Eucalyptus globulus Essential Oil as a Natural Food Preservative: Antioxidant, Antibacterial and Antifungal Properties In Vitro and in a Real Food Matrix (Orangina Fruit Juice)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Distillation of Eucalyptus globulus Essential Oil
2.1.2. Food Spoilage Microorganisms
2.1.3. Chemicals and Reagents
2.2. Methods
2.2.1. Chemical Composition of Eucalyptus globulus Essential Oil by GC-MS Analysis
2.2.2. In Vitro Antioxidant Activity
DPPH Radical Scavenging Assay
Metal Chelating Activity
2.2.3. In Vitro Antimicrobial Effect of EGEO
Disc Diffusion Method
Disc Volatilization Assay
2.2.4. Orangina Juice Preservation by Eucalyptus globulus Oil and Moderate Heat Processing
Preparation of Orangina Juice Inoculated with a Yeast Strain (Saccharomyces cerevisiae)
Influence of Eucalyptus globulus Essential Oil Alone
Influence of Eucalyptus globulus Essential Oil and Moderate Heat Processing: Combined Action
2.3. Statistical Analysis
3. Results
3.1. Chemical Composition of Eucalyptus globulus Essential Oil
3.2. Antioxidant Effect of the EGEO
3.3. In vitro Antibacterial and Antifungal Effects
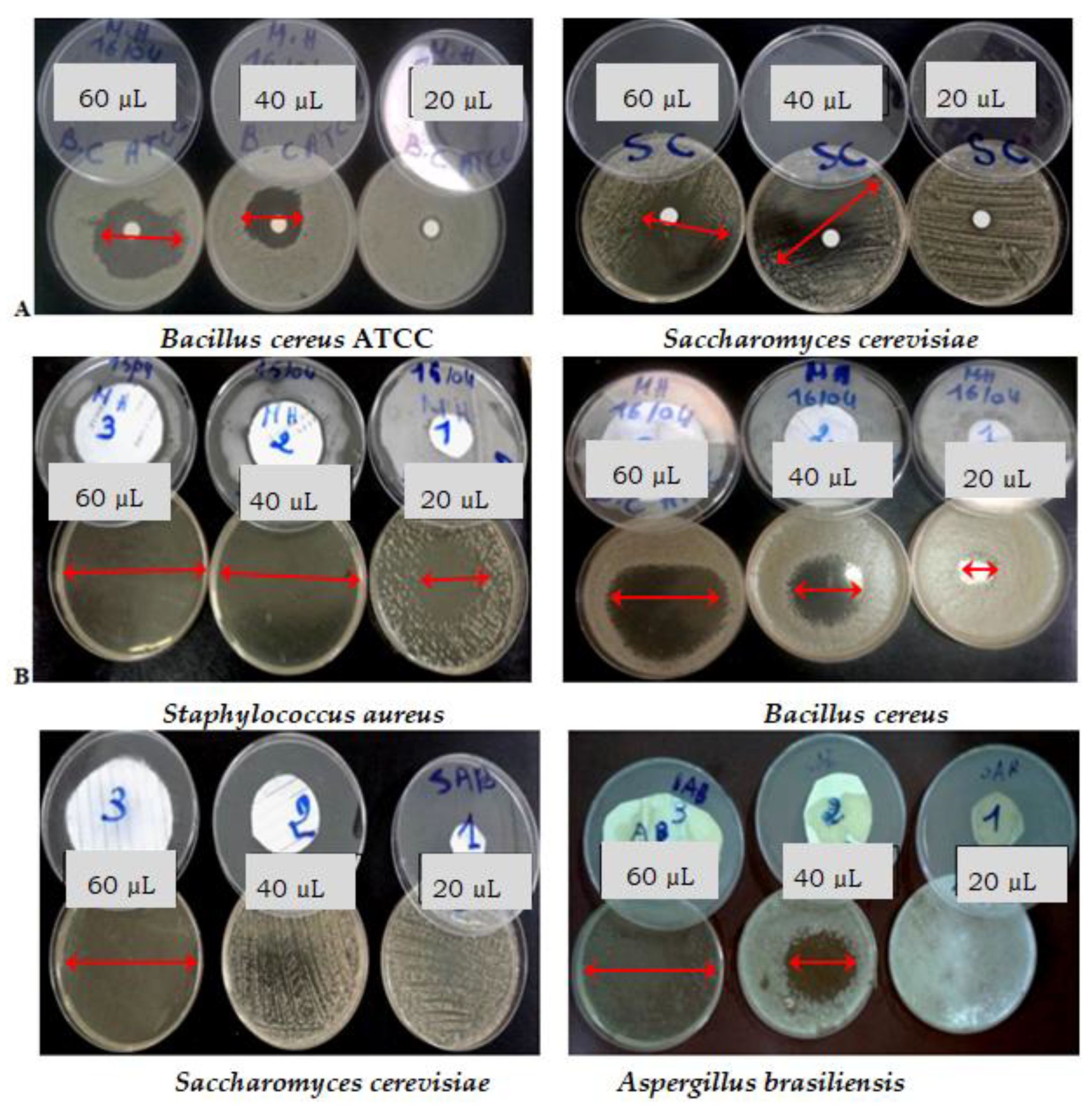
3.3.1. Disc Diffusion Assay
3.3.2. Disc Volatilization Method
3.4. Orangina Fruit Juice Preservation
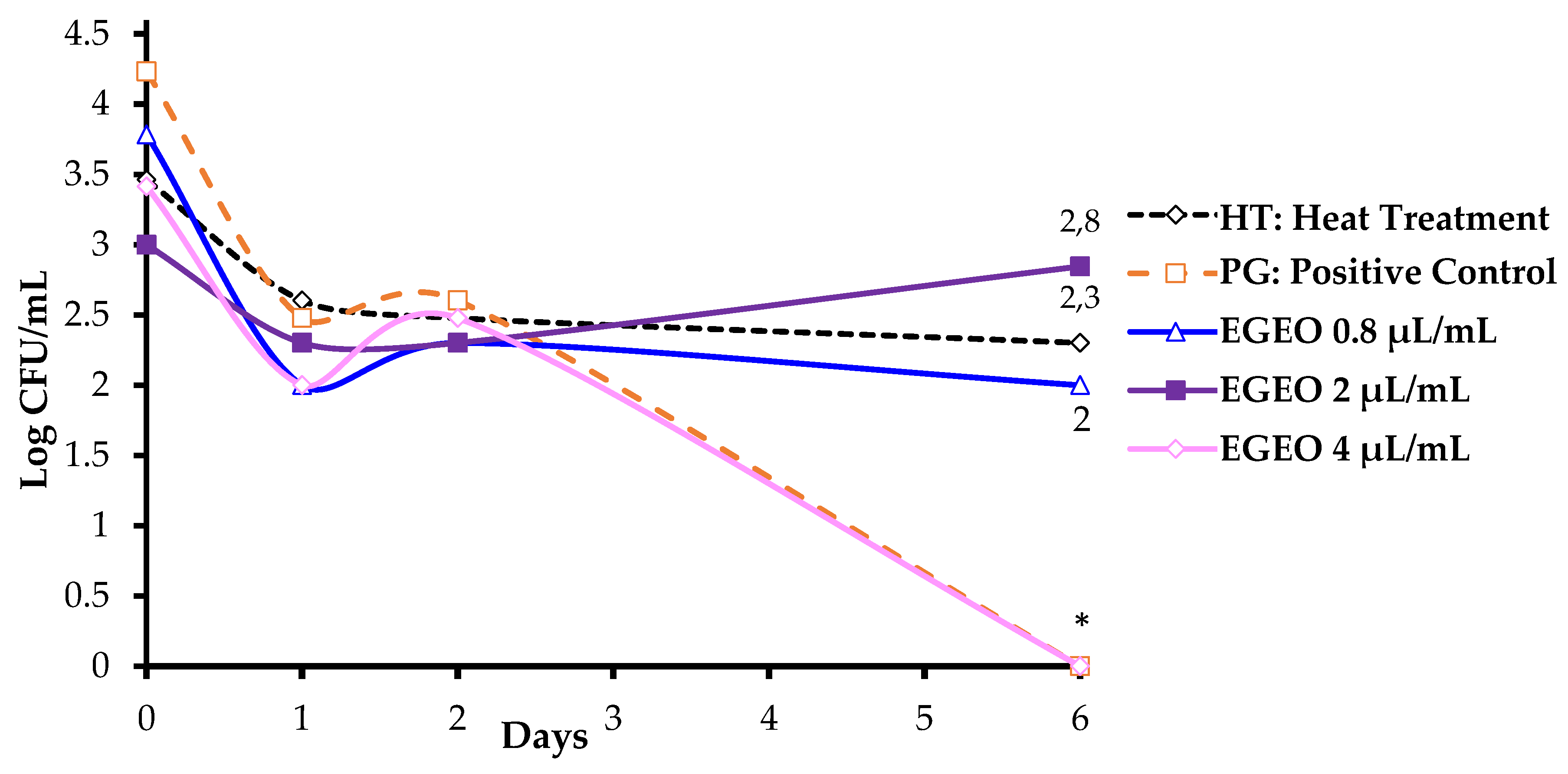
3.4.1. Effect of Varying Dose of EGEO
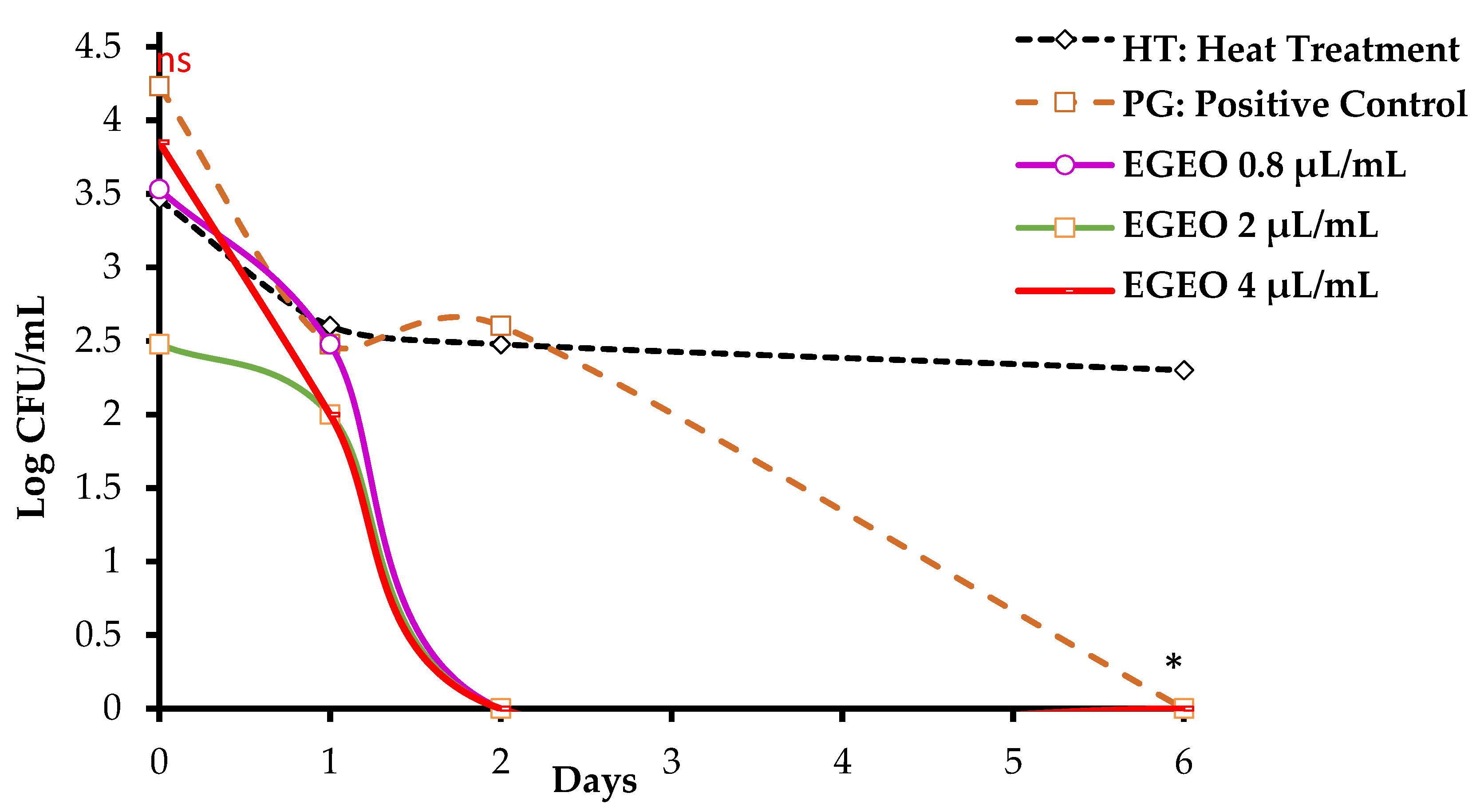
3.4.2. Combined Effect of EGEO and Moderate Heat Processing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomar, R.S.; Sharma, B.; Kaushik, S.; Mishra, R.K. Potential Antifungal Activity of Essential Oils and their Application in Food Preservation. Asian J. Pharm. Clin. Res. 2018, 11, 54–57. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.D.C. Use of Herbs, Spices and Their Bioactive Compounds in Active Food Packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant Essential Oils as Food Preservatives to Control Moulds, Mycotoxin Contamination and Oxidative Deterioration of Agri-Food Commodities–Potentials and Challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Tyagi, K.A.; Bukvicki, D.; Gottardi, D.; Tabanelli, G.; Montanari, C.; Malik, A.; Guerzoni, M.E. Eucalyptus Essential Oil as a Natural Food Preservative: In Vivo and In Vitro Antiyeast Potential. BioMed Res. Int. 2014, 2014, 969143. [Google Scholar] [CrossRef]
