Revisiting the Sodiation Mechanism of TiO2 via Operando X-ray Absorption Spectroscopy
Abstract
Featured Application
Abstract
1. Introduction
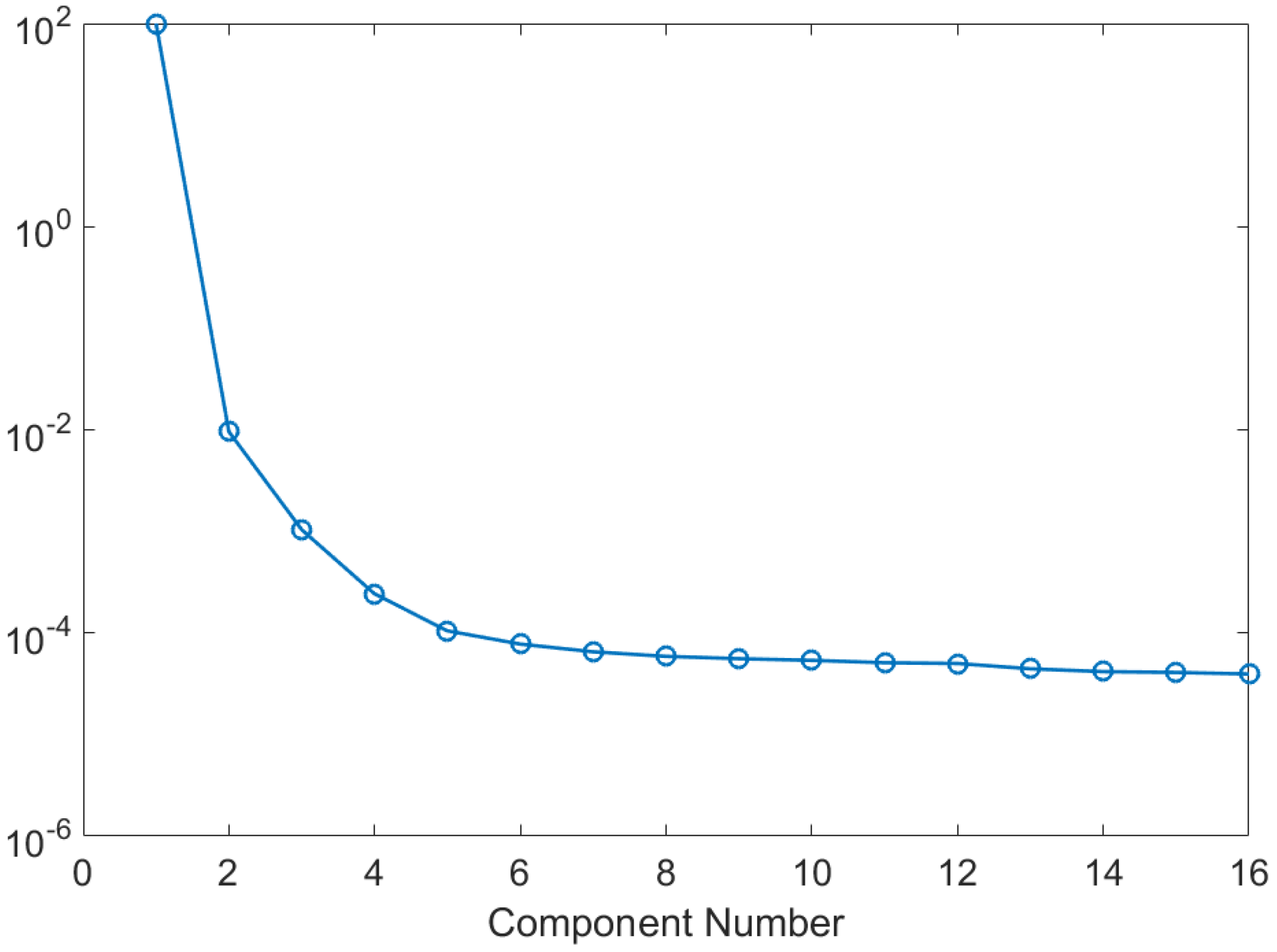
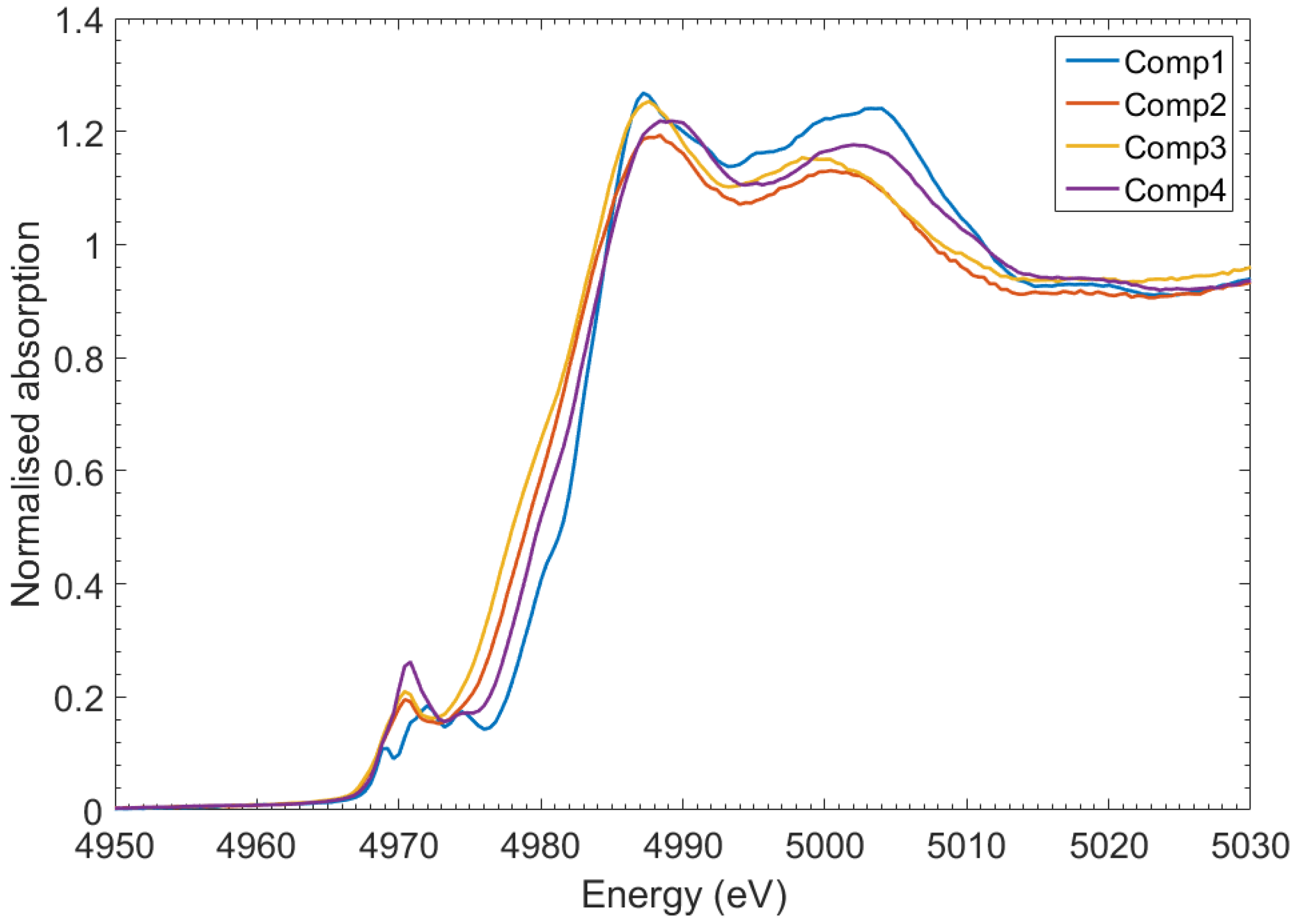
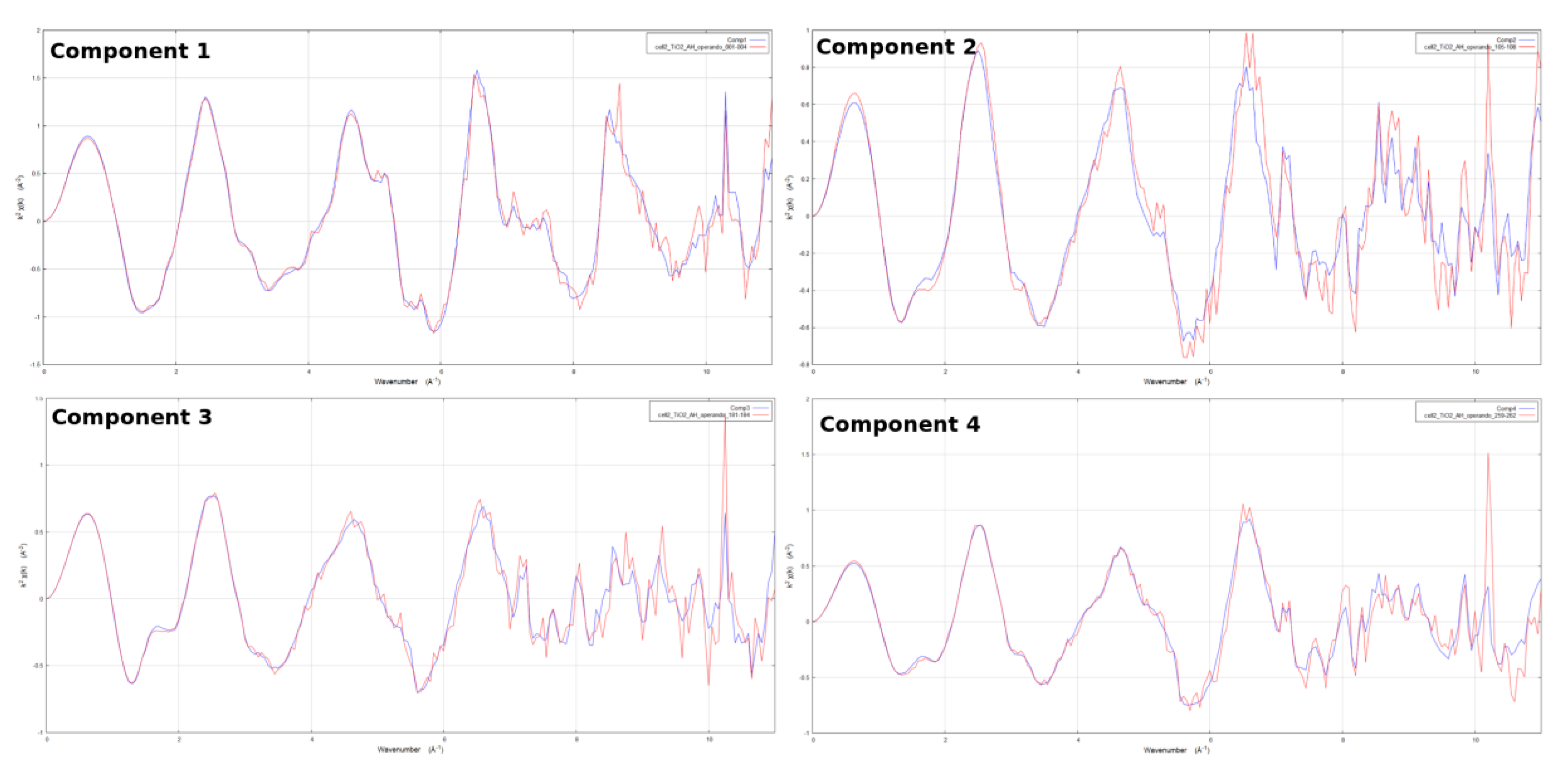
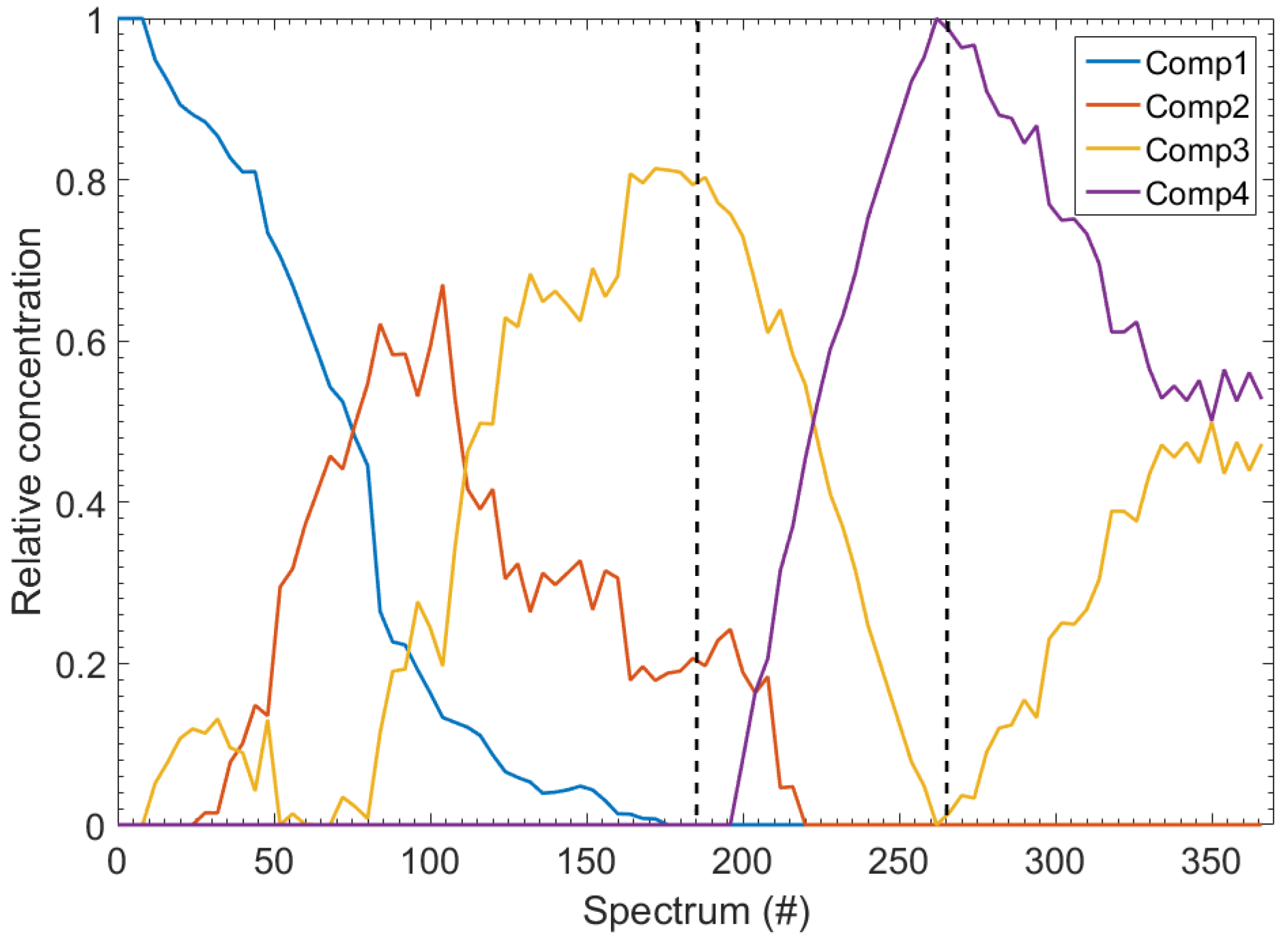
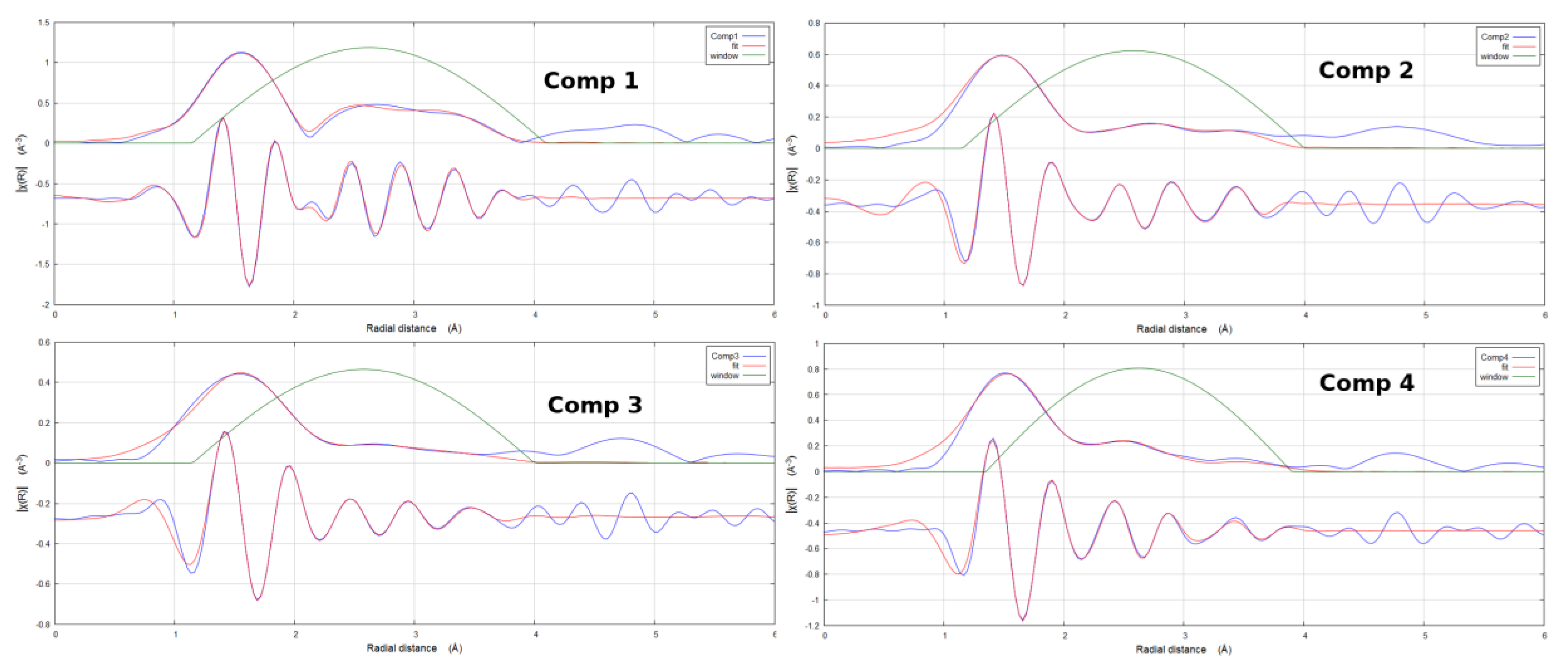
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Material Synthesis and Electrode Formulation
5.2. Operando X-ray Absorption Spectroscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EOC | End of Charge |
| EOD | End of Discharge |
| EXAFS | Extended X-ray Absorption Fine Structure |
| MCR-ALS | Multivariate Curve Resolution - Alternating Least Squares |
| PCA | Principal Component Analysis |
| Pair Distribution Function | |
| XANES | X-ray Absorption Near-Edge Structure |
| XAS | X-ray Absorption Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
Appendix A
References
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Fehse, M.; Ventosa, E. Is TiO2(B) the future of titanium-based battery materials? ChemPlusChem 2015, 80, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yi, J.; Sun, Y.; Zhou, H. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries. Energy Environ. Sci. 2016, 9, 2978–3006. