen Peroxide Vapour Aerial Decontamination Systems for Enhanced Disinfection of Meticillin-Resistant Staphylococcus Aureus, Klebsiella Pneumoniae and Clostridium Difficile in Single Isolation Rooms. J. Hosp. Infect. 2016, 93, 70–77. [Google Scholar] [CrossRef]
- Montazeri, N.; Manuel, C.; Moorman, E.; Khatiwada, J.R.; Williams, L.L.; Jaykus, L.A. Virucidal Activity of Fogged Chlorine Dioxide- and Hydrogen Peroxide-Based Disinfectants against Human Norovirus and Its Surrogate, Feline Calicivirus, on Hard-to-Reach Surfaces. Front. Microbiol. 2017, 8, 1031. [Google Scholar] [CrossRef]
- Mohagheghzadeh, A.; Faridi, P.; Shams-Ardakani, M.; Ghasemi, Y. Medicinal smokes. J. Ethnopharmacol. 2006, 108, 161–184. [Google Scholar] [CrossRef]
- Vishnuprasad, C.N.; Pradeep, N.S.; Cho, Y.W.; Gangadharan, G.G.; Han, S.S. Fumigation in Ayurveda: Potential strategy for drug discovery and drug delivery. J. Ethnopharmacol. 2013, 149, 409–415. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.; Ma, Y.; Jin, Y.H.; Meng, F.J. Efficacy of combining traditional Chinese medicine fumigation with Western medicine for diabetic peripheral neuropathy: A systematic review and meta-analysis. Int. J. Nurs. Sci. 2015, 2, 295–303. [Google Scholar] [CrossRef]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored. Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Shukla, P.; Srivastava, R.K.; Mondal, R.; Anupam, R. Validation of environmental disinfection efficiency of traditional Ayurvedic fumigation practices. J. Ayurveda Integr. Med. 2009, 10, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Chauhan, P.S.; Nene, Y.L. Medicinal smoke reduces airborne bacteria. J. Ethnopharmacol. 2007, 114, 446–451. [Google Scholar] [CrossRef]
- Bisht, L.S.; Brindavanam, N.B.; Kimothic, P. Comparative study of herbal agents used for fumigtation in relations to formulation. Anc. Sci. Life 1988, 8, 125–132. [Google Scholar]
- Bartlett, D.F.; Goldhagen, P.E.; Phillips, E.A. Experimental Test of Coulomb’s Law. Available online: https://www.princeton.edu/~romalis/PHYS312/Coulomb%20Ref/BartlettCoulomb (accessed on 12 October 2016).
- Tang, K.; Smith, R.D. Physical/chemical separations in the break-up of highly charged droplets from electrosprays. J. Am. Soc. Mass Spectrom. 2001, 12, 343–347. [Google Scholar] [CrossRef]
- Robertson, J.T. Electrostatic Technology for Surface Disinfection in Healthcare Facilities. Infection Control. Tips. Available online: https://emist.com/electrostatic-technology-for-surface-disinfection/ (accessed on 14 October 2016).
- Sasaki, R.S.; Teixeira, M.M.; Fernandes, H.C.; Monteiro, P.M.d.; Rodrigues, D.E.; de Alvarenga, C.B. Parameters of electrostatic spraying and its influence on the application efficiency. Rev. Ceres Viçosa 2013, 60, 474–479. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Jencson, A.L.; Livingston, S.H.; Li, D.; Redmond, S.N.; Pearlmutter, B.; Wilson, B.M.; Donskey, C.J. Evaluation of an electrostatic spray disinfectant technology for rapid decontamination of portable equipment and large open areas in the era of SARS-CoV-2. Am. J. Infect. Control 2020, 48, 951–954. [Google Scholar] [CrossRef]
- COVID-19: Interim Guidance; World Health Organization. Available online: https://apps.who.int/iris/bitstream/handle/10665/332096/WHO-2019-nCoV-Disinfection-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 15 May 2020).
- Centers for Disease Control and Prevention (U.S.); Public Health Service (U.S.); National Institutes of Health (Eds.) Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; HHS Publication: Washington, DC, USA, 1999.
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef]
- Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-09/documents/fogger-mister-final-signed-letter.pdf (accessed on 27 August 2020).