- Jirovetz, L.; Bail, S.; Buchbauer, G.; Stoilova, I.; Krastanov, A.; Stoyanova, A.; Schmidt, E. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of the Leaf Essential Oil of Corymbia citriodora (Hook) from China. Nat. Prod. Commun. 2007, 2, 1934578X0700200518. [Google Scholar] [CrossRef]
- Bhagat, M.; Gupta, S.; Jamwal, V.S.; Sharma, S.; Kattal, M.; Dawa, S.; Bindu, K. Comparative Study on Chemical Profiling and Antimicrobial Properties of Essential Oils from Different Parts of Eucalyptus lanceolatus. Indian J. Tradit. Knowl. 2016, 15, 425–432. [Google Scholar]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts Against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ušjak, L.; Petrović, S.; Drobac, M.; Soković, M.; Stanojković, T.; Ćirić, A.; Niketić, M. Essential Oils of Three Cow Parsnips–Composition and Activity Against Nosocomial and Foodborne Pathogens and Food Contaminants. Food Funct. 2017, 8, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Belotti, L.; Vecchi, S.; Testa, C.; Beretta, G. Air Dispersed Essential Oils Combined with Standard Sanitization Procedures for Environmental Microbiota Control in Nosocomial Hospitalization Rooms. Complement. Ther. Med. 2016, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.N.; Saha, S.; Ali, M.K. Antioxidant, Antimicrobial, Cytotoxic and Analgesic Activities of Ethanolic Extract of Mentha arvensis L. Asian Pac. J. Trop. Biomed. 2014, 4, 792–797. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of Essential Oils: A Review on their Interaction with Food Components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Mekarnia, M. Eucalyptus globulus (Labill.): Un Arbre à Essence aux Mille Vertus. Phytothérapie 2018, 16, 203–214. [Google Scholar] [CrossRef]
- Goetz, P.; Ghedira, K. Phytothérapie Anti-Infectieuse; Springer: Paris, France, 2012; pp. 271–279. [Google Scholar]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.H.; Khouja, M.L. Chemical composition of 8 Eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12, 81. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Compositional Analysis and Insecticidal Activity of Eucalyptus globulus (Family: Myrtaceae) Essential Oil against Housefly (Musca domestica). Acta Trop. 2012, 122, 212–218. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Fairouz, S.; Mekarnia, M. Liquid and vapour phase antibacterial activity of Eucalyptus globulus essential oil: Susceptibility of selected respiratory tract pathogens. Am. J. Infect. Dis. 2014, 10, 105. [Google Scholar]