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Giffin, G.A.; Castro, C.R.; Ochel, A.; Passerini, S. Unfolding the Mechanism of Sodium Insertion in Anatase TiO2 Nanoparticles. Adv. Energy Mater. 2015, 5, 1401142. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Y.; Wang, J.; Yin, G.; Du, C.; Sun, X. Ti?Based Oxide Anode Materials for Advanced Electrochemical Energy Storage: Lithium/Sodium Ion Batteries and Hybrid Pseudocapacitors. Small 2019, 15, 1904740. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Buchholz, D.; Bresser, D.; Gomes Chagas, L.; Passerini, S. Anatase TiO2 nanoparticles for high power sodium-ion anodes. J. Power Sources 2014, 251, 379–385. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Anatase TiO2: Better Anode Material Than Amorphous and Rutile Phases of TiO2 for Na-Ion Batteries. Chem. Mater. 2015, 27, 6022–6029. [Google Scholar] [CrossRef]
- Chen, C.; Wen, Y.; Hu, X.; Ji, X.; Yan, M.; Mai, L.; Hu, P.; Shan, B.; Huang, Y. Na(+) intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling. Nat. Commun. 2015, 6, 6929. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Wang, C.; Hou, H.; Zhang, Y.; Wang, C.; Zou, G.; Ji, X. Black Anatase Titania with Ultrafast Sodium-Storage Performances Stimulated by Oxygen Vacancies. ACS Appl. Mater. Interfaces 2016, 8, 9142–9151. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Bai, Y.; Wang, Z.; Ni, Q.; Chen, G.; Zhou, Z.; Wu, C. Remarkable Effect of Sodium Alginate Aqueous Binder on Anatase TiO2 as High-Performance Anode in Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5560–5568. [Google Scholar] [CrossRef]
- Ni, Q.; Dong, R.; Bai, Y.; Wang, Z.; Ren, H.; Sean, S.; Wu, F.; Xu, H.; Wu, C. Superior sodium-storage behavior of flexible anatase TiO2 promoted by oxygen vacancies. Energy Storage Mater. 2020, 25, 903–911. [Google Scholar] [CrossRef]
- Kim, K.T.; Ali, G.; Chung, K.Y.; Yoon, C.S.; Yashiro, H.; Sun, Y.K.; Lu, J.; Amine, K.; Myung, S.T. Anatase titania nanorods as an intercalation anode material for rechargeable sodium batteries. Nano Lett. 2014, 14, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Nohira, T.; Hagiwara, R. A new sodiation-desodiation mechanism of the titania-based negative electrode for sodium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 30770–30776. [Google Scholar] [CrossRef] [PubMed]
- Louvain, N.; Henry, A.; Daenens, L.; Boury, B.; Stievano, L.; Monconduit, L. On the electrochemical encounter between sodium and mesoporous anatase TiO 2 as a Na-ion electrode. CrystEngComm 2016, 18, 4431–4437. [Google Scholar] [CrossRef]
- Li, W.; Fukunishi, M.; Morgan, B.J.; Borkiewicz, O.J.; Chapman, K.W.; Pralong, V.; Maignan, A.; Lebedev, O.I.; Ma, J.; Groult, H.; et al. A Reversible Phase Transition for Sodium Insertion in Anatase TiO2. Chem. Mater. 2017, 29, 1836–1844. [Google Scholar] [CrossRef]
- Fehse, M.; Sougrati, M.T.; Darwiche, A.; Gabaudan, V.; La Fontaine, C.; Monconduit, L.; Stievano, L. Elucidating the origin of superior electrochemical cycling performance: New insights on sodiation-desodiation mechanism of SnSb from: Operando spectroscopy. J. Mater. Chem. A 2018, 6, 8724–8734. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Greco, G.; Mazzio, K.A.; Dou, X.; Gericke, E.; Wendt, R.; Krumrey, M.; Passerini, S. Structural Study of Carbon-Coated TiO2 Anatase Nanoparticles as High-Performance Anode Materials for Na-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 7142–7151. [Google Scholar] [CrossRef]
- Fehse, M.; Iadecola, A.; Sougrati, M.T.; Conti, P.; Giorgetti, M.; Stievano, L. Applying chemometrics to study battery materials: Towards the comprehensive analysis of complex operando datasets. Energy Storage Mater. 2019, 18, 328–337. [Google Scholar] [CrossRef]
