Preparation and Electrochemical Properties of Functionalized Multi-Walled Carbon Nanotubes @ Carbon Quantum Dots @ Polyaniline Ternary Composite Electrode Materials
Abstract
1. Introduction
2. Experimental
2.1. Materials and Preparation Method
2.2. Characterization
3. Results and Discussion
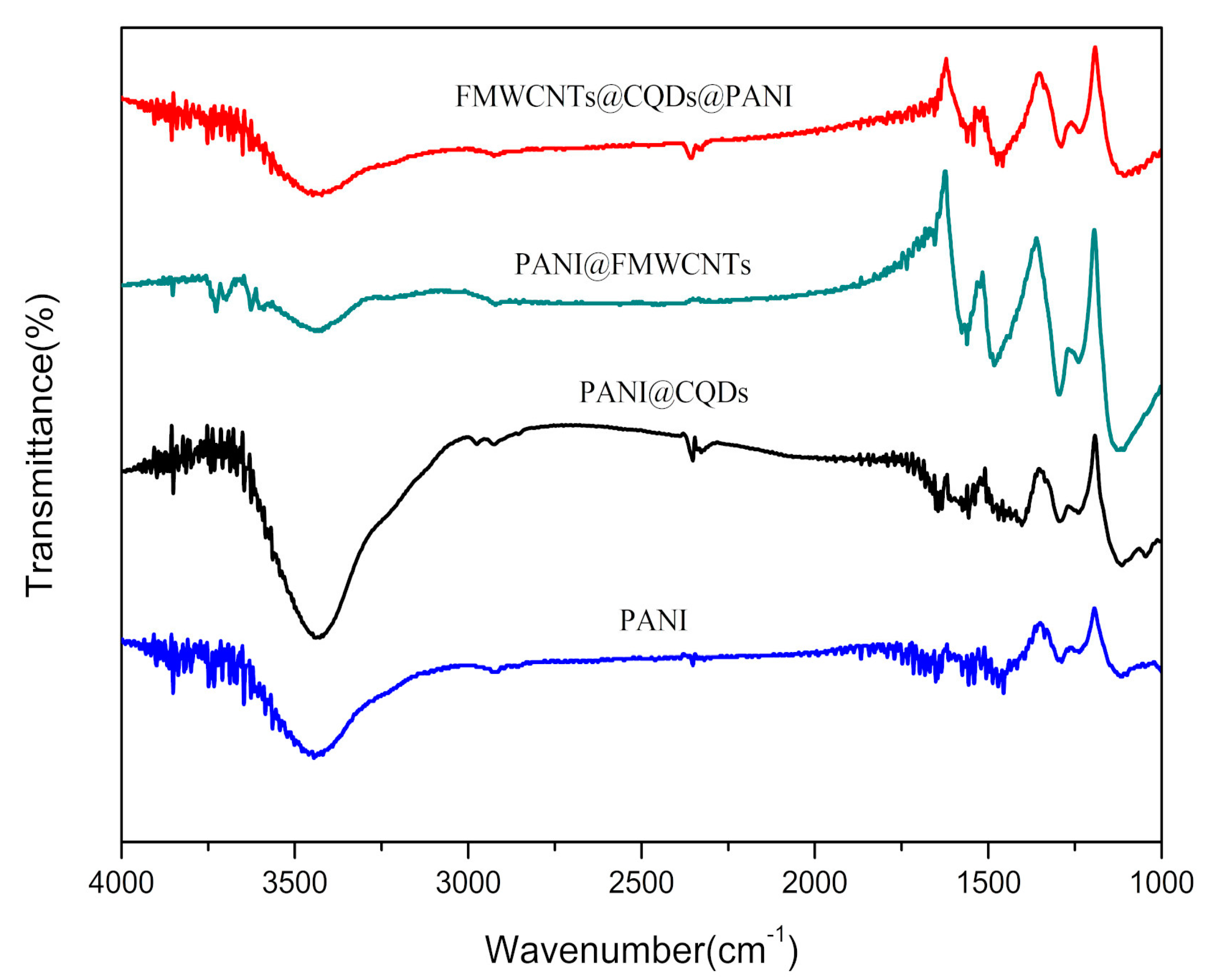
3.1. FTIR Analysis
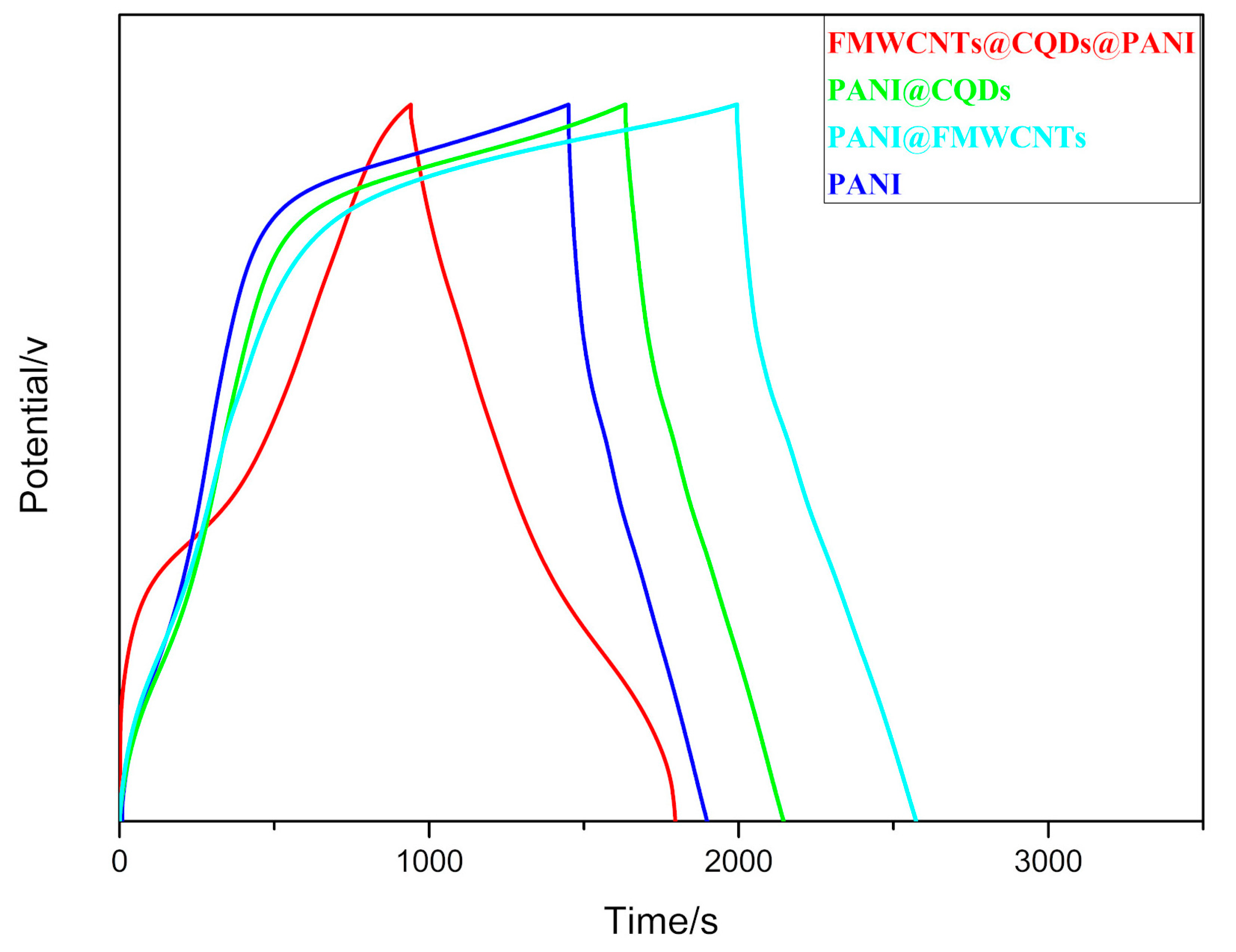
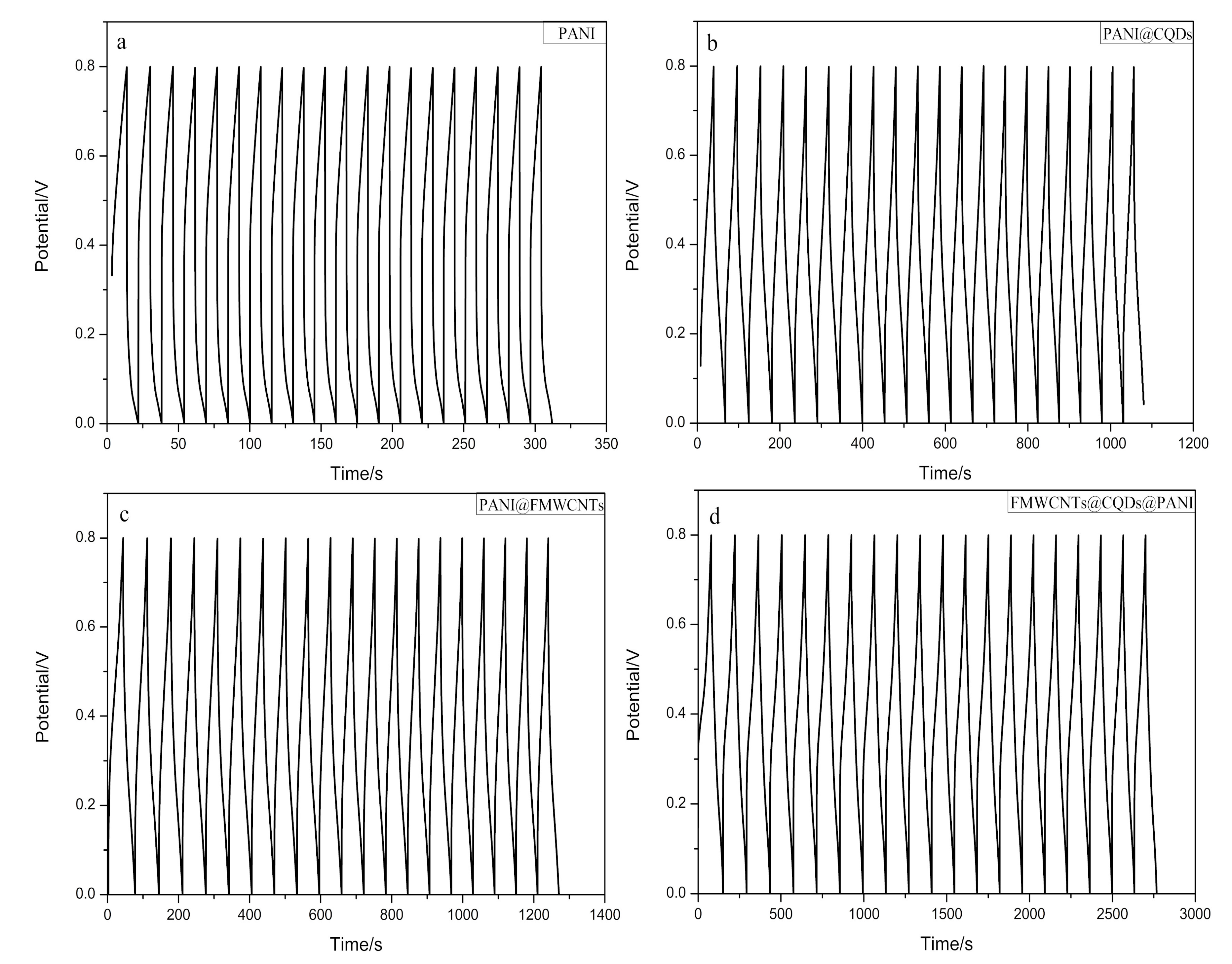
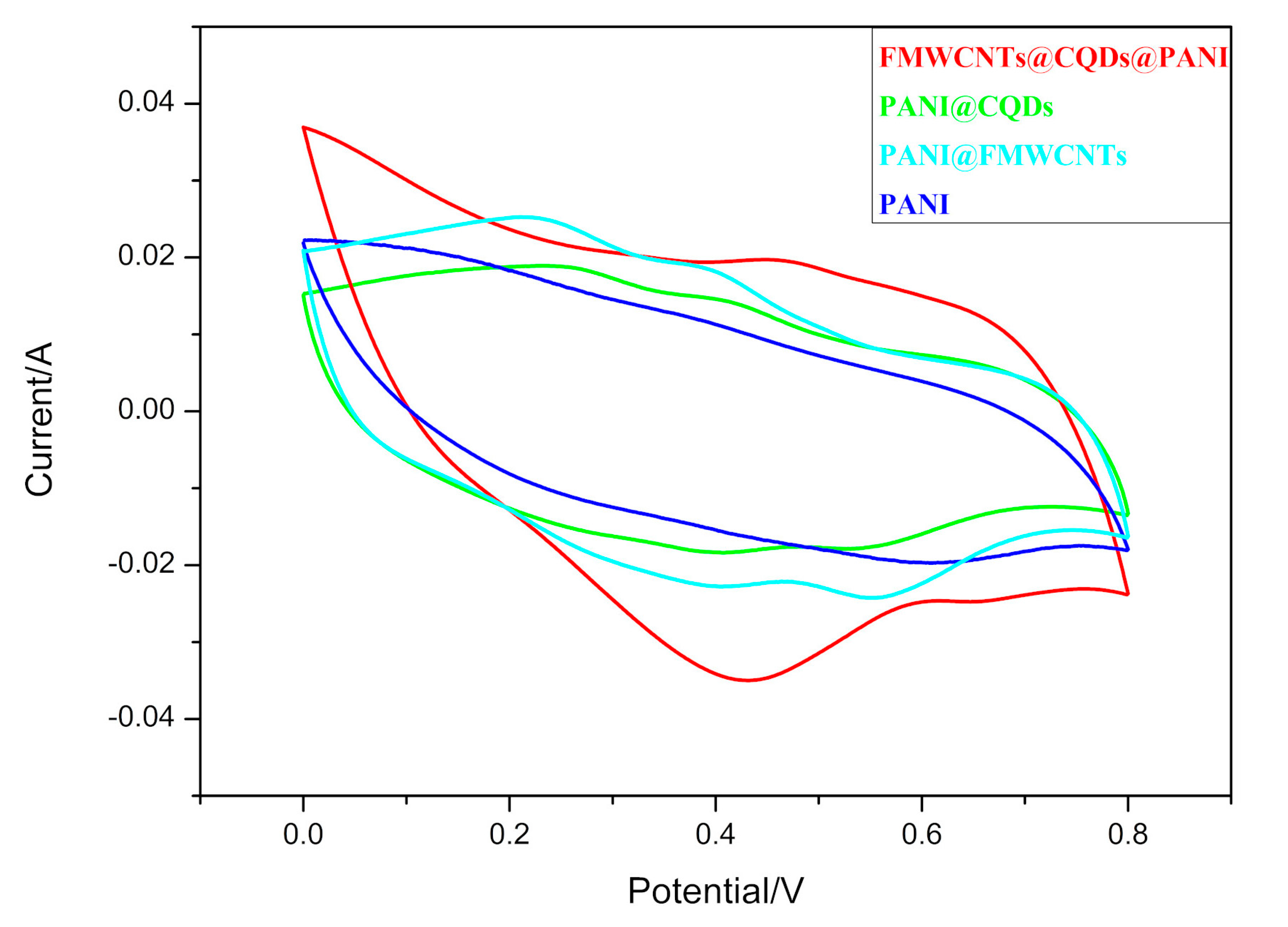
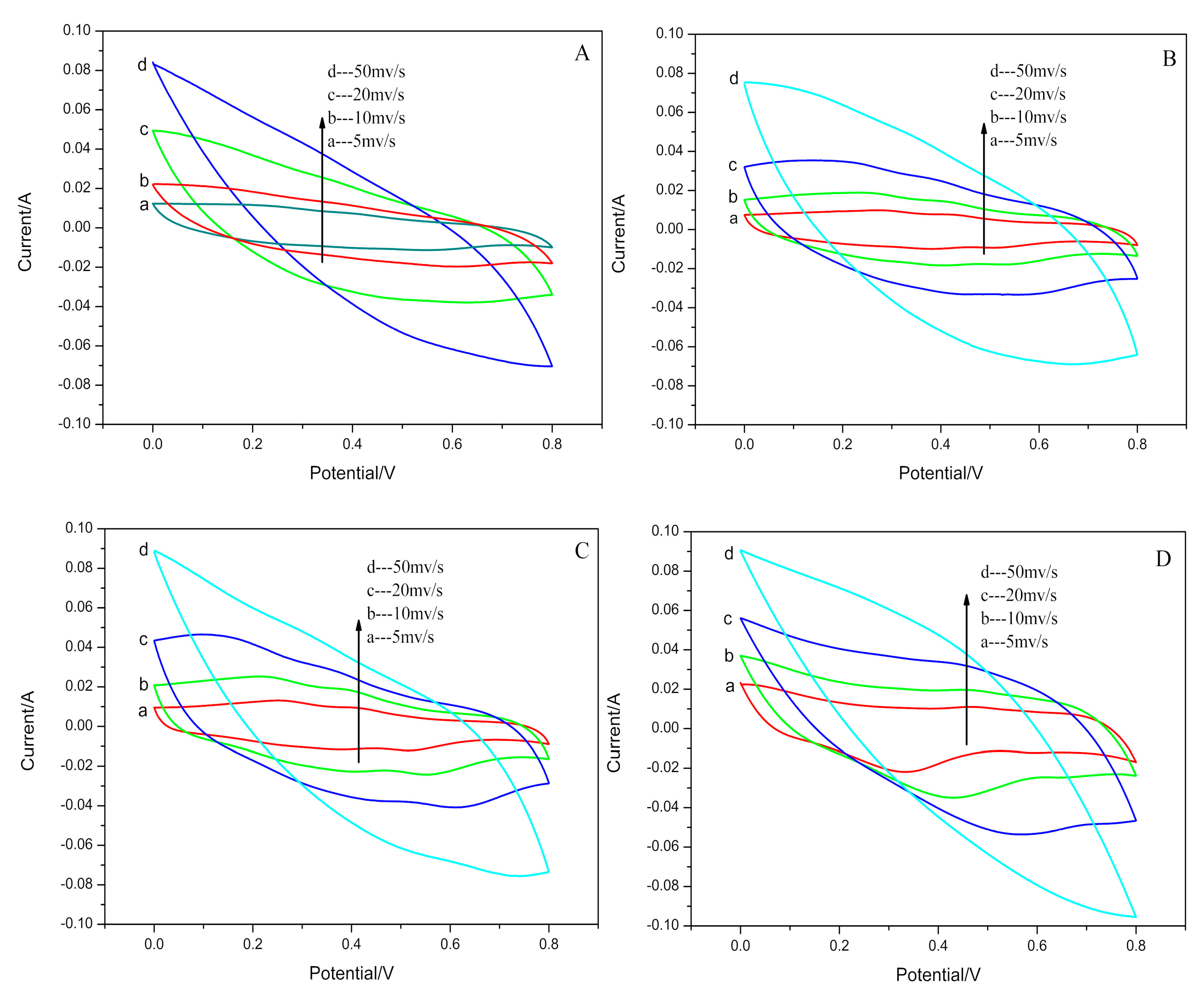
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, F.; Jamal, R.; Ubul, A.; Shao, W.; Abdiryim, T. Characterization and electrochemical properties of poly(aniline-co-o-methoxyaniline)/multi-walled carbon nanotubes composites synthesized by solid-state method. Fibers Polym. 2013, 14, 8–15. [Google Scholar] [CrossRef]
- Dyachkova, T.P.; Anosova, I.V.; Tkachev, A.G.; Chapaksov, N.A. Synthesis of composites from functionalized carbon nanotubes and polyaniline. Inorg. Mater. Appl. Res. 2018, 9, 305–310. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, W.; Huang, W.; Liu, B.; Lin, H.; Dong, C. Carbon nanotube-graphene hybrid electrodes with enhanced thermo-electrochemical cell properties. Nanomaterials 2019, 9, 1450. [Google Scholar] [CrossRef]
