1. Introduction
According to the World Health Organization (WHO), chronic diseases such as chronic obstructive pulmonary disease (COPD) will become the third-main cause of death worldwide by 2020 [
1]. The environment (e.g., gases, sound level, and ultraviolet radiation), the patient’s behavior (e.g., activity), and the physiological state of the subject (e.g., vital signs) are important factors of disease development. Furthermore, the simultaneous monitoring of these domains has great importance in the safety monitoring of workplaces. Under the rapid development of technologies, the world has entered into a new era of interconnected networks via the Internet of Things (IoT) as one of the most powerful communication paradigm [
2]. The IoT supports the connection of objects putting forward technologies and networking architectures. Numerous objects in houses or vehicles are connected and share information and resources. Therefore, IoT has become a key component in healthcare applications and environmental monitoring [
3]. Wireless sensors can also measure environmental conditions and parameters related to the health status [
4]. Continuous health monitoring is available and with the methodology of mobile health (mHealth) or electronic health (eHealth), the medical paradigm shifts from diagnostics and treatment towards predictive, preventive, personalized, and participatory (P4) medicine [
5,
6,
7]. In contrast to reactive traditional medicine, P4 medicine is proactively seeking causes rather than symptoms of diseases. It addresses patients, consumers, and physicians. Personalized medicine motivates actively participating lifestyle observation, leading to improved quality of life. Studies have shown that wearable technology has many advantages and benefits in healthcare [
8,
9]. Wearable sensors gained attention of researchers, vendors, and consumers [
10,
11,
12]. Based on wearable devices and their advantages in healthcare, the medical IoT (mIoT) is the most acceptable solution reaching proactive and personalized medicine [
13,
14]. Miniaturization and user convenience boosted mass production of non-invasive sensors with the applications in well-being, lifestyle, and personalized healthcare [
15,
16].
Wireless body area networks (WBAN) are widely used to combine wearables for measuring physiological parameters such as the heart rate (HR), respiration rate (RR), electrocardiography (ECG), body temperature, and blood pressure (BP). WBAN is also applied to measure physical activities (e.g., body positioning, motion tracking) and environmental conditions. Such applications are of great importance for chronic disease management (e.g., COPD) and safety of workplace monitoring (e.g., workers on construction sites). Then health and safety (H&S) are interwoven domains [
17,
18].
Several publications reported the influence of air pollutants including carbon monoxide (CO), nitric dioxide (
), and particle matter (
and
) [
19,
20] on health. However, such ambient parameters are not limited to hazardous gases. The loudness of environmental sound is important, too. Long-term exposure yields hearing loss or psychological impairments [
21]. Overexposure to ultraviolet (UV) radiation causes skin cancer, eye irritation, and aging skin [
22]. Furthermore, air temperature, humidity, and air pressure are usually delivered by wearables (
Table 1). Skin temperature and photoplethysmography (PPG) are the commonly monitored physiological signals [
23]. Long-term and continuous monitoring of safety conditions with respect to a combination of (i) environmental conditions, (ii) behavioural parameters, and vital signs can significantly contribute protecting health in hazardous situations by early warning systems [
24].
Nevertheless, there is lack of efficient wearable systems to monitor environmental, behavioral, and physiological parameters at the same time [
2]. In safety monitoring and industrial environments, the current approach is to deploy static wireless sensor nodes for the monitoring of ambient and physiological parameters. Several works report on hybrid systems in the monitoring of environmental-physiological domains based on wireless sensor networks (WSN) [
25].
Chen et al. present a smart clothing for ECG, blood oxygen saturation, and temperature assessment. Bluetooth Low Energy (BLE) is adopted to transmit the data to a smartphone as gateway to the cloud [
28].
Wu et al. introduced a hybrid distributed system for health and safety monitoring [
2]. Two sensor nodes, one for environmental measurement (safety node) and the other for vital signs (health node) are deployed utilizing WBAN. Data from the safety node (temperature, humidity, UV, and
) are transmitted to the health node (HR and body temperature) by BLE. For the long-range data transmission to the cloud, low-power wireless area network (LPWAN) is used.
A hybrid and decentralized system including one wrist-worn and one chest-worn device for environmental and physiological monitoring is reported by Dieffendefer et al. [
29]. The chest-worn measures ECG, pulse oxygen, skin impedance, and strain; the wrist-worn volatile organic compounds (VOCs) and ozone (
), temperature, and humidity. The data are transmitted via smartphone to the cloud.
Haghi et al. present a distributed wearables system for monitoring of environmental, physiological, and behavioural. The authors implement a three tier IoT solution within the scope of WBAN. They describe convenient and comprehensive system including several wearables on wrist and chest. Data are transmitted via a flexible IoT-gateway (i.e., smartphone) to the cloud [
30].
The work reported in Reference [
25] presents the implementation of a wearable wireless sensor network measuring the harmful gases (e.g.,
) in industrial environments. The solution is a customized WSN using a low-power low-rate wireless personal area network (LR-WPAN) communications protocol.
Martin et al. propose heart rate and breathing rate monitoring of construction workers by the integration of a noninvasive vital sign monitoring into the workers’ hearing protection devices (HPDs). The solution is based on using a microphone positioned within the ear canal under the HPD followed with developing digital signal processing algorithms [
31].
With respect to chronic-disease management, COPD is of particular relevance. COPD patients usually are experiencing an altered skin temperature, making their skin more sensitive to UV light [
32]. Many patients are suffering from sleep disorders. Thus, physicians seek a better understanding of the ambient living conditions [
33].
As of today, remote or tele-monitoring of COPD patients usually is limited to special programs, a short period of time, or a special parameter such as the trans-cutaneous oxygen saturation (
) [
34]. Contrarily, COPD is impacted by a variety of parameters, for instance, cardiovascular co-morbidity, environmental factors such as exposure to pollutants, and physiological factors [
35]. Furthermore, the activity profile is found to be more important for elderly who are suffering from muscle weakness as respiratory parameters [
36]. Therefore, multi-dimensional monitoring is necessary and shall not be restricted to particular target populations or parameters, but a wide range of environmental, physiological, and behavioral factors have to be considered [
37].