- Matsuo, S.; Sakaguchi, N.; Wakita, H. Pre-edge features of Ti K-edge X-ray absorption near-edge structure for the local structure of sol-gel titanium oxides. Anal. Sci. 2005, 21, 805–809. [Google Scholar] [CrossRef]
- Wan, J.; Chen, W.; Jia, C.; Zheng, L.; Dong, J.; Zheng, X.; Wang, Y.; Yan, W.; Chen, C.; Peng, Q.; et al. Defect Effects on TiO2 Nanosheets: Stabilizing Single Atomic Site Au and Promoting Catalytic Properties. Adv. Mater. 2018, 30, 1705369. [Google Scholar] [CrossRef] [PubMed]
- Lafont, U.; Carta, D.; Mountjoy, G.; Chadwick, A.V.; Kelder, E.M. In Situ Structural Changes upon Electrochemical Lithium Insertion in Nanosized Anatase TiO2. J. Phys. Chem. C 2010, 114, 1372–1378. [Google Scholar] [CrossRef]
- Fehse, M.; Monconduit, L.; Fischer, F.; Tessier, C.; Stievano, L. Study of the insertion mechanism of lithium into anatase by operando X-ray diffraction and absorption spectroscopy. Solid State Ionics 2014, 268, 252–255. [Google Scholar] [CrossRef]
- Fehse, M.; Darwiche, A.; Sougrati, M.T.; Kelder, E.M.; Chadwick, A.V.; Alfredsson, M.; Monconduit, L.; Stievano, L. In-Depth Analysis of the Conversion Mechanism of TiSnSb vs Li by Operando Triple-Edge X-ray Absorption Spectroscopy: A Chemometric Approach. Chem. Mater. 2017, 29, 10446–10454. [Google Scholar] [CrossRef]