- Abalyaeva, V.V.; Vershinin, N.N.; Shul’ga, Y.M.; Efimov, O.N. The composites of polyaniline with multiwall carbon nanotubes: Preparation, electrochemical properties, and conductivity. Russ. J. Electrochem. 2009, 45, 1266–1275. [Google Scholar] [CrossRef]
- Nikzad, L.; Vaezi, M.R.; Yazdani, B. Synthesis of carbon nanotube–Poly aniline nano composite and evaluation of electrochemical properties. Int. J. Mod. Phys. Conf. Ser. 2012, 5, 527–535. [Google Scholar] [CrossRef]
- Agyemang, F.O.; Tomboc, G.M.; Kwofie, S.; Kim, H. Electrospun carbon nanofiber-carbon nanotubes coated polyaniline composites with improved electrochemical properties for supercapacitors. Electrochim. Acta 2018, 259, 1110–1119. [Google Scholar] [CrossRef]
- Grodzka, E.; Pieta, P.; Dłużewski, P.; Kutner, W.; Winkler, K. Formation and electrochemical properties of composites of the C60–Pd polymer and multi-wall carbon nanotubes. Electrochim. Acta 2009, 54, 5621–5628. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Alshareefn, H.N. A general strategy for the fabrication of high performance microsupercapacitors. Nano Energy 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Chen, H.S.; Drag, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Li, Y.L.; Zheng, Y.Y. Preparation and electrochemical properties of polyaniline/reduced graphene oxide composites. J. Appl. Polym. Sci. 2018, 135, 235–262. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Flexible supercapacitors based on carbon nanotubes. Chin. Chem. Lett. 2018, 29, 571–581. [Google Scholar] [CrossRef]
