Does Platelet-Rich Fibrin Decrease Dimensional Changes and Improve Postoperative Comfort in Post-Extraction Sockets? An Overview of Systematic Reviews
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.1.1. Focused Question: PICO Criteria [22]
2.1.2. Outcome Measures
2.2. Search Strategy
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Screening Process
2.5. Data Extraction
2.6. Quality Assessment of the Systematic Reviews
2.7. Statistical Analysis
3. Results
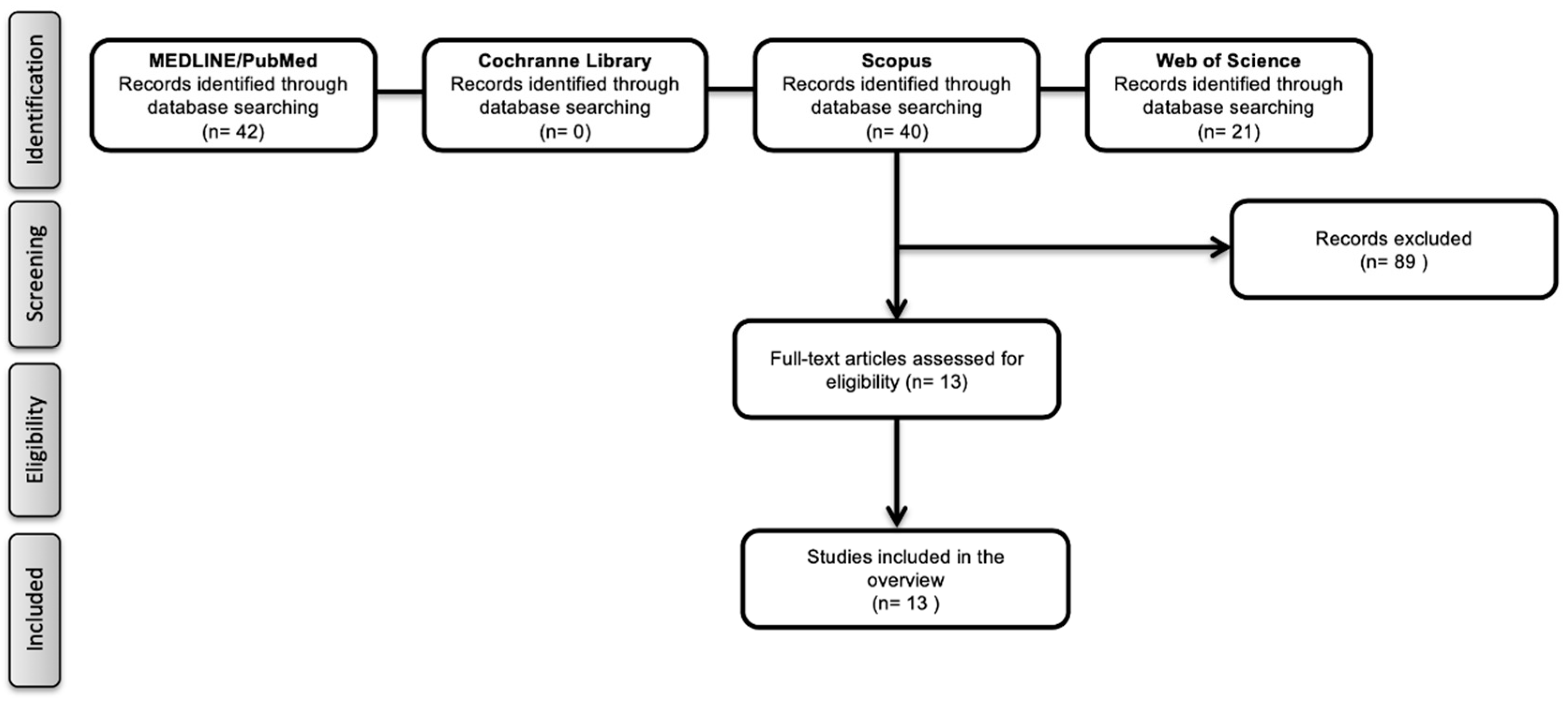
3.1. Literature Search
3.2. Study Characteristics
3.3. Findings Based on Previous Focused Questions
3.3.1. Primary Outcome Measures
3.3.2. Secondary Outcome Measures
- Soft tissue healing
- Pain
- Swelling
- Osteitis
- Trismus
3.4. Quality Assessment
4. Discussion
4.1. Study Strengths and Limitations/Implications for Clinical Practice
4.2. Recommendations for Further Research
5. Conclusions
- The use of PRF improves soft tissue healing around the extraction socket;
- The use of PRF in post-extraction sockets significantly reduces postoperative pain;
- The use of PRF in post-extraction sockets decreases the level of postoperative bleeding;
- The use of PRF in post-extraction sockets reduces the number of osteitis occurrences;
- There is no evidence for the positive interrelation between PRF and the occurrence of trismus.