- Horn, M.; Schwebdtfeger, C.F.; Meagher, E.P. Refinement of the structure of anatase at several temperatures. Z. Kristallogr. 1972, 136. [Google Scholar] [CrossRef]
- Leriche, J.B.; Hamelet, S.; Shu, J.; Morcrette, M.; Masquelier, C.; Ouvrard, G.; Zerrouki, M.; Soudan, P.; Belin, S.; Elkaïm, E.; et al. An electrochemical cell for operando study of lithium batteries using synchrotron radiation. J. Electrochem. Soc. 2010, 157, A606–A610. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]

| Component | Shell | N * | R [Å] | [Å] |
|---|---|---|---|---|
| MCR #1 | Ti-O | 4 | 1.91(1) | 0.0014(6) |
| Ti-O | 2 | 2.32(2) | 0.0014(6) | |
| Ti-Ti | 4 | 3.02(8) | 0.008(1) | |
| Ti-Ti | 4 | 3.87(8) | 0.008(1) | |
| Ti-O | 8 | 3.79(3) | 0.004(10) | |
| MCR #2 | Ti-O | 4 | 1.91(1) | 0.008(1) |
| Ti-O | 2 | 2.33(1) | 0.008(1) | |
| Ti-Ti | 6 | 3.09(3) | 0.022(3) | |
| Ti-Na | 3 | 3.20(3) | 0.005(2) | |
| Ti-Ti | 3 | 3.89(3) | 0.012(2) | |
| MCR #3 | Ti-O | 4 | 2.00(2) | 0.014(1) |
| Ti-O | 2 | 2.47(2) | 0.014(1) | |
| Ti-Ti | 4 | 3.18(3) | 0.018(3) | |
| Ti-Na | 3 | 3.34(4) | 0.015(4) | |