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Crawford, K.H.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; Veesler, D.; Bloom, J.D. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. CellPress 2020, 182, 1–16. [Google Scholar] [CrossRef]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, W.G. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm. Sin. 2014, 4, 18–25. [Google Scholar] [CrossRef]
- Brough, C.; Williams, R.O., III. Amorphous solid dispersions, and nano-crystal technologies for poorly watersoluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- Lam, J.; Vaughan, S.; Parkins, M.D. Tobramycin Inhalation Powder (TIP): An Efficient Treatment Strategy for the Management of Chronic Pseudomonas Aeruginosa Infection in Cystic Fibrosis. Clin. Med. Insights. Circ. Respir. Pulm. Med. 2013, 7, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Geller, D.E.; Minić, P.; Brockhaus, F.; Zhang, J.; Angyalosi, G. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: The EVOLVE trial. Pediatr. Pulmonol. 2011, 46, 230–238. [Google Scholar] [CrossRef]
- Anderson, S.D.; Daviskas, E.; Brannan, J.D.; Chan, H.K. Repurposing excipients as active inhalation agents: The mannitol story. Adv. Drug Deliv. Rev. 2018, 133, 45–56. [Google Scholar] [CrossRef]
- Guntur, V.P.; Dhand, R. Inhaled Insulin: Extending the Horizons of Inhalation therapy. Respir. Care 2007, 52, 911–922. [Google Scholar]
- Arpagaus, C. Pharmaceutical Particle Engineering via Nano Spray Drying-Process Parameters and Application Examples on the Laboratory-scale. Arpagaus. Int. J. Med. Nano Res. 2018, 5, 26. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi nano spray dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Schafroth, N.; Arpagaus, C.; Jadhav, U.Y.; Makne, S.; Douroumis, D. Nano and microparticle engineering of water insoluble drugs using a novel spray-drying process. Colloids Surf. B Biointerfaces 2012, 90, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Seid, M.J., Ed.; Elsevier Science: Amsterdam, The Netherland, 2017; pp. 346–401. ISBN 978-0-12-809436-5. [Google Scholar]
- Schmid, K.; Arpagaus, C.; Friess, W. Evaluation of the Nano Spray Dryer B-90 for pharmaceutical applications. Pharm. Dev. Technol. 2011, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C.; Schafroth, N. Laboratory scale spray drying of biodegradable polymers. In Conference, Respiratory Drug Delivery Europe; RDD Europe 2009; RDD: Lisbon, Portugal, 2009; ISBN 1933722312. [Google Scholar]
- Haggag, Y.A.; Faheem, A.M. Evaluation of nanospray drying as a method for drying and formulation of therapeutic peptides and proteins. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.P.; Stigliani, M.; Del Gaudio, P.; Mencherini, T.; Sansone, F.; Russo, P. Nanospray drying as a novel technique for the manufacturing of inhalable NSAID powders. Hindawi Publ. Corp. Sci. World J. 2014, 2014, 838410. [Google Scholar]
- Arpagaus, C. Nano Spray Drying of Pharmaceuticals. In IDS’2018—21st International Drying Symposium; Editorial Universitat Politècnica de València: Valencia, Spain, 2018; pp. 1–8. [Google Scholar]
- Fang, Z.; Bhandari, B. Encapsulation techniques for food ingredient systems. In Food Materials Science and Engineering; Bhandari, B., Roos, Y.H., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; Chapter 12; pp. 320–348. [Google Scholar]
- Couvreur, P.; Dubernet, C.; Puisieux, F. Controlled drug delivery with nanoparticles—Current possibilities and future trends. Eur. J. Pharm. Biopharm. 2005, 41, 2–13. [Google Scholar]
- Tantawy, A.S.; Salama, Y.A.M.; Abdel–Mawgoud, A.M.R.; Ghoname, A.A. Comparison of chelated calcium with nano-calcium on alleviation of salinity negative effects on tomato plants. Middle East J. Agric. Res. 2014, 3, 912–916. [Google Scholar]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv. Red Delicious. Sci. Hortic. 2008, 240, 57–64. [Google Scholar] [CrossRef]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.A. Coronavirus: Can Nano-Coatings and Sprays Help? Available online: https://kashmirlife.net/coronavirus-can-nano-coatings-and-sprays-help-227570/ (accessed on 30 March 2020).