- Pei, X.Y.; Mo, D.C.; Lyu, S.S.; Zhang, J.H.; Fu, Y.X. Synthesis of MnCO(3)/Multiwalled carbon nanotube composite as anode material for lithium-ion batteries. J. Nanosci. Nanotechnol. 2019, 19, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, S.A.; Widiyandari, H.; Subagio, A. Synthesis and characterization carboxyl functionalized Multi-Walled Carbon Nanotubes (MWCNT-COOH) and NH2 functionalized Multi-Walled Carbon Nanotubes (MWCNTNH2). J. Phys. Conf. Ser. 2018, 1025, 12005. [Google Scholar] [CrossRef]
- Aziz, S.A.A.; Mazlan, S.A.; Ismail, N.I.N.; Choi, S.B. Implementation of functionalized multiwall carbon nanotubes on magnetorheological elastomer. J. Mater. Sci. 2018, 53, 10122–10134. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Dou, C.; Ma, G.; Wang, Y.; Feng, N.; Wang, W.; Fang, L. Synthesis and biocompatibility assessment of polyaniline nanomaterials. J. Bioact. Compat. Polym. 2018, 34, 16–24. [Google Scholar] [CrossRef]
- Zhou, H.; Zhi, X.; Zhai, H.-J. A facile approach to improve the electrochemical properties of polyaniline-carbon nanotube composite electrodes for highly flexible solid-state supercapacitors. Int. J. Hydrogen Energy 2018, 43, 18339–18348. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Wang, Y.; Cheng, H.; Zhang, X.; Xu, S.; Liu, H.; Liu, W.; Hu, C. Excellent electrochemical performances of intrinsic polyaniline nanofibers fabricated by electrochemical deposition. J. Wuhan Univ. Technol. Mater Sci. Ed. 2019, 34, 216–222. [Google Scholar] [CrossRef]
- Robertson, J.; Dalton, J.; Wiles, S.; Gizdavic-Nikolaidis, M.; Swift, S. The tuberculocidal activity of polyaniline and functionalised polyanilines. PeerJ 2016, 4, e2795. [Google Scholar] [CrossRef]
- Koluaçik, E.; Karabiberoğlu, Ş.U.; Dursun, Z. Electrochemical determination of serotonin using pre-treated multi-walled carbon nanotube-polyaniline composite electrode. Electroanalysis 2018, 30, 2977–2987. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.-X. Carbon nanotube-polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Bharadiya, P.; Jainm, R.; Chaudhari, V.; Mishra, S. Graphene oxide-wrapped polyaniline nanorods for supercapacitor applications. Polym. Compos. 2019, 40, E1716–E1724. [Google Scholar] [CrossRef]
- Zhou, L.; Qiao, M.; Zhang, L.; Sun, L.; Zhang, Y.; Liu, W. Green and efficient synthesis of carbon quantum dots and their luminescent properties. J. Lumin. 2019, 206, 158–163. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharm. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Ladrón de Guevara, A.; Boscá, A.; Pedrós, J.; Climent-Pascual, E.; De Andrés, A.; Calle, F.; Martínez, J. Reduced graphene oxide/polyaniline electrochemical supercapacitors fabricated by laser. Appl. Surf. Sci. 2019, 467, 691–697. [Google Scholar] [CrossRef]