Author Contributions
Funding
Conflicts of Interest
References
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of Postextraction Sockets Preserved With Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J. Oral Maxillofac Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Bortolin, M.; Taschieri, S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Int. J. Oral Maxillofac Surg. 2011, 40, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Ricci, M.; Tonelli, P.; Santini, S.; Covani, U. Tissue changes of extraction sockets in humans: A comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin. Oral Implants Res. 2013, 24, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, L.; Devlin, H.; Ting, K.; Nishimura, I. Current perspectives in residual ridge remodeling and its clinical implications: A review. J. Prosthet. Dent. 1998, 80, 224–237. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine 2018. Stem Cells Int. 2018. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef]
- Marrazzo, P.; Paduano, F.; Palmieri, F.; Marrelli, M.; Tatullo, M. Highly Efficient In Vitro Reparative Behaviour of Dental Pulp Stem Cells Cultured with Standardised Platelet Lysate Supplementation. Stem Cells Int. 2016. [Google Scholar] [CrossRef]
- Barry, M.; Pearce, H.; Cross, L.; Tatullo, M.; Gaharwar, A.K. Advances in Nanotechnology for the Treatment of Osteoporosis. Curr. Osteoporos 2016, 14, 87–94. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Corbella, S.; Taschieri, S.; Francetti, L.; Weinstein, R. Autologous platelet concentrate for post-extraction socket healing: A systematic review. Eur. J. Oral Implantol. 2014, 7, 333–344. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med. Oral Pathol Oral Radiol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent. 2013, 16, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Canellas, J.; Ritto, F.G.; Medeiros, P.J.D. Evaluation of postoperative complications after mandibular third molar surgery with the use of platelet-rich fibrin: A systematic review and meta-analysis. Int. J. Oral Maxillofac Surg. 2017, 46, 1138–1146. [Google Scholar] [CrossRef]
- Canellas, J.; Medeiros, P.J.D.; Figueredo, C.; Fischer, R.G.; Ritto, F.G. Platelet-rich fibrin in oral surgical procedures: A systematic review and meta-analysis. Int. J. Oral Maxillofac Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef]
- Castro, A.B.; Herrero, E.R.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Huang, Y.; Pan, Q.; Nie, M. Local Application of Platelet-Rich Fibrin During Lower Third Molar Extraction Improves Treatment Outcomes. J. Oral Maxillofac Surg. 2017, 75, 2497–2506. [Google Scholar] [CrossRef]
- Moraschini, V.; Barboza, E.S. Effect of autologous platelet concentrates for alveolar socket preservation: A systematic review. Int. J. Oral Maxillofac Surg. 2015, 44, 632–641. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Al-Hamed, F.S.; Tawfik, M.A.; Abdelfadil, E.; Al-Saleh, M.A.Q. Efficacy of Platelet-Rich Fibrin After Mandibular Third Molar Extraction: A Systematic Review and Meta-Analysis. J. Oral Maxillofac Surg. 2017, 75, 1124–1135. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Balli, G.; Ioannou, A.; Powell, C.A.; Angelov, N.; Romanos, G.E.; Soldatos, N. Ridge Preservation Procedures after Tooth Extractions: A Systematic Review. Int. J. Dent. 2018, 2018, 8546568. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Guida, L.; Nastri, L.; Piccirillo, A.; Sommese, L.; Napoli, C. The Role of Autologous Platelet Concentrates in Alveolar Socket Preservation: A Systematic Review. Transfus. Med. Hemother. 2018, 45, 195–203. [Google Scholar] [CrossRef]
- Niu, W.; Wang, P.; Ge, S.; Ji, P. Effects of Platelet Concentrates Used in Alveolar Ridge Preservation: A Systematic Review. Implant. Dent. 2018, 27, 498–506. [Google Scholar] [CrossRef]