| Ti-O | 6 | 3.98(5) | 0.024(5) | |
| MCR #4 | Ti-O | 4 | 1.97(1) | 0.007(1) |
| Ti-O | 2 | 2.41(2) | 0.007(1) | |
| Ti-Ti | 4 | 3.07(3) | 0.013(2) | |
| Ti-Ti | 2 | 3.98(3) | 0.013(2) | |
| Ti-Na (O) | 3 | 3.85(3) | 0.023(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehse, M.; Henry, A.; Zitolo, A.; Boury, B.; Louvain, N.; Stievano, L. Revisiting the Sodiation Mechanism of TiO2 via Operando X-ray Absorption Spectroscopy. Appl. Sci. 2020, 10, 5547. https://doi.org/10.3390/app10165547
Fehse M, Henry A, Zitolo A, Boury B, Louvain N, Stievano L. Revisiting the Sodiation Mechanism of TiO2 via Operando X-ray Absorption Spectroscopy. Applied Sciences. 2020; 10(16):5547. https://doi.org/10.3390/app10165547
Chicago/Turabian StyleFehse, Marcus, Aurélien Henry, Andrea Zitolo, Bruno Boury, Nicolas Louvain, and Lorenzo Stievano. 2020. "Revisiting the Sodiation Mechanism of TiO2 via Operando X-ray Absorption Spectroscopy" Applied Sciences 10, no. 16: 5547. https://doi.org/10.3390/app10165547
APA StyleFehse, M., Henry, A., Zitolo, A., Boury, B., Louvain, N., & Stievano, L. (2020). Revisiting the Sodiation Mechanism of TiO2 via Operando X-ray Absorption Spectroscopy. Applied Sciences, 10(16), 5547. https://doi.org/10.3390/app10165547