- Cowan, S.T.; Steel, K.J. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993; 216p. [Google Scholar]
- Davey, K.G.; Chant, P.M.; Downer, C.S.; Campbell, C.K.; Warnock, D.W. Evaluation of the AUXACOLOR System, a New Method of Clinical Yeast Identification. J. Clin. Pathol. 1995, 48, 807–809. [Google Scholar] [CrossRef][Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ye, C.L.; Dai, D.H.; Hu, W.L. Antimicrobial and Antioxidant Activities of the Essential Oil From Onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- NCCLS (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Disc Susceptibility Tests: Approved Standard M2-A8; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- Goldbeck, J.C.; do Nascimento, J.E.; Jacob, R.G.; Fiorentini, Â.M.; da Silva, W.P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Manika, N.; Chanotiya, C.S.; Negi, M.P.S.; Bagchi, G.D. Copious Shoots as a Potential Source for the Production of Essential Oil in Eucalyptus globulus. Ind. Crops Prod. 2013, 46, 80–84. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.; Mendes, A.; Belo, A.D. Chemical Composition, Antibacterial, Antibiofilm and Synergistic Properties of Essential Oils from Eucalyptus globulus Labill. and seven Mediterranean Aromatic Plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef]
- Si Said, Z.B.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Rigou, P.; Remini, H.; Adjaoud, A.; Madani, K. Essential Oil Composition, Antibacterial and Antioxidant Activities of Hydrodistillated Extract of Eucalyptus globulus fruits. Ind. Crops Prod. 2016, 89, 167–175. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Sghaier, A. Variation in Chemical Composition of Eucalyptus globulus Essential Oil under Phenological Stages and Evidence Synergism with Antimicrobial Standards. Ind. Crops Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In vitro Antimicrobial Activity of Essential Oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016. [Google Scholar] [CrossRef]