Furthermore, long-term monitoring of the vital signs such as oxygen saturation, heart rate, and near-body temperature indicates the health status [
38]. In our aging population, continuous tele-monitoring of patients’ suffering from chronic diseases is important. WHO statistics show chronic diseases are among the leading causes of death [
39]. Wearable devices support not only unobtrusive real-time and long-term monitoring of physiological parameters, vital signs, and motion activities but can also track ambient parameters from the environment [
40,
41]. However, the required sensor integration and data fusion still is missing. For many diseases, and in particular for COPD, we need multi-functional, economic, and user-friendly wearables that are feasible in daily routine of clinical practice to identify individual’s health status and its changes. Such devices should not be limited to a specific group of individuals but applicable to anyone and anywhere [
42]. Nonetheless, previous works on wearable devices and technological platforms for the monitoring of COPD patients are either focused on physical activities, or physiology and vital signs, but only a few approaches actually combine several domains. Long-term physical activities are considered most, as body positioning and mobility indicate the health status [
15]. Therefore, several works are published in this area, mainly utilizing accelerometers and gyroscopes [
43,
44].
Contrarily, Kollmann et al. developed an ear-worn prototype of pulse oximeter. The prototype is designed for low-cost, convenient, and long-term continuous monitoring. Technological, it is based on a vertical cavity surface emitting laser and shaped in style of hearing aids [
45].
In “VTAMN” four electrodes for ECG and two for skin temperature measurement are combined with accelerometer and gyroscope for fall detection. Events are either launched manually by the patient or generated automatically from remote signal analytic for fall, hyper- and hypothermia, and tachycardia detection [
46].
“SweetAge” is a distributed system that monitors heart rate, skin and near-body temperatures, and physical activity by a wrist band, which is connected via Bluetooth to a smartphone. A commercial pulse-oximeter is coupled as second component. The phone operates as gateway receiving data from the wearable sensors and transmitting it to the monitoring system [
38].
Likewise, “HealthWear” is based on wearable garment with several textile-embedded biomedical sensors including 6-lead ECG, pulse rate, and respiratory movements and rate. Data is transmitted to a so-called portable patient unit (PPU). The PPU further connects external sensors such as an oximeter. All data is transmitted via the general packet radio service (GPRS) to a centralized server [
47].
In Reference [
48], motion activities, heart rate, and breathing rate are measured through a combination of wearable devices for vital signs and a camera for motion tracking and gesture detection. Heart rate and respiratory-induced intensity variation (RIIV) are extracted from a photo-plethysmographic headset. Data are transmitted via BLE to a personal computer (PC).
Bellos et al. introduced “CHRONIOUS” as an integrated platform composed of several wearable devices. Data are obtained from several wearable sensors, fused, and transmitted to a server as decision support system (DSS). The smart T-shirt integrates 3-lead ECG, a microphone as context-audio sensor, a pulse oximeter, two respiration bands (thoracic and abdominal), an accelerometer, and sensors to measure humidity, body and ambient temperature. Additionally, external sensors capture body weight, blood pressure, respiratory volume, and glucose level [
49].
The “WELCOME” platform is proposed by Chouvarda et al. as a bi-directional approach between the COPD patient and a care center. The platform is composed of three modules named patient hub, cloud, and application. The platform measures parameters in the domains of heart failure, diabetes, anxiety, and lifestyle issues. The patient hub interacts with the patient via a light vest-jacket including in total 26 non-invasive sensors for:
, electrical impedance tomography (EIT), chest sound, multi-lead ECG, and activity tracking. The cloud implements storage server, feature extraction, and DSS [
50].
In summary, comprehensive and efficient monitoring of COPD patients requires simultaneous assessment of all three domains of influencing factors: environmental, physiological, and behavioral parameters (
Table 2).
However, the state-of-the-art is focused on physiological and behavioral monitoring, or on designing platforms to combine separated devices, which all come with their individual overhead, synchronization, calibration, power supply and data transmission (distributed approach). However, chronic diseases in general and COPD in particular need convenient and integrated measures of environmental parameters such as hazardous and/or toxic gases (e.g., , , ) and other pollutants, as they cause breathing and heart problems; UV radiation exposure, as COPD patients have an increased risk of skin cancer; and ambient sound level, as this impacts sleep quality and causes sleep disorders. Furthermore, conditions and priorities of COPD patients demand centralized data collection and processing with ubiquitous devices.
Our previous work was concentrated on the design of wrist-worn hardware for monitoring environmental parameters. Hardware design, sensor calibration and fusion, power consumption, and data logging in internal memory while BLE disconnections have been discussed in Reference [
51]. In this paper, we aim to design and implement a low-cost and convenient prototype for simultaneously monitoring of environmental, behavioral, and physiological parameters. As a focus of this work and an important aspect of wearables, we prolong the monitoring through a careful and seamless hardware design and software development. The work concentrates on technical development and its features rather than its clinical application to improve the flexibility and applicability of the prototype by enhancing the configurability and improving the wearability via downsizing the design. Therefore, we:
extend environmental and behavioral to physiological monitoring of skin temperature and heart rate,
re-design the hardware to downsize the prototype and significantly improve its wearability,
add a sensor layer that sticks at the bottom of the prototype directly touching the user’s wrist,
improve the signal quality of gas sensors by additional voltage reference,
remove the sound pressure level module, design, and integrate the respective electronics circuit,
eliminate mismatching impedance via a gain unity buffer, and
add a UV sensor to complete the requirements for COPD patients and safety workplaces monitoring.
To the best of our knowledge, we are the first to contribute a wrist-worn prototype that integrates all three (environmental, physiological, and behavioral) domains of health monitoring for COPD patients and industrial workers. Therefore, our concept and prototypical realization of an all-in-one and wrist-worn device may significantly contribute to personalized healthcare monitoring.
2. Materials and Methods
The basic idea behind this work is to design a ubiquitous, cost effective, convenient, and easy to use prototype as an all-in-one approach to support people identifying their health status as well as to predict and prevent adverse events. Hence, we design our prototype according to four principle pillars. Comprehensive, Convenience, Continuous, and Cost-effective (4C) are taken into consideration as the principles. We provide the strict definitions for each component of this term as: Comprehensive—indicates multi-parameters monitoring and the combination of the effective areas in personalized healthcare [
52]. Convenience—discusses and addresses the mode of wearability including the size and weight of the prototype [
53]. Continuous—refers to prolonged monitoring which is a serious concern in wearable monitoring [
40]. Cost-effective—targets the final cost of the prototype which is a concern. The personalized healthcare becomes more and more important and popular and wearables as the main means are receiving more attention. Thus to encourage the users to use them, these devices must be affordable [
54].