- Armes, S.P.; AIdissi, M. Potassium iodate oxidation route to polyaniline: An optimization study. Polymer 1991, 32, 2043–2048. [Google Scholar] [CrossRef]
- Feng, W.; Bai, X.D.; Lian, Y.Q.; Liang, J.; Wang, X.G.; Yoshino, K. Well-aligned polyaniline/carbon-nanotube composite films grown by in-situ aniline polymerization. Carbon 2003, 41, 1551–1557. [Google Scholar] [CrossRef]
- Kovalyshyn, Y.; Konovska, M.; Milanese, C.; Saldan, I.; Serkiz, R.; Pereviznyk, O.; Reshetnyak, O.; Kuntyi, O. Electrochemical properties of the composites synthesized from polyaniline and modified mwcnt. Chem. Chem. Technol. 2017, 11, 261–269. [Google Scholar] [CrossRef]
- Mezdour, D. Dielectric properties of polyaniline composites. Spectrosc. Lett. 2017, 50, 214–219. [Google Scholar] [CrossRef]
- Faraji, M.; Mohammadzadeh, A.H. Flexible free-standing polyaniline/graphene/carbon nanotube plastic films with enhanced electrochemical activity for an all-solid-state flexible supercapacitor device. New J. Chem. 2019, 43, 4539–4546. [Google Scholar] [CrossRef]