- de Almeida Barros Mourao, C.F.; de Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Craniomaxillofac Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef]
- Gassling, V.L.; Acil, Y.; Springer, I.N.; Hubert, N.; Wiltfang, J. Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2009, 108, 48–55. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Rademacher, F.; Gross, J.; Siggelkow, M.; Lippross, S.; Kluter, T.; Varoga, D.; Tohidnezhad, M.; Pufe, T.; et al. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp. Dermatol. 2016, 25, 460–465. [Google Scholar] [CrossRef]
- Afat, I.M.; Akdogan, E.T.; Gonul, O. Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on early soft tissue healing after surgical extraction of impacted mandibular third molars: A prospective clinical study. J. Craniomaxillofac Surg. 2019, 47, 280–286. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: Sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Pinto, N.R.; Quirynen, M.; Ghanaati, S. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J. Periodontol. 2019, 90, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Choukroun, J.; Ghanaati, S. Reply from authors: RE: Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response: Necessity for standardization of relative centrifugal force values in studies on platelet-rich fibrin. J. Periodontol. 2019, 90, 122–125. [Google Scholar] [CrossRef] [PubMed]
| Authors | Focused Question | No. of Included Studies Design of Studies (No.) | Outcome Measures | Meta-Analysis Results | No. of Sockets Evaluated |
|---|---|---|---|---|---|
| Al-Hamed et al., 2017 | Is platelet-rich fibrin effective after mandibular third molar extraction? | 6 RCT (SM)—4 RCT (P)—1 CC—1 | Pain and analgesic consumption, swelling, trismus, alveolar osteitis, periodontal pocket depth, bone healing, soft tissue healing | Bone regeneration: MD 1.43 (95% CI −0.5 to 0.49), p = 0.98 (The use of PRF did not significantly affect the bone regeneration) | 335 |
| Annunziata et al., 2018 | What is the effect of platelet concentrates on alveolar socket preservation compared with spontaneous alveolar socket healing | 4 RCT (SM)—3 RCT (P)—1 | Qualitative and/or quantitative changes of soft and hard tissues. | NP | 189 |
| Canellas et al., 2017 | Is there a difference in postoperative complications (pain, alveolar osteitis, swelling, and bone healing impairment) when PRF is used in mandibular third molar surgery? | 7 RCT (SM)—5 RCT (P)—2 | Pain, alveolar osteitis, swelling, bone healing | Alveolar osteitis: OR 0.31 (95% CI 0.13 to 0.77), p = 0.01 (The use of PRF significantly decreases the occurrence of alveolar osteitis) | 485 |
| Canellas et al., 2019 | What indications has PRF shown to be effective in oral surgical procedures? | 16 RCT (SM)—11 RCT (P)—5 | Bone healing, soft tissue healing, bone density and alveolar dimensions | Alveolar osteitis: OR 0.33 (95% CI 0.14 to 0.76), p = 0.009 (The use of PRF significantly decreases the occurrence of osteitis) | 810 |
| Castro et al., 2017 | Does L-PRF promote regeneration in systemically healthy patients (ASA I) during guided bone regeneration techniques and implant surgery compared to traditional techniques? | 7 RCT(SM)—4 RCT (P)—3 | Bone healing, soft tissue healing, pain | NP | 227 |
| Del Fabbro et al., 2014 | In patients undergoing tooth extraction, does the local application of autologous platelet concentrate improve clinical, radiographic, and histological outcomes related to socket healing as compared to control? | 1 RCT (SM) | Bone healing | Alveolar bone regeneration: MD 20.4 (95% CI 13.2 to 27.5), p < 0.00001 (The use of PRF significantly affects the alveolar bone regeneration) | 44 |
| Del Fabbro et al., 2011 | Is autologous platelet concentrate beneficial for post-extraction socket healing? | 1 RCT (SM)—1 | Bone healing | NP | 40 |