The fundamental concept is to construct the hardware in
z-direction (3D-MPL approach). Each task and parameter is implemented through mounting the sensor/actuator (or number of sensors from the same domain) on the same physical layer. These layers are located one on top of another through a board-to-board connector. Therefore,
-plane remains unchanged (in contrast to other prototypes/products) but the height of the prototype is expanded slightly—but still appropriate for a wrist-worn device. The connectors provide signaling between layers and the main platform. The measurements are displayed on the prototype and transmitted to the smartphone in real-time. The smartphone is used as mid-term storage and gateway to a server for permanent storage and data analysis. We support the prototype configuration in BLE connected mode by sensor activation/deactivation. Thus, we develop a smartphone application for bidirectional data communication between the prototype and the smartphone. User activates the intended sensor by pressing the respective button on the application and the data are measured, processed, and transmitted to the smartphone. Upon the pressing the button, a string including the sensor’s name is transmitted from the smartphone which is handled on the prototype. This string is used as a condition for sensor activation. We apply centralized data processing and a strict data protection policy. All sensors are connected and communicate to microcontroller via Inter-Integrated Circuit (IIC), Analogue to Digital Converter (ADC), or a combination of both interfaces. Behavioral sensors, air condition sensors, and skin temperature are supported with IIC, PPG sensor, loudness, and UV are supported with ADC, and gas sensors are taking the combination of both for initialization and configuration. The data from all sensors are pre-processed and processed on the prototype prior than transmission to the smartphone. This includes filtering the noise, pattern creation, and data conversion to the target data unit. All data are either transmitted directly via BLE or stored in the integrated memory, if BLE connection temporarily is unavailable (see
Figure 1 and Algorithm 1).
| Algorithm 1: Operational Strategy of the Device |
![Applsci 10 05470 i001 Applsci 10 05470 i001]() |
2.1. Concept and Architecture
Hardware: eleven sensors measure 15 parameters from three domains: (i) ambient parameters (toxic/hazardous gases (e.g., , ), UV, sound level, air pressure, air temperature, and air humidity), (ii) vital signs (skin temperature, heart rate), and (iii) motion tracking (raw data from gyroscope, magnetometer, and accelerometer) in nine degrees of freedom (DoF). Our prototype follows the common approaches for behavioral data using accelerometer, magnetometer, and gyroscopes and hence is not particularly tested. The hardware is modular and each sensor’s functionality is independent. We present an innovative three-dimensional (3D) multi-physical-layer (MPL) approach.
2.2. Hardware Development and Layer Description
We distribute 11 sensors on 5 physical layers. A detailed description of sensors, companies, and technical specifications is given in
Table 3.
2.2.1. Layer 1: Gas Sensor
The gas sensor (Spec, CA, USA) is placed on the very top for free exposure to pollutants. It is small (
mm) and has high resolution (0.1 PPM), low response time (between 1 and 15 s), low cost (20€), fast recovery time, ideal cross sensitivity, and low power consumption. Gas sensors can be easily replaced by the 3D-MPL design. In fact, we build a multi-gas sensor, but always only one particular detector is operated (
Figure 2).
2.2.2. Layer 2: Gas Sensor Driver
We design the driver in a universal configuration mode, which is compatible with wide range of hazardous/toxic gas sensors (2–3 lead gas sensors). The driver can be used for both, oxidizing and reducing gas sensors (
Figure 2).
It includes a 24-pin board-to-board connector, a gain adjuster, a front-end amplifier (LMP91000, Texas Instruments, Dallas, TX, USA), and a signal filter. To improve signal quality during calibration, the voltage reference is strictly held. Thus, in addition to the microcontroller voltage reference, we use a voltage reference chip (Intersil ISL60002DIH320Z-TK). We implement a low-pass filter to eliminate ripples. To tackle the possible mismatch impedance between the output signal of the gas sensor and the ADC micro-controller, a gain unity buffer (MicroChip MCP6041T-I/OT) is used (
Figure 2).
2.2.3. Layer 3: Hardware Interface
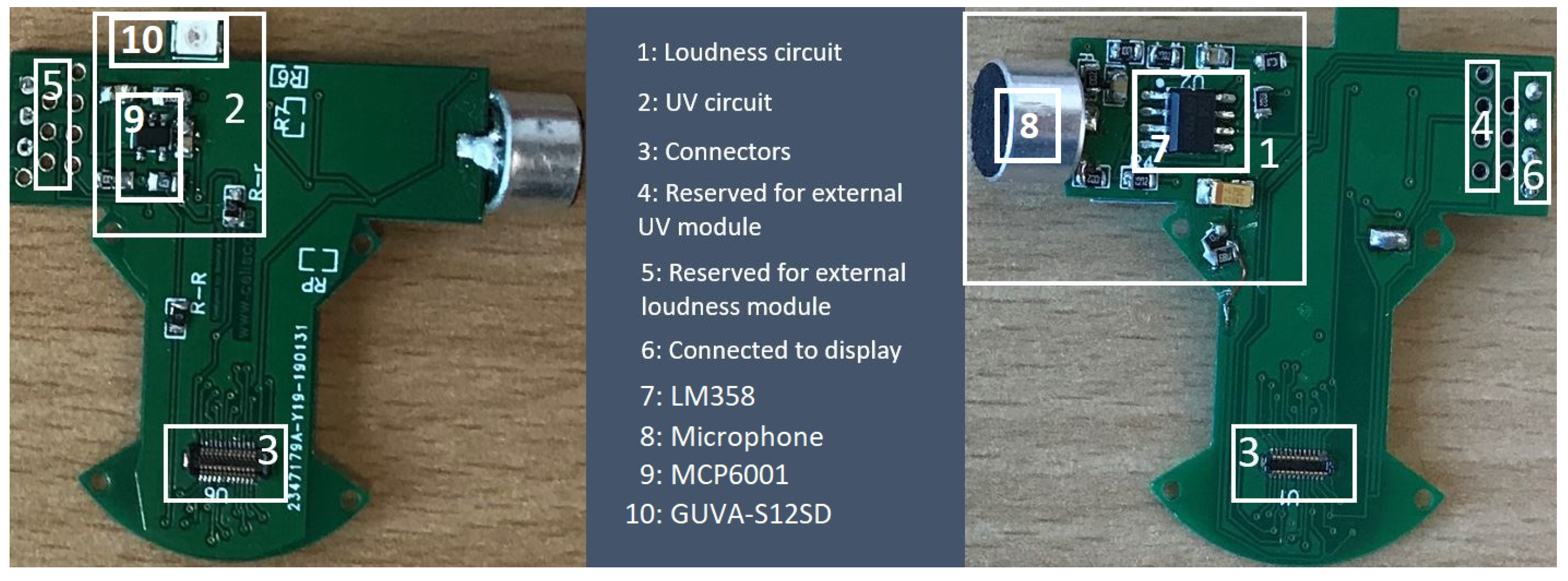
The physical layers, sensors, and the display are connected to this layer from three sides. It interconnects the gas layers (Layers 1 & 2) to main platform (Layer 4). Furthermore, it hosts the microphone and UV sensor. We designed and implemented sound (LM358) and UV circuits (GUAV-S12SD) to fit the board (
Figure 3).