- Soonwera, M.; Sittichok, S. Adulticidal Activities of Cymbopogon citratus (Stapf.) and Eucalyptus globulus (Labill.) Essential Oils and of their Synergistic Combinations against Aedes aegypti (L.), Aedes albopictus (Skuse), and Musca domestica (L.). Environ. Sci. Pollut. Res. 2020, 27, 20201–20214. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Hajlaoui, H.; Trabelsi, N.; Ksouri, R.; Valentin, E.; Bakhrouf, A. Chemical Composition, Antioxidant and Antifungal Potential of Melaleuca alternifolia (Tea Tree) and Eucalyptus globulus Essential Oils Against Oral Candida Species. J. Med. Plants Res. 2011, 5, 4147–4156. [Google Scholar]
- Singh, H.P.; Kaur, S.; Negi, K.; Kumari, S.; Saini, V.; Batish, D.R.; Kohli, R.K. Assessment of In Vitro Antioxidant Activity of Essential Oil Of Eucalyptus citriodora (Lemon-Scented Eucalypt; Myrtaceae) and its Major Constituents. LWT-Food Sci. Technol. 2012, 48, 237–241. [Google Scholar] [CrossRef]
- Horvathova, E.; Navarova, J.; Galova, E.; Sevcovicova, A.; Chodakova, L.; Snahnicanova, Z.; Slamenova, D. Assessment of Antioxidative, Chelating, and DNA-Protective Effects of Selected Essential Oil Components (Eugenol, Carvacrol, Thymol, Borneol, Eucalyptol) of Plants and Intact Rosmarinus officinalis Oil. J. Agric. Food Chem. 2014, 62, 6632–6639. [Google Scholar] [CrossRef] [PubMed]
- El-Ghorab, A.H.; El-Massry, K.F.; Marx, F.; Fadel, H.M. Antioxidant Activity of Egyptian Eucalyptus camaldulensis var. Brevirostris Leaf Extracts. Food 2003, 47, 41–45. [Google Scholar] [PubMed]
- Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P.; Angioni, A. Chemical Variability, Antifungal and Antioxidant Activity of Eucalyptus camaldulensis Essential Oil From Sardinia. Nat. Prod. Commum. 2010, 5, 329–335. [Google Scholar] [CrossRef]
- Su, Y.C.; Ho, C.L.; Wang, E.I.C.; Chang, S.T. Antifungal Activities and Chemical Compositions of Essential Oils From Leaves of four Eucalyptus. Taiwan J. For. Sci. 2006, 21, 9–61. [Google Scholar]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential Oil of Algerian Eucalyptus citriodora: Chemical Composition, Antifungal Activity. J. Mycol. Med. 2015, 25, e128–e133. [Google Scholar] [CrossRef]