- Trchová, M.; Matějka, P.; Brodinová, J.; Kalendova, A.; Prokeš, J.; Stejskal, J. Structural and conductivity changes during the pyrolysis of polyaniline base. Polym. Degrad. Stab. 2006, 91, 114–121. [Google Scholar] [CrossRef]
- Plonska-brzezinska, M.E.; Breczko, J.; Palys, B.; Echegoyen, L. The electrochemical properties of nanocomposite films obtained by chemical in situ polymerization of aniline and carbon nanostructures. Chemphyschem 2013, 14, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shao, C.; Li, X.; Wang, K.; Lu, N.; Liu, Y. Electrospun Carbon nanofibers/carbon nanotubes/polyaniline ternary composites with enhanced electrochemical performance for flexible solid-state supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 1689–1696. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dhibar, S.; Kundu, M.K.; Hatui, G.; Das, C.K. Graphene and MWCNT based bi-functional polymer nanocomposites with enhanced microwave absorption and supercapacitor property. Mater. Res. Bull. 2015, 66, 200–212. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Cheng, L.; Xia, Y.-Y. Electrochemical profile of nano-particle CoAl double hydroxide/active carbon supercapacitor using KOH electrolyte solution. J. Power Sources 2006, 153, 191–196. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Ding, H.-Y.; Zhang, M.-L. Preparation and electrochemical properties of nickel oxide as a supercapacitor electrode material. Mater. Res. Bull. 2009, 44, 403–407. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Huang, T.-H.; Lin, T.-W. Fabrication of 3D hierarchically structured carbon electrode for supercapacitors by carbonization of polyaniline/carbon nanotube/graphene composites. Inorg. Chim. Acta 2019, 489, 217–223. [Google Scholar] [CrossRef]
- Liang, J.S.; Su, S.J.; Fang, X.; Wang, D.Z.; Xu, S.C. Electrospun fibrous electrodes with tunable microstructure made of polyaniline/multi-walled carbon nanotube suspension for all-solid-state supercapacitors. Mater. Sci. Eng. B 2016, 211, 61–66. [Google Scholar] [CrossRef]
- Chaudhari, S.; Sharma, Y.; Archana, P.S.; Jose, R.; Ramakrishna, S.; Mhaisalkar, S.; Srinivasan, M. Electrospun polyaniline nanofibers web electrodes for supercapacitors. J. Appl. Polym. Sci. 2013, 129, 1660–1668. [Google Scholar] [CrossRef]
- Dhibar, S.; Sahoo, S.; Das, C.K. Copper chloride-doped polyaniline/multiwalled carbon nanotubes nanocomposites: Superior electrode material for supercapacitor applications. Polym. Compos. 2013, 34, 517–525. [Google Scholar] [CrossRef]

| Sample/Scan Rates | 5 mv/s | 10 mv/s | 20 mv/s | 50 mv/s |
|---|---|---|---|---|
| PANI | 185.24 F/g | 155.12 F/g | 157 F/g | 100.75 F/g |
| PANI @ CQDs | 245.45 F/g | 236.36 F/g | 215.34 F/g | 162.73 F/g |
| PANI @ FMWCNTs | 293.98 F/g | 296.30 F/g | 251.16 F/g | 169.44 F/g |