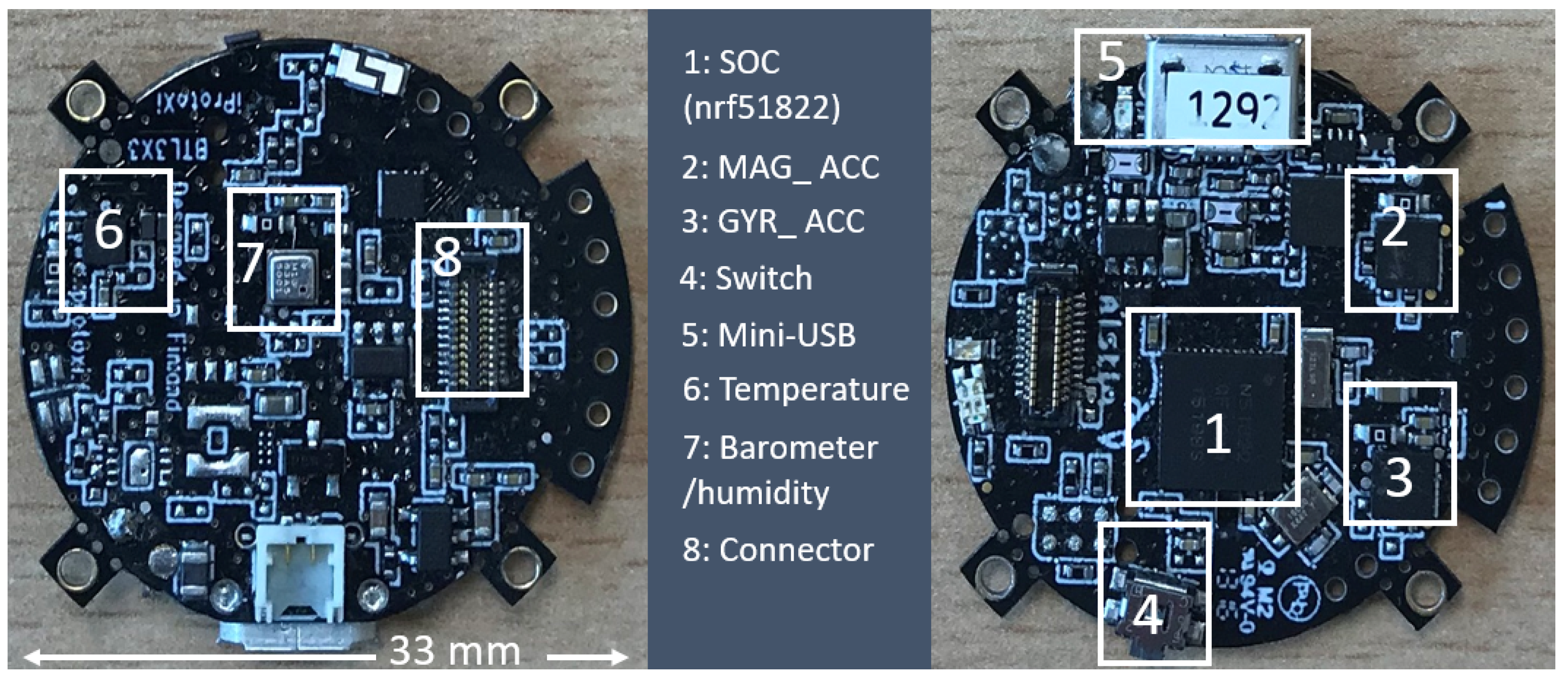
2.2.4. Layer 4: Host Platform
This layer performs data collection, processing, calibration, conversion, and transmission. We selected a
-based microcontroller (
, iProtoxi, Oulu, Finland). The circular platform (33 mm in diameter) is enriched with accelerometer, gyroscope, and magnetometer for motion tracking, air temperature, pressure, and humidity sensors, as well as internal memory. The sensors and memory components utilize the IIC bus with different sampling rates (
Figure 4). We also apply the ambient physical parameters (air pressure, air temperature, and air humidity) for gas sensor calibration.