- Sun, Z.; Ostrikov, K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Miranda-Linares, V.; Quintanar-Guerrero, D.; Del Real, A.; Zambrano-Zaragoza, M.L. Spray-drying method for the encapsulation of a functionalized ingredient in alginate-pectin nano- and microparticles loaded with distinct natural actives: Stability and antioxidant effect. Food Hydrocoll. 2020, 101, 105560. [Google Scholar] [CrossRef]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr. Polym. 2017, 177, 369–377. [Google Scholar] [CrossRef]
- Anna, G.; Elizaveta, K.; Elena, Y.; Galina, L.; Natalia, K.; Denis, K. Stability study of ZnO nanoparticles in aqueous solutions of carboxylate anions. J. Nanopart. Res. 2015, 17, 123. [Google Scholar]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.K.; Tan, R.B. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Schweiger, C.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J. Control. Release 2012, 158, 329–335. [Google Scholar] [CrossRef]
- Gonzattia, M.B.; Sousaa, M.E.P.; Tunissib, A.S.; Mortaraa, R.A.; de Oliveirab, A.M.; Cerizeb, N.N.P.; Kellera, A.D. Nano spray dryer for vectorizing α-galactosylceramide in polymeric nanoparticles: A single step process to enhance invariant Natural Killer T lymphocyte responses. Int. J. Pharm. 2019, 565, 123–132. [Google Scholar] [CrossRef]
- Arpagaus, C. Nano Spray Dryer B-90: Literature review and applications. Inf. Bull. 2011, 63, 2011. [Google Scholar]
- Arpagaus, C.; Schafroth, N.; Meuri, M. Laboratory scale spray drying of lactose: A review. Inf. Bull. 2010, 57, 1–12. [Google Scholar]
- Büchi Labortechnik, A.G. Nano Spray Dryer B-90. Tech. Data Sheet 2013, en 1311. Available online: http://static2.buchi.com/sites/default/files/technical-data-pdf/B-90_Data_Sheet_en_A_0.pdf (accessed on 2 June 2020).
- Giannossa, L.C.; Longano, D.; Cioffi, N.; Nitti, M.A.; Paladini, F.; Pollini, M.; Rai, M.; Sannino, A.; Valentini, A.; Cioffi, N. Metal nanoantimicrobials for textile applications. Nanotechnol. Rev. 2013, 2, 307–331. [Google Scholar] [CrossRef]
- Zendehdel, R.; Goli, F.; Hajibabaei, M. Comparing the microbial inhibition of nanofibres with multi-metal ion exchanged nano-zeolite Y in air sampling. J. Appl. Microbiol. 2020, 128, 202–208. [Google Scholar] [CrossRef]
- De Jong, B.; Meeder, A.M.; Koekkoek, K.W.A.C.; Schouten, M.A.; Westers, P.; Van Zanten, A.R.H. Pre-post evaluation of effects of a titanium dioxide coating on environmental contamination of an intensive care unit: The TITANIC study. J. Hosp. Infect. 2018, 99, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Ilić, K.; Juganson, K.; Ivask, A.; Ahonen, M.; Vrček, I.V.; Kahru, A. Potential ecotoxicological effects of antimicrobial surface coatings: A literature survey backed up by analysis of market reports. PeerJ 2019, 7, e6315. [Google Scholar] [CrossRef] [PubMed]
- Biller, K. Focus on Powder Coatings; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2020. [Google Scholar]
- Ghosh, S.K. Anti-Viral Surface Coating to Prevent Spread of Novel Coronavirus (COVID-19) Through Touch. Coatings World. Available online: https://www.coatingsworld.com/content-microsite/cw_covid-19/2020-04-15/anti-viral-surface-coating-to-prevent-spread-of-novel-coronavirus-covid-19-through-touch (accessed on 15 April 2020).