- Damjanović-Vratnica, B.; Đakov, T.; Šuković, D.; Damjanović, J. Antimicrobial Effect of Essential Oil Isolated From Eucalyptus globulus Labill. From Montenegro. Czech J. Food Sci. 2011, 29, 277–284. [Google Scholar] [CrossRef]
- Vilela, G.R.; de Almeida, G.S.; D’Arce, M.A.B.R.; Moraes, M.H.D.; Brito, J.O.; da Silva, M.F.D.G.; da Gloria, E.M. Activity of Essential Oil and its Major Compound, 1, 8-Cineole, from Eucalyptus globulus Labill., Against the Storage Fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009, 45, 108–111. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial Activity of Essential Oils and Their Major Constituents Against Respiratory Tract Pathogens by Gaseous Contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef]
- Goni, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial Activity in the Vapour Phase of a Combination of Cinnamon and Clove Essential Oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Cavanagh, H.M. Antibacterial Activity of Essential Oils From Australian Native Plants. Phytother. Res. 2005, 19, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Krisch, J. Anti Yeast Activities of Some Essential Oils in Growth Medium, Fruit Juices and Milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial Activity of Plant Essential Oils using Food Model Media: Efficacy, Synergistic Potential and Interactions with Food Components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Belletti, N.; Kamdem, S.S.; Patrignani, F.; Lanciotti, R.; Covelli, A.; Gardini, F. Antimicrobial Activity of Aroma Compounds against Saccharomyces cerevisiae and Improvement of Microbiological Stability of Soft Drinks as Assessed by Logistic Regression. Appl. Environ. Microbiol. 2007, 73, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellvi, S.; Pagán, R.; Garcia-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, 0056769. [Google Scholar] [CrossRef] [PubMed]
- Cherrat, L.; Espina, L.; Bakkali, M.; García-Gonzalo, D.; Pagán, R.; Laglaoui, A. Chemical Composition and Antioxidant Properties of Laurus nobilis L. and Myrtus communis L. Essential Oils from Morocco and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes for Food Preservation. J. Sci. Food Agric. 2014, 94, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Char, C.; Guerrero, S.; Alzamora, S.M. Survival of Listeria innocua in Thermally Processed Orange Juice as Affected by Vanillin Addition. Food Control 2009, 20, 67–74. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Gottardi, D.; Malik, A.; Guerzoni, M.E. Anti-Yeast Activity of Mentha Oil and Vapours Through In Vitro and In Vivo (Real Fruit Juices) Assays. Food Chem. 2013, 137, 108–114. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Pagán, R.; Mackey, B. Relationship Between Sublethal Injury and Microbial Inactivation by the Combination of High Hydrostatic Pressure and Citral or Tert-Butyl Hydroquinone. Appl. Environ. Microbiol. 2008, 74, 7570–7577. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. New Insights in Mechanisms of Bacterial Inactivation by Carvacrol. J. Appl. Microbiol. 2013, 114, 173–185. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, D.; Bukvicki, D.; Prasad, S.; Tyagi, A.K. Beneficial Effects of Spices in Food Preservation and Safety. Front. Microbiol. 2016, 7, 1394. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N. Scientific Findings: The Amazing Use of Essential Oils and Their Related Terpenes as Natural Preservatives to Improve the Shelf-Life of Food. Food Sci. Nutr. Technol. 2020, 5, 00021. [Google Scholar]
- Belletti, N.; Kamdem, S.S.; Tabanelli, G.; Lanciotti, R.; Gardini, F. Modeling of Combined Effects of Citral, Linalool and β-Pinene Used Against Saccharomyces cerevisiae in Citrus-Based Beverages Subjected to a Mild Heat Treatment. Int. J. Food Microbiol. 2010, 136, 283–289. [Google Scholar] [CrossRef]
| Retention Time (RT, min) | RI § | Name # | Concentration (%) |
|---|---|---|---|
| 8.777 | 925 | α-Pinene | 2.93 ± 0.1 |
| 9.685 | 969 | β-Pinene | 0.20 ± 0.02 |
| 10.110 | 983 | Myrcene | 0.19 ± 0.07 |
| 10.282 | 1000 | α-Phellandrene | 0.59 ± 0.02 |
| 10.886 | 1033 | Eucalyptol (1,8-Cineole) | 94.03 ± 0.23 |
| 11.282 | 1053 | γ-Terpinene | 1.93 ± 0.17 |
| Oxygenated monoterpenes | 94.03 | ||
| Monoterpene hydrocarbons | 05.86 |
| Sample | DPPH Radical Scavenging IC50 (mg/mL) | Chelation IC50 (mg/mL) |
|---|---|---|
| Eucalyptus globulus Essential Oil | 2.48 ± 2.24 C | 8.43 ± 0.03 A |
| Positive Control (BHA) | 0.13 ± 0.05 B | 104.73 ± 7.30 B |
| Positive Control (Ascorbic acid) | 0.018 ± 0.004 A | 140.99 ± 3.13 C |
| Positive Control (Gallic Acid) | — | 136.97 ± 9.09 C |