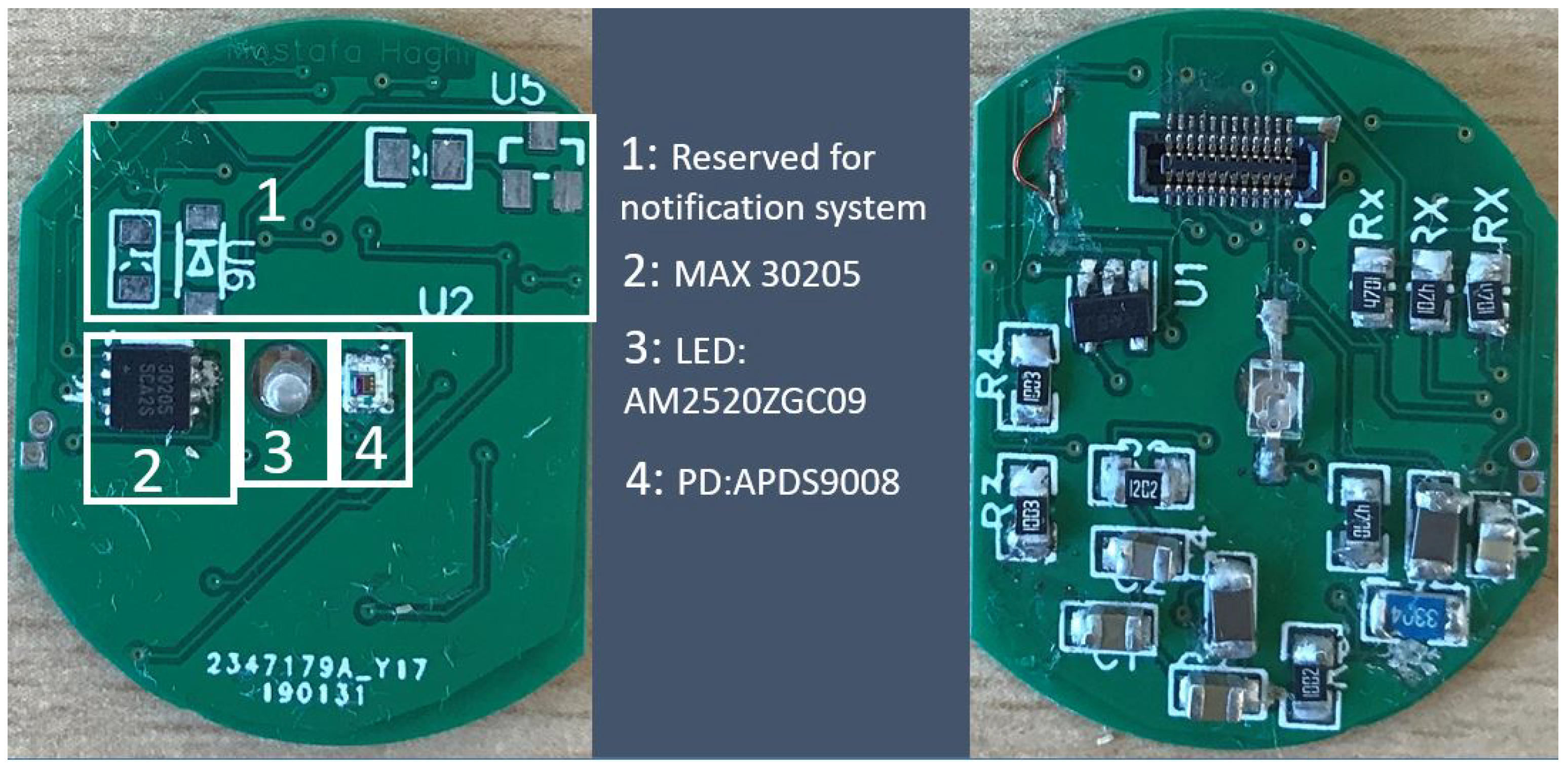
2.2.5. Layer 5: Physiological Sensors
This layer holds the biomedical sensors. We locate the layer at the very bottom in direct contact to the user’s skin. Skin temperature (MAX30205) and Photoplethysmography (PPG) are measured by the sensors mounted on this layer (
Figure 5). It connects the bottom of the host platform and communicates with the microcontroller via the IIC bus at a 15 s time interval. We detect the PPG by optical sensing. The PPG sensor consists of light emitting diode (AM2520ZGC09) and photo diode (APDS9008). Currently, we process the PPG signal to analyse and determine the heart rate only but further parameter extraction (e.g., breathing rate) is planned.
After the detail description of layers of 1 to 5 and also with respect to general strategy of operation described in
Figure 1 and Algorithm 1, the assembled version of the prototype is seen in
Figure 6.
2.3. Software Architecture and Bidirectional Communication
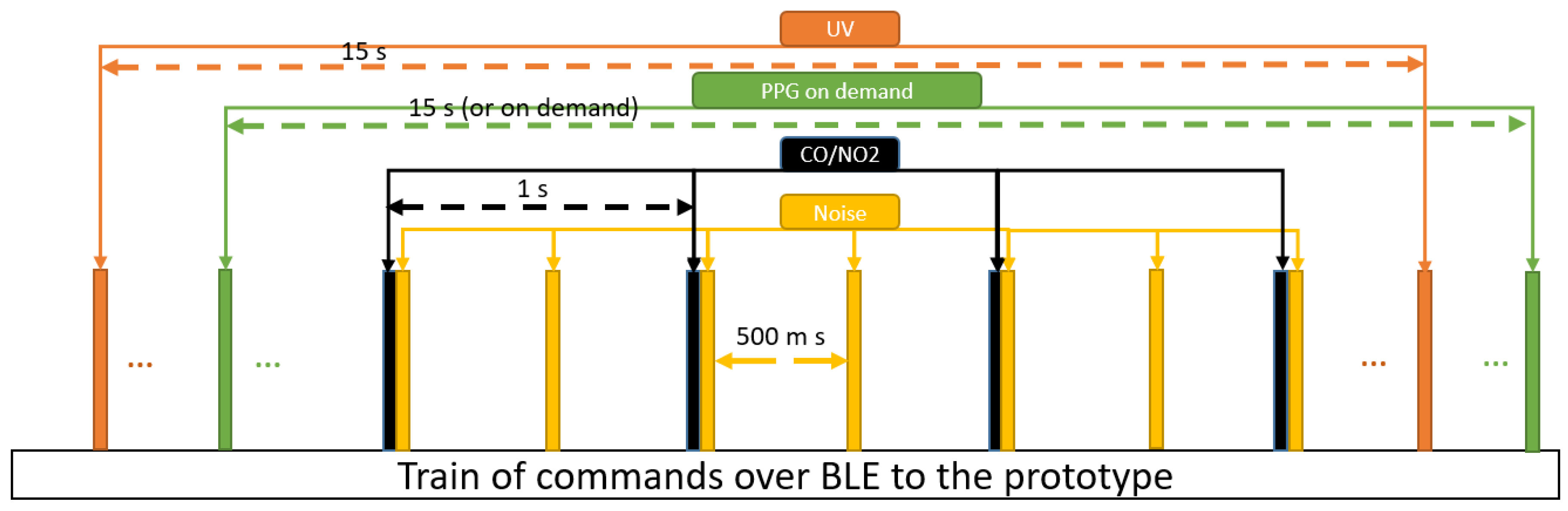
Software: the prototype is flexible. Smartphone’s commands are sent via BLE to activate/deactivate the sensor nodes (
Figure 7). Such comprehensive wearable with sensor activation on demand is the first-of-its-kind. Implementing an efficient software is the second part of our work. Therefore, we have developed an interactive application. The application has three major parts: parameters and values- data-, interactive buttons to activates the sensors, and the status bar which indicate the status of sensors whether are activated (1) or deactivated (0). Re-pressing an interactive button changes the status (see
Figure 8). The application has been developed in Visual studio with C# programming languages. The software shall enable synchronization of data for further analysis and save power consumption. Therefore, communication of device and smartphone is implemented in seven packets:
F1: The motion tracking parameters includes gyroscope, magnetometer, and accelerometer. Each of this parameter is three dimensional and thus, nine values are transmitted. Packet F1 starts with one identifying byte followed by the sequence number (one byte). Each dimension of parameter is coded with 12 bits and packets are separated with another 4 bits. Therefore, the total length of F1 is 17 bytes.