| FMWCNTs @ CQDs @ PANI | 507.81 F/g | 441.41 F/g | 355.47 F/g | 224.22 F/g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, Y.; Hu, Z.; Ge, Y.; Dong, G.; Hu, T.; Jhun, C.G. Preparation and Electrochemical Properties of Functionalized Multi-Walled Carbon Nanotubes @ Carbon Quantum Dots @ Polyaniline Ternary Composite Electrode Materials. Appl. Sci. 2020, 10, 5462. https://doi.org/10.3390/app10165462
Wang J, Chen Y, Hu Z, Ge Y, Dong G, Hu T, Jhun CG. Preparation and Electrochemical Properties of Functionalized Multi-Walled Carbon Nanotubes @ Carbon Quantum Dots @ Polyaniline Ternary Composite Electrode Materials. Applied Sciences. 2020; 10(16):5462. https://doi.org/10.3390/app10165462
Chicago/Turabian StyleWang, Jing, Youyang Chen, Zhihao Hu, Ye Ge, Guotao Dong, Tianhao Hu, and Chul Gyu Jhun. 2020. "Preparation and Electrochemical Properties of Functionalized Multi-Walled Carbon Nanotubes @ Carbon Quantum Dots @ Polyaniline Ternary Composite Electrode Materials" Applied Sciences 10, no. 16: 5462. https://doi.org/10.3390/app10165462
APA StyleWang, J., Chen, Y., Hu, Z., Ge, Y., Dong, G., Hu, T., & Jhun, C. G. (2020). Preparation and Electrochemical Properties of Functionalized Multi-Walled Carbon Nanotubes @ Carbon Quantum Dots @ Polyaniline Ternary Composite Electrode Materials. Applied Sciences, 10(16), 5462. https://doi.org/10.3390/app10165462