| DIZ (mm) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Disc Diffusion Technique | Disc Vapor Technique | ||||||||
| Bacterial Strains | Volume of EGEO (µL) per Disc | Positive Control b | |||||||
| Gram-negative bacteria | 20 | 40 | 60 | 20 | 40 | 60 | AMC | E | C |
| Pseudomonas aeruginosa | - | - | - | - | - | - | - | 16 | 14 |
| Enterobacter sakazakii | 12 | 15 | 25 | - | 85 | 85 | - | 19 | 27 |
| Klebsiella ornithinolytica | - | 10 | 19 | - | - | - | - | 22 | 12 |
| Escherichia coli | 11 | 19 | 34 | - | - | 36 | 12 | 19 | 7 |
| Gram-positive bacteria | |||||||||
| Bacillus cereus | 15 | 35 | 50 | 24 | 40 | 59 | 25 | 35 | 24 |
| Staphylococcus aureus | 18 | 48 | 85 | 41 | 85 | 85 | 26 | R | 16 |
| DIZ (mm) a | |||||||
|---|---|---|---|---|---|---|---|
| Disc Diffusion Assay | Disc Volatilization Assay | ||||||
| Fungal Strains | Quantity of EGEO (µL) per Disc | Hex b | |||||
| Yeasts | 20 | 40 | 60 | 20 | 40 | 60 | 40 |
| Candida albicans ATCC | - | 11 | 40 | - | - | - | 30 |
| Candida albicans (Ca1) | 10 | 25 | 27 | - | - | 53 | 85 |
| Candida albicans (Ca2) | 14 | 19 | 24 | 85 | 85 | 85 | 13 |
| Candida parapsilosis | 12 | 17 | 20 | 85 | 85 | 85 | 30 |
| Saccharomyces cerevisiae | 11 | 37 | 49 | - | - | 59 | - |
| Trichosporon sp. | 16 | 34 | 39 | 21 | 85 | 85 | 42 |
| Molds | |||||||
| Aspergillus niger (An1) | 12 | 21 | 29 | - | - | - | - |
| Aspergillus niger (An2) | - | 10 | 15 | - | - | - | - |
| Aspergillus fumigatus | - | - | - | - | - | - | - |
| Aspergillus flavus | - | - | - | - | - | - | - |
| Aspergillus brasiliensis ATCC | - | - | 14 | - | 43 | 85 | 14 |
| Plants | Country | Period | Method | Composition (%) | Authors |
|---|---|---|---|---|---|
| E. globulus | Spain | Hydrodistillation of leaves and small branches of the tree. Certified as biological products to be used in humans. | Eucalyptol = 63.81 α-Pinene = 16.06 Aromadendrene = 3.68 o-Cymene = 2.35 | Luís et al. [28] | |
| E. radiata | Australia | Limonene = 68.51 α-Terpineol = 8.60 α-Terpinyl acetate = 6.07 α-Pinene = 3.01 | |||
| E. globulus leaves | Alentejo (Portugal) | Spring 2014 | Hydrodistillation in a modified Clevenger-type apparatus | Eucalyptol = 4.6% Metileugenol = 3.5 α-Pinene = 2.9 Globulol = 3.2 Terpinene-4-ol = 2 | Vieira et al. [29] |