F2: Air pressure, temperature, and humidity also are merged in one packet. Again, the first byte is an identifier followed by the sequence number and total of 6 bytes of data (two bytes for each parameter). Hence, 8 bytes per second are transmitted.
F3 – F7: These packets contain the identifier, sequence number, and 2 bytes of data for UV radiation, sound level, pollutants, skin temperature and heart rate, respectively. The total of 4 bytes is transmitted for each.
Sensors as well as data communication consume energy. For instance, the PPG uses an optical sensor that requires high energy. In contrast to other wearable devices, we suggest bidirectional communication to activate/deactivate particular sensors through commands from the smartphone. In our implementation, the user presses buttons to send these commands, but task- or measurement-depending automatic commands can easily be implemented, too. This feature supports an anytime-anywhere-anything-anyone concept in wearable devices. In the manual setting, the wearer can decide at what time and in which location what type of data is collected. In our prototype, we use it for gas, sound level, UV light, and PPG (PPG implementation is tested in debugging mode). This concept targets the applicability of the prototype and the monitoring status. We briefly describe, how the developed software application, the feature, and prototype are acting as the complementary items to support this concept.
Anytime: the smartphone application as an interactive and flexible feature can activate/deactivate the sensors at any time, when the user is willing, by pressing the button.
Anywhere: the compact structure of the prototype with a diameter of 33 mm and a height of 20 mm, is very convenient to be worn at anywhere without user daily routine interference.
Anything: covering a large effective range of parameters from major areas of interest in healthcare, broadband the range of application of this prototype. However, this feature needs to be further developed and extended.
Anyone: Combination of all the previous three features that supports convenient, comprehensive plus cost-effective and continuous monitoring device make the prototype applicable by users from different categories.
Due to the high accuracy requirements of the data in motion tracking, no sensor activation is applied for gyroscope, accelerometer, and magnetometer. Here, the data are transmitted to the smartphone, immediately after BLE establishes the channel. As the air pressure, temperature, and humidity values are used for calibration, their data are also transmitted continuously without sensor activation/deactivation. Furthermore, we strictly aim at protecting measured data. We integrated memory (256 kB) to buffer data when BLE is temporarily unavailable. Due to the limited capacity of the memory, it is used only if necessary. A specific scenario for retrieving data from the internal memory has been implemented, too. So far, the transmission of buffered data requires a user decision. Once the user has pressed the “Data log” button on the smartphone, data are transmitted in a first-in-first-out (FIFO) mode as they have been stored. The last packet of stored data is followed with an empty packet that indicates the re-start of real-time data acquisition (Algorithm 1).
2.4. Experimental Conditions and Setup
We tested the functionality of the all layers and prototype comprehensively, indoors as well as outdoors. The test lasts from several minutes to a few hours up to days (30 h is the longest after battery charging). In general, six prototypes have been tested by five volunteers including three males and two females aged 26 to 48 years old. Users have been from different occupations ranging from ordinary workers with no technical information on the functionalities of the prototype to scientists in the fields. In every experiment, the user has been asked to wear the prototype on the top side of left-hand with outside-oriented microphone, in a good skin contact and comfortable. Users have been recommended to wear the prototype in a fix location on the left-hand to avoid moving the prototype. Besides, we have strongly asked the subjects to avoid covering the surface and microphone of the prototype. All users have been trained to only switch on/off the prototype, pair BLE, and configure the prototype with the application. Additionally, each user has been asked to avoid interruption of the data transmission unless otherwise necessary. At the end of each experiment, user has reported the technical problems and data interruption, if any. Each user also submitted the written protocol, specifying the activities and switching time between, if applicable. In each test, the prototype was BLE-paired with a smartphone, which starts the bidirectional data transmission. If activated, the sensors have sampling rates of sound level (2 Hz), gas (1 Hz), UV radiation (15 s time interval), air temperature (1 Hz), air humidity (1 Hz), air pressure (1 Hz), skin temperature (15 s time interval), PPG (15 s time interval), gyroscope (50 Hz), magnetometer (50 Hz), and accelerometer (50 Hz). During the tests, the prototype measured a surrounding sound level of 32 to 85 dB, with the resolution of 1 dB.
and
are limited to 52 and 1680 ppm with the resolution of 1 and 5 ppm, respectively. This specification for UV index is restricted up to 9 with the resolution of 1. For all tests, we extracted the presented data from the smartphone in Comma-Separated Value (CSV) format. The sampling rates, specified above are constant and have been applied in every experiment. We have not imposed any specific conditions/restrictions to users, unless otherwise it is clearly stated. Hereafter, we will support the functionaries of the prototype with two types of tests: from
Section 3.1,
Section 3.2,
Section 3.3,
Section 3.4 and
Section 3.5, the experimental tests are focused on the detail description of the tests for each sensor and the respective parameters. In these sections, the general conditions and specifications mentioned in
Section 2.4 are applicable. However, if additional conditions have been available due to the nature of experiment(s), will be reported in the that particular subsection. We support the comprehensive functionaries of the prototype, data synchronization, accurate time stamp, and labeling the activities in
Section 3.6.
4. Discussion
There is large agreement that the safety monitoring of workplaces and COPD requires the monitoring of environmental, behavioral, and physiological parameter [
68,
69]. However, the majority of scientific work in mobile health and safety monitoring is limited to one at most two of these domain [
2,
46]. Furthermore, the authors still use decentralized data collection and processing systems [
47,
49]. Usually, they integrate sensors into wearables and combine with commercial devices (mostly for vital signs) to enhance the range of monitored parameters [
30,
50]. In addition, many existing platforms offer data collection with several single-parameter devices integrated into a wireless body area network (WBAN) [
70], and in some cases (unavoidable) stationary medical equipment [
71]. However, there are common concerns regarding the practical application of these platforms in terms of convenience, economic, reliability, long-term usage, user-friendly, pervasive monitoring, and general performance [
72].