| Del Fabbro et al., 2017 | Does the adjunct of autologous platelet concentrates produce benefits to post-extraction socket healing for hard and soft tissue parameters, postoperative complications, and patient’s postoperative quality of life? | 12 RCT (SM)—9 RCT (P)—2 CCT—1 | Complications and adverse events, discomfort, quality of life, bone healing, soft tissue healing | Soft tissue healing: MD—1.63 (95% CI −2.05 to 1.22), p < 0.00001 (The use of PRF significantly increases the alveolar soft tissue healing) Probing depth: MD 20.4 (95% CI 13.2 to 27.5), p < 0.00001 (The use of PRF significantly decreases the probing depth) Inflammation/infection: MD 0.27 (95% CI 0.06 to 1.27), p = 0.10 (The use of PRF did not significantly affect the alveolar inflammation or infection) Alveolar bone regeneration: MD—1.58 (95% CI −2.83 to −0.32), p = 0.01 (The use of PRF significantly affects the alveolar bone regeneration) | 712 |
| He et al., 2017 | Is the application of platelet-rich fibrin (PRF) during tooth extraction able to accelerate wound healing, stimulate osseous and soft tissue regeneration, and reduce unwanted side effects? | 10 RCT (SM)—7 RCT (P)—2 Retrospective—1 | Pain, swelling, trismus, alveolar osteitis, osteoblastic activity | Pain: MD—1.58 (95% CI −2.83 to −0.32, p = 0.01 (The use of PRF significantly relieves the pain) 1-day postoperative swelling: MD—0.46 (95% CI −1.03 to 0.10), p = 0.11 (The use of PRF did not significantly affect the 1-day postoperative swelling) Trismus: MD—4.33 (95% CI −10.9 to 2.29), p = 0.20 (The use of PRF did not significantly affect the trismus) Alveolar osteitis: OR 0.22 (95% CI 0.10 to 0.46), p < 0.0001 (The use of PRF significantly decreases the occurrence of alveolar osteitis) Osteoblastic activity: MD 0.03 (95% CI −0.46 to 0.53), p = 0.90 (The use of PRF did not significantly affect osteoblastic activity | 935 |
| Miron et al., 2017 | Has platelet rich fibrin (PRF) been shown effective for tissue repair/regeneration of either soft or hard tissues in dentistry? | 4 RCT (SM)—2 RCT (P)—1 Retrospective—1 | Bone healing | NP | 501 |
| Moraschini and Barboza, 2015 | What is the effect of autologous platelet concentrates for alveolar socket preservation using autologous plasma concentrates when compared with natural (spontaneous) socket healing? | 2 RCT (P)—1 CCT—1 | Soft tissue healing, bone healing, pain | NP | 37 |
| Niu et al., 2018 | Are platelet concentrates effective for alveolar ridge preservation? | 1 RCT (P)—1 | Soft tissue healing, bone healing | NP | 44 |
| Balli et al., 2018 | Is leukocyte-platelet-rich fibrin effective for ridge preservation procedures after tooth extractions? | 2 RCT (SM)—1 CCT—1 | Bone healing | NP | 104 |
| Authors | Methodology for PRF Production | AMSTAR 2 Score (16 Items) | Comments | ||
| Al-Hamed et al., 2017 | 1; 3000 rpm; 10 min 2030 rpm; 10 min (1 author) | 13 | The qualitative and meta-analysis results showed no significant improvement in bone healing with PRF-treated sockets compared with the naturally healing sockets. | ||
| Annunziata et al., 2018 | 3000 rpm; 10 min (1 author) 2700 rpm; 12 min (3 authors) | 14 | There is growing evidence that platelet concentrates may be advantageously used in post-extraction sites, mainly to improve soft tissue healing and to reduce postoperative symptoms | ||
| Canellas et al., 2017 | 3000 rpm; 10 min 2030 rpm; 10 min (1 author) | 16 | The use of PRF in mandibular third molar surgery is an alternative method to decrease postoperative pain, swelling, and reduce the risk of alveolar osteitis | ||
| Canellas et al., 2019 | 3000 rpm; 10 min 2700 rpm; 12 min (3 authors) 2030 rpm; 10 min (1 author) | 15 | The available literature suggests that PRF has a positive effect on improving alveolar preservation in extraction sockets | ||