- Li, Y.; Leung, W.K.; Yeung, K.L.; Lau, P.S.; Kwan, J.K. A Multilevel Antimicrobial Coating Based on Polymer-Encapsulated ClO2. Langmuir 2009, 25, 13472–13480. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Cristancho, D.E.; Rachford, A.A.; Reder, A.L.; Williamson, A.; Grzesiak, A.L. Controlled Release of Antimicrobial ClO2 Gas from a Two-Layer Polymeric Film System. J. Agric. Food Chem. 2016, 64, 8647–8652. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future…? Open Chem. 2016, 14, 76. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- EPA-Approved Antimicrobial Surface Coating Represents Breakthrough in the Control and Spread of Infectious Diseases. Press Release. 19 March 2020. Available online: https://www.prnewswire.com/news-releases/epa-approved-antimicrobial-surface-coating-represents-breakthrough-in-the-control-and-spread-of-infectious-diseases-301027074.html (accessed on 22 June 2020).
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Mario, D.; Sovereto, D.; Illuzzi, G.; Laneve, E.; Raddato, B.; Arena, C.; Caponio, V.C.A.; Caloro, G.A.; Zhurakivska, K.; Troiano, G.; et al. Management of Instrument Sterilization Workflow in Endodontics: A Systematic Review and Meta-Analysis. Int. J. Dent. 2019, 2020, 5824369. [Google Scholar]
- Centers for Disease Control and Prevention. An Introduction to Applied Epidemiology and Biostatistics. Lesson 3: Measures of Risk. Section 2: Morbidity Frequency Measures. In Principles of Epidemiology in Public Health Practice, 3rd ed. Available online: https://www.cdc.gov/csels/dsepd/ss1978/index.html (accessed on 7 July 2020).
- Brühwasser, C.; Heinrich, H.; Lass-Flörl, C.; Mayr, A. Self-disinfecting surfaces and activity against Staphyloccocus aureus ATCC 6538 under real-life conditions. J. Hosp. Infect. 2017, 97, 196–199. [Google Scholar] [CrossRef]
- George, L.; Müller, A.; Röder, B.; Santala, V.; Efimov, A. Photodynamic self-disinfecting surface using pyridinium phthalocyanine. Dye. Pigment. 2017, 147, 334–342. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. Self-Disinfecting Surfaces, Infect. Control Hosp. Epidemiol. 2012, 33, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Badrossamay, M.; Sun, G. Enhancing hygiene/antimicrobial properties of polyolefins. In Polyolefin Fibres; Woodhead Publishing: Cambridge, UK, 2017; pp. 265–284. [Google Scholar]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Zoccolillo, M.L.; Rogers, S.C.; Mang, T.S. Antimicrobial photodynamic therapy of S. mutansbiofilms attached to relevant dental materials. Lasers Surg. Med. 2016, 48, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Adán, C.; Marugán, J.; Mesones, S.; Casado, C.; van Grieken, R. Bacterial inactivation and degradation of organic molecules by titanium dioxide supported on porous stainless-steel photocatalytic membranes. Chem. Eng. J. 2017, 318, 29–38. [Google Scholar] [CrossRef]
- Choi, H.; Castillo, B.; Seminario-Vidal, L. Silver absorption in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis treated with silver-impregnated dressings. A case series. Int. Wound J. 2018, 15, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Liu, S. Antibacterial surface design—Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Gjorgievska, E.S.; Nicholson, J.W.; Coleman, N.J.; Booth, S.; Dimkov, A.; Hurt, A. Component Release and Mechanical Properties of Endodontic Sealers following Incorporation of Antimicrobial Agents. Biomed. Res. Int. 2017, 2017, 2129807. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.D.; Brooks, A.E.; Grainger, D.W. Antimicrobial Medical Devices in Preclinical Development and Clinical Use. In Biomaterials Associated Infection; Moriarty, T., Zaat, S., Busscher, H., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Weng, D.; Qi, H.; Wu, T.T.; Yan, M.; Sun, R.; Lu, Y. Visible light powered self-disinfecting coatings for influenza viruses. Nanoscale 2012, 4, 2870. [Google Scholar] [CrossRef] [PubMed]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent, and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S.; Sanjines, R.; Pulgarin, C. TiO2 and TiO2-Doped Films Able to Kill Bacteria by Contact: New Evidence for the Dynamics of Bacterial Inactivation in the Dark and under Light Irradiation. Int. J. Photoenergy 2014, 2014, 785037. [Google Scholar] [CrossRef]