The wrist-worn prototype proposed in this work introduces an innovative hardware solution with the 3D MPL approach. We intend to provide COPD patients an efficient companion for multi-parameter monitoring and real-time data transmission. However, patients can use our prototype also within sensor platforms [
73,
74]. Nevertheless, with respect to the convenient wearability of the prototype, it can be applied for safety monitoring of workers in construction sites without interfering in the daily working routine and reducing the maneuverability.
In comparison with the state-of-the-art in safety [
2,
30] and COPD monitoring devices [
49,
50], the proposed device improves wearability and increases the dimensions of monitoring (
Table 9). Indeed, for the first time, we consider environmental monitoring and combine it with behavioral and physiological domains, all embedded into a lightweight wrist-worn device. Despite work that focuses on monitoring via distributed approaches and WBAN [
29,
50] or integration of commercial devices [
30,
49], we concentrate on standalone and independent monitoring with central data processing. The design supports convenient (wrist-worn) monitoring without imposing difficulties from weight [
46], wire based communication [
49], and interfering the users routine [
50]. Moreover, our prototype supports sensor (de-) activation. This feature allows patient (user)-specific customization, for example, turning from single to multiple hazardous gas monitoring. Thus, there is bi-directional interaction of smartphone (as the gateway) and the device. In the long term, medical caregivers will configure the device.
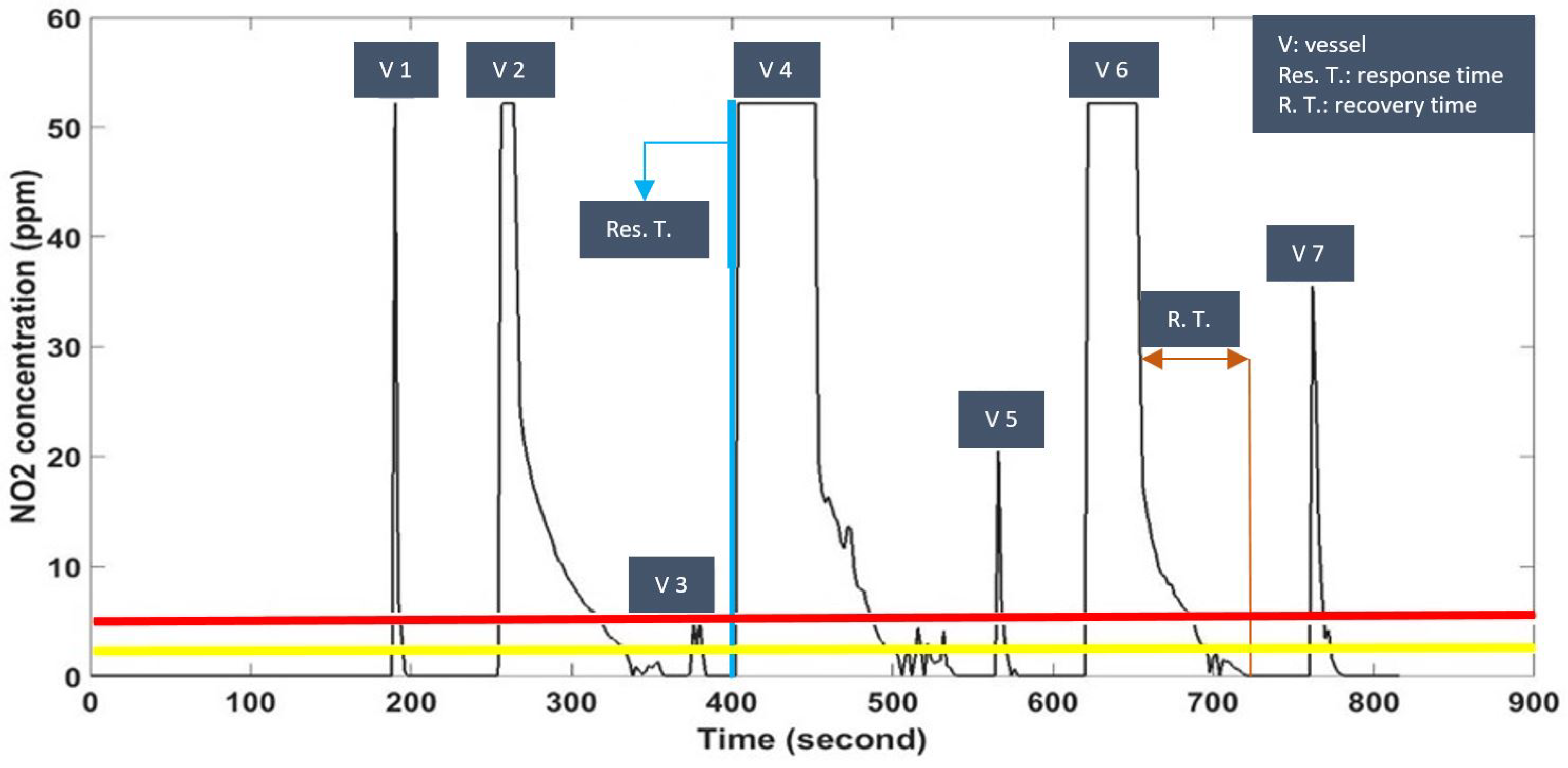
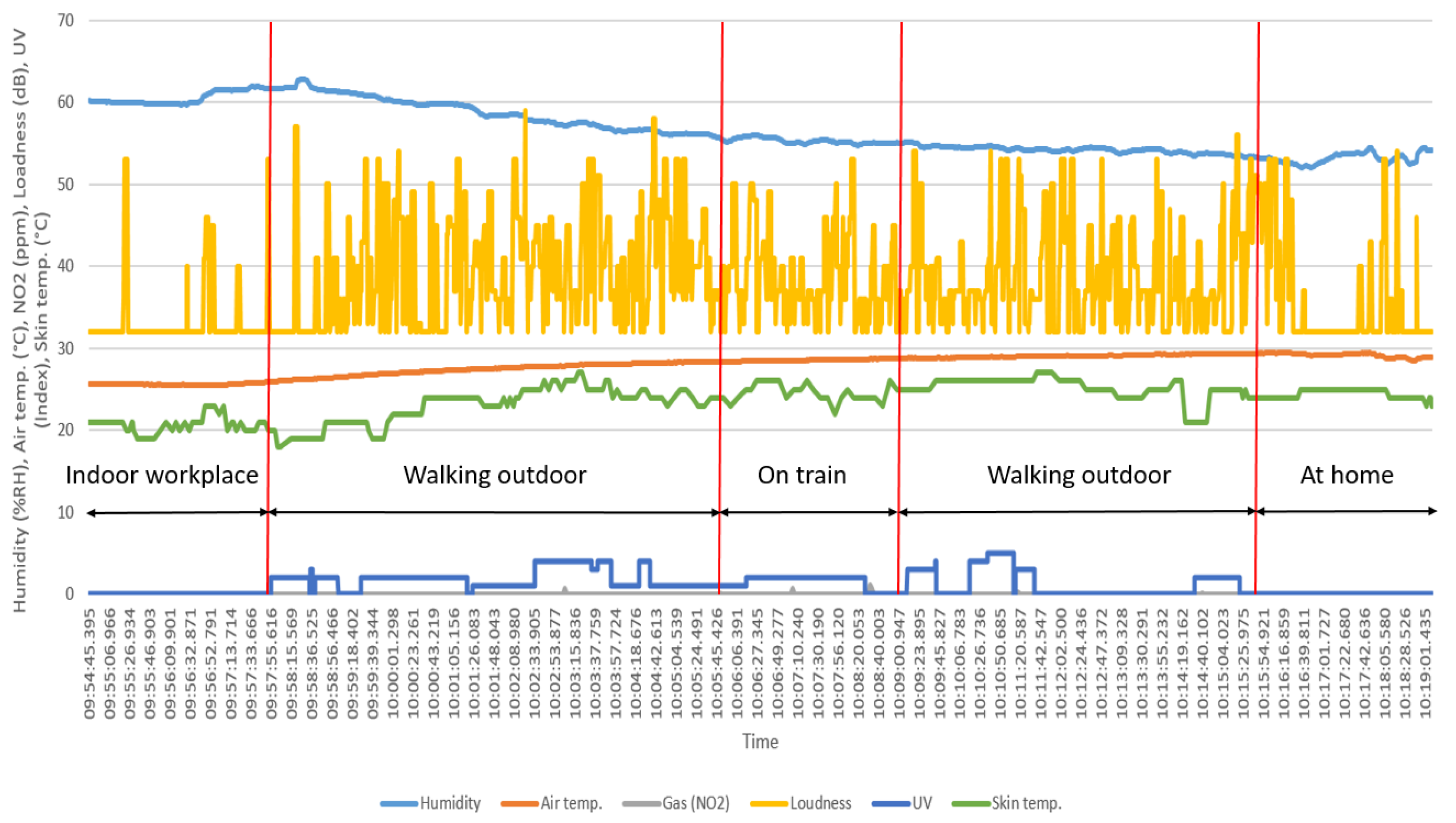
We briefly discuss the interpretation of the results and experimental tests. The output results of
Section 3.1 shows a large range of loudness swinging. The respective sensor was set to high sensitivity to detect any changes (record the peaks) but taking the average changes into consideration and comparing with the delivered protocol, indicate the mode of transportation changes. Performing this experiment in a coastal city and large difference in humidity and temperature within a limited period of test is considered as the travel direction (e.g., toward sthe coast or in opposite direction) and the fine performance of the prototype. The specified resolution, response time, and recovery time of the prototype with the respect to air pollutants, depend on concentration of the exposed target gases. Higher concentration of pollutant and shorter distance to the source, reduce the response time and consequently increased the recovery time. As a result, the visualized saturation state of
Figure 10 exhibits the concentrations beyond the boundary of the prototype. The results of skin temperature as a physiological parameter are influenced by external players. Environmental parameters (e.g., air temperature) and the mode and location of wearability affect the measured output. Comparing the recorded skin temperature with the written protocol during the tests, indicates the effect of air temperature on the output. Swinging the data under the constant environmental conditions can be due to movement of the prototype caused by a loose attachment.