| Castro et al., 2017 | 400 g/10 min (1 author) 3000 rpm; 10 min (4 authors) 2700 rpm; 12 min (2 authors) | 12 | PRF might have a positive effect on alveolar regeneration | ||
| Del Fabbro et al., 2014 | 1×; 360–400 rpm; 20 min | 15 | There is a positive effect of platelet concentrates on bone formation in post-extraction sockets | ||
| Del Fabbro et al., 2011 | 1×; 400 g (2030 rpm); 10 min | 14 | Based on the reports of the selected studies, the use of platelet concentrates may be beneficial for reducing postoperative pain and inflammation | ||
| Del Fabbro et al., 2017 | 1×: 360–400 rpm 1×: NR 1×: 3000 rpm (7 authors) 1×: 2700 rpm (2 authors) 1×: 2030 rpm (1 author) | 13 | PRF should be used in post-extraction sites to improve clinical and radiographic outcomes such as bone density and soft tissue healing and postoperative symptoms | ||
| He et al., 2017 | 3000 rpm: 10 min (7 authors) 2030 rpm: 10 min (1 author) 2700 rpm: 10–12 min (2 authors) | 14 | Local application of PRF after lower third molar extraction is a valid method for relieving pain and 3-day postoperative swelling and reducing the incidence of alveolar osteitis | ||
| Miron et al., 2017 | NR | 9 | PRF was shown to improve soft tissue generation and limit dimensional changes post-extraction | ||
| Moraschini and Barboza, 2015 | 1×: 3000 rpm × 10 min 1×: 2700 rpm × 12 min | 4 | The use of plasma concentrates seems to accelerate healing and soft tissue epithelialization in extraction sockets and reduce postoperative pain and discomfort | ||
| Niu et al., 2018 | NR | 13 | PRF might have a more positive effect on alveolar width and height preservation than does platelet-rich plasma | ||
| Balli et al., 2018 | NR | 14 | The PRF prevented both the horizontal and vertical crestal bone resorption | ||
| AMSTAR Questions | Al-hamed et al., 2017 | Canellas et al., 2019 | Castro et al., 2017 | Del Fabbro et al., 2014 | Del Fabbro et al., 2011 | Del Fabbro et al., 2017 | |
| (1) Was an “a priori” design provided? | no | yes | yes | yes | no | yes | |
| (2) Was there duplicate study selection and data extraction? | yes | yes | yes | yes | Yes | yes | |
| (3) Was a comprehensive literature search performed? | yes | yes | yes | yes | yes | yes | |
| (4) Was the status of publication (i.e., grey literature) used as an inclusion criterion? | no | yes | no | yes | yes | no | |
| (5) Was a list of studies (included and excluded) provided? | yes | yes | no | no | no | no | |
| (6) Were the characteristics of the included studies provided? | yes | yes | yes | yes | yes | yes | |
| (7) Was the scientific quality of the included studies assessed and documented? | yes | yes | yes | yes | yes | yes | |
| (8) Was the scientific quality of the included studies used appropriately in formulating conclusions? | yes | yes | yes | yes | yes | no | |
| (9) Were the methods used to combine the findings of studies appropriate? | yes | no | no | yes | yes | yes | |
| (10) Was the likelihood of publication bias assessed? | yes | yes | yes | yes | yes | yes | |
| (11) Was the conflict of interest stated? | yes | no | yes | yes | yes | yes | |
| (12) Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | yes | yes | yes | yes | yes | yes | |
| (13) Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? | yes | yes | yes | yes | yes | yes | |
| (14) If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | yes | yes | NP | yes | NP | yes | |
| (15) If meta-analysis was justified, did the review authors use appropriate methods for statistical combination of results? | no | yes | NP | yes | NP | yes | |
| (16) Did the review authors use a satisfactory technique for assessing the risk of bias in individual studies that were included in the review? | yes | yes | yes | yes | yes | yes | |
| Total (yes) | 13 | 14 | 12 | 15 | 13 | 13 | |