- Krumdieck, S.P.; Boichot, R.; Gorthy, R.; Land, J.G.; Lay, S.; Gardecka, A.J.; Polson, M.I.; Wasa, A.; Aitken, J.E.; Heinemann, J.A.; et al. Nanostructured TiO2 anataserutile-carbon solid coating with visible light antimicrobial activity. Sci. Rep. 2019, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, S.O. Antibacterial Activity of Novel Self-Disinfecting Surface Coatings. Ph.D. Thesis, The University of Salford, Salford, UK, 2014; 289p. [Google Scholar]
- Mayr, A.; Orth-Holler, D.; Heinrich, H.; Hinterberger, G.; Wille, I.; Naschberger, V.; Lass-Florl, C.; Binder, U. Galleria mellonella as a Model to Study the Effect of Antimicrobial Surfaces on Contamination by Staphylococcus aureus. Arch. Clin. Biomed. Res. 2019, 3, 315–325. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Kanamori, H.; Anderson, D.; CDC Prevention Epicenters Program. Continuous room decontamination technologies. Am. J. Infect. Control 2019, 47, A72–A78. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.H.; Carlino, S.; Gerba, C.P. Long-term efficacy of a self-disinfecting coating in an intensive care unit. Am. J. Infect. Control 2014, 42, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A. Self-disinfecting surfaces: Review of current methodologies, and future prospects. Am. J. Infect. Control 2013, 41, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Quaranta, D.; Grass, G. Antimicrobial Metallic Copper Surfaces Kill Staphylococcus haemolyticus Via Membrane Damage. Microbiologyopen 2012, 1, 46–52. [Google Scholar] [CrossRef]
- Waman, P.; Swapnil, S.; Krishna, K.; Rajshree, C.; Chetan, K.; Naresh, B.; Udit, P.; Ram, P.; Ghanshyam, L.B. Future prospects of plasma treatment technology for disinfection. In Engineering Technology of the 21st Century, 1st ed.; Roy, A.K., Ed.; New India Publishing Agency: New Delhi, India, 2015; Chapter 10. [Google Scholar]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.R.W.; Ostrikov, K.K. The Emerging Role of Gas Plasma in Oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Parra, F.S.; Collins, J.; Bruggeman, P.; Goyal, S.M. Ìn situ inactivation of human norovirus GII.4 by cold plasma: Ethidium monoazide (EMA)-coupled RT-qPCR underestimates virus reduction and fecal material suppresses inactivation. Food Microbiol. 2020, 85, 103307. [Google Scholar] [CrossRef] [PubMed]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2018, 52, 034002. [Google Scholar] [CrossRef]
- Ben Belgacem, Z.; Carré, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.M.; Polidor, F.; Gangloff, S.C.; Boudifa, M.; et al. Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms. PLoS ONE 2017, 12, e0180183. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von dem Hagen, T.; Weltmann, K.D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS cov-2 as compared with SARS cov-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Rideout, K. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 2005, 59, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Henneberger, P.K.; Braun, B.; Delclos, G.L.; Fagan, K.; Huang, V.; Knaack, J.L.; Kusek, L.; Lee, S.J.; Le Moual, N.; et al. Cleaning and disinfecting environmental surfaces in health care: Toward an integrated framework for infection and occupational illness prevention. Am. J. Infect. Control 2015, 43, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wang, M.; Qin, C.; Tan, L.; Ran, L.; Chen, D.; Zhang, H.; Shang, K.; Xia, C.; Wang, S.; et al. Coronavirus Disease 2019 (COVID-2019) Infection Among Health Care Workers and Implications for Prevention Measures in a Tertiary Hospital in Wuhan, China. JAMA Netw. Open 2020, 3, e209666. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Dell’Erba, A.; Migliore, G.; De Maria, L.; Caputi, A.; Quarato, M.; Stefanizzi, P.; Cavone, D.; Ferorelli, D.; Sponselli, S.; et al. Prevention and protection measures of healthcare workers exposed to SARS-CoV-2 in a university hospital in Bari, Apulia, Southern Italy. J. Hosp. Infect. 2020, 105, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Anderson, D.J.; Chen, L.F.; Sickbert-Bennett, E.E.; Boyce, J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am. J. Infect. Control 2016, 44, e77–e84. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).