However, there are limitations of the device. The boundary measurement of
,
, UV index, and loudness are 0 to 1680 ppm, 0 to 52.8 ppm, 0 to 9, and 32 to 85 dB, respectively. The resolution is 5 ppm, 0.1 ppm, 1, and 1 dB, respectively. It is also worth mention that the multi-gas monitoring works only in BLE-paired status. In contrast to existing approaches (
Table 9), our device supports internal data storage in BLE-disconnected mode for approximately 10 h. So far, we recommend the immediate data transmission of the logged data when BLE is resumed, which will be performed automatically in future versions of the device software. While conducting the experimental tests, we observed dependencies of PPG, skin temperature, and UV measurements regarding the wearing location, tight or loose mounting, and relative angle of sensors. Furthermore, external material must not cover the device. Hence, the user must make sure that the physiological layer is appropriately located and tight on the wrist. The prototype is required to be outside-oriented, as the used microphone is omni-directional. Also, more comprehensive evaluations and field test are required before the device can be confirmed in clinical trials.
In future, if a user is willing to extend his/her health monitoring conditions and already has an integrated diagnostic system in the private spaces, our prototype can be readily integrated to smart-homes [
75] and smart-cars [
76] for data correlation, expanding the application usage, and improving the reliability of collected data. Assuming stable communication between device and smart environments, the off-line time can be extended significantly when the device is worn in such environments.
Furthermore, medical application is not limited to COPD. The acute respiratory distress syndrome (ARDS), for instance, also needs monitoring of environmental, behavioral, and physiological parameters [
77], as ARDS patients are encountering an often severe shortness of breath, have a cough, and may have fever [
78]. Furthermore, ARDS patients have difficulties when inhaling and exhaling, and thus a chest-worn device is infeasible [
79]. Most people with ARDS stay in bed, many in critical states [
80]. Therefore, embedding our device in smart environments will further broadband the monitoring and link vital signs to non-medical data (e.g., environmental and behavioral) for status assessment and early detection of abnormalities. In future studies we plan to shift the concentration from testing the functionality to improve the accuracy of measured data. Thus we will compare the data measured with the developed prototype with those measured with gold standard instruments to enhance the performance in terms of accuracy and uncertainty. For the next version of the prototype, we plan to implement a notification system to alert abnormalities detected from domain-fused sensor data. Currently, we have reserved the required circuit on Layer 5. Furthermore, we aim at driving research towards self-powered devices and consider a hybrid power-harvesting scenario. With a more comprehensive evaluation, we also plan to use the prototype for COPD patients in the clinical setting.