| AMSTAR Questions (cont) | He et al., 2017 | Canellas et al., 2017 | Miron et al., 2017 | Moraschini and Barboza, 2015 | Niu et al., 2018 | Annunziata et al., 2018 | Balli et al., 2018 |
| (1) Was an “a priori” design provided? | no | yes | yes | yes | yes | yes | yes |
| (2) Was there duplicate study selection and data extraction? | yes | yes | yes | yes | yes | yes | yes |
| (3) Was a comprehensive literature search performed? | yes | yes | yes | yes | yes | yes | yes |
| (4) Was the status of publication (i.e., grey literature) used as an inclusion criterion? | no | yes | no | no | no | yes | no |
| (5) Was a list of studies (included and excluded) provided? | yes | yes | no | yes | yes | yes | yes |
| (6) Were the characteristics of the included studies provided? | yes | yes | yes | yes | yes | yes | yes |
| (7) Was the scientific quality of the included studies assessed and documented? | yes | yes | no | Yes | yes | yes | yes |
| (8) Was the scientific quality of the included studies used appropriately in formulating conclusions? | yes | yes | no | yes | no | yes | yes |
| (9) Were the methods used to combine the findings of studies appropriate? | yes | yes | yes | yes | yes | no | yes |
| (10) Was the likelihood of publication bias assessed? | yes | yes | no | yes | yes | yes | yes |
| (11) Was the conflict of interest stated? | yes | yes | yes | yes | yes | yes | yes |
| (12) Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | yes | yes | yes | yes | yes | yes | yes |
| (13) Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? | yes | yes | no | yes | yes | yes | yes |
| (14) If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | yes | yes | NP | NP | NP | NP | NP |
| (15) If meta-analysis was justified, did the review authors use appropriate methods for statistical combination of results? | yes | yes | yes | NP | NP | NP | NP |
| (16) Did the review authors use a satisfactory technique for assessing the risk of bias in individual studies that were included in the review? | yes | yes | yes | yes | yes | yes | yes |
| Total (yes) | 14 | 16 | 9 | 14 | 13 | 14 | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraschini, V.; Mourão, C.F.d.A.B.; Machado, R.C.d.M.; Nascimento, J.R.B.; Javid, K.; Calasans-Maia, M.D.; Cardarelli, A.; Montemezzi, P.; Calasans-Maia, J.d.A. Does Platelet-Rich Fibrin Decrease Dimensional Changes and Improve Postoperative Comfort in Post-Extraction Sockets? An Overview of Systematic Reviews. Appl. Sci. 2020, 10, 5750. https://doi.org/10.3390/app10175750
Moraschini V, Mourão CFdAB, Machado RCdM, Nascimento JRB, Javid K, Calasans-Maia MD, Cardarelli A, Montemezzi P, Calasans-Maia JdA. Does Platelet-Rich Fibrin Decrease Dimensional Changes and Improve Postoperative Comfort in Post-Extraction Sockets? An Overview of Systematic Reviews. Applied Sciences. 2020; 10(17):5750. https://doi.org/10.3390/app10175750
Chicago/Turabian StyleMoraschini, Vittorio, Carlos Fernando de Almeida Barros Mourão, Rafael Coutinho de Mello Machado, Jhonathan Raphaell Barros Nascimento, Kayvon Javid, Monica Diuana Calasans-Maia, Angelo Cardarelli, Pietro Montemezzi, and Jose de Albuquerque Calasans-Maia. 2020. "Does Platelet-Rich Fibrin Decrease Dimensional Changes and Improve Postoperative Comfort in Post-Extraction Sockets? An Overview of Systematic Reviews" Applied Sciences 10, no. 17: 5750. https://doi.org/10.3390/app10175750
APA StyleMoraschini, V., Mourão, C. F. d. A. B., Machado, R. C. d. M., Nascimento, J. R. B., Javid, K., Calasans-Maia, M. D., Cardarelli, A., Montemezzi, P., & Calasans-Maia, J. d. A. (2020). Does Platelet-Rich Fibrin Decrease Dimensional Changes and Improve Postoperative Comfort in Post-Extraction Sockets? An Overview of Systematic Reviews. Applied Sciences, 10(17), 5750. https://doi.org/10.3390/app10175750