| E. globulus fruits | Bejaia (Algeria) | 2013 | Hydrodistillation using a Clevenger type apparatus | Globulol = 23.6 Eucalyptol = 19.8 α-Pinene = 3.8 iso-Valeradehyde = 2.4 α-Phellandrene = 1.9 | Si Said et al. [30] |
| E. globulus aerial parts | Takelsa (Tunisia) | Vegetative stages (Feb. 2017) | Hydrodistillation by Clevenger apparatus | Eucalyptol = 13.2 p-Cymene = 12.5 α-Pinene = 12.1 β-Pinene = 10.6 | Salem et al. [31] |
| Full Flowering Stages (March 2017) | p-Cymene = 32.1 α-Pinene = 10.4 Eucalyptol = 7.7 β-Pinene = 7.4 | ||||
| Fructification stages (May 2017) | p-Cymene = 37.8 α-Pinene = 13.3 Eucalyptol = 10.3 L-Phellandrene = 8.2 | ||||
| Fresh leaves | Ankober Ethiopia | Hydrodistilled in a Clevenger-type apparatus | Eucalyptol = 63.001 𝛼-Pinene 16.101 Camphor 3.422 | Mekonnen et al. [32] | |
| Fresh stems and leaves | Nakhon Nayok, (Thailand) | Rainy Season June–Aug. 2018 | Hydrodistilled | Eucalyptol = 44.54 𝛼-terpinene = 19.83 𝛼-Pinene = 4.95 Terpinene-4-ol = 3.49 | Soonwera and Sittichok [33] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M.; Mousa, S.A. Eucalyptus globulus Essential Oil as a Natural Food Preservative: Antioxidant, Antibacterial and Antifungal Properties In Vitro and in a Real Food Matrix (Orangina Fruit Juice). Appl. Sci. 2020, 10, 5581. https://doi.org/10.3390/app10165581
Boukhatem MN, Boumaiza A, Nada HG, Rajabi M, Mousa SA. Eucalyptus globulus Essential Oil as a Natural Food Preservative: Antioxidant, Antibacterial and Antifungal Properties In Vitro and in a Real Food Matrix (Orangina Fruit Juice). Applied Sciences. 2020; 10(16):5581. https://doi.org/10.3390/app10165581
Chicago/Turabian StyleBoukhatem, Mohamed Nadjib, Asma Boumaiza, Hanady G. Nada, Mehdi Rajabi, and Shaker A. Mousa. 2020. "Eucalyptus globulus Essential Oil as a Natural Food Preservative: Antioxidant, Antibacterial and Antifungal Properties In Vitro and in a Real Food Matrix (Orangina Fruit Juice)" Applied Sciences 10, no. 16: 5581. https://doi.org/10.3390/app10165581
APA StyleBoukhatem, M. N., Boumaiza, A., Nada, H. G., Rajabi, M., & Mousa, S. A. (2020). Eucalyptus globulus Essential Oil as a Natural Food Preservative: Antioxidant, Antibacterial and Antifungal Properties In Vitro and in a Real Food Matrix (Orangina Fruit Juice). Applied Sciences, 10(16), 5581. https://doi.org/10.3390/app10165581






