Investigation of the Maturity Changes of Cherry Tomato Using Magnetic Resonance Imaging
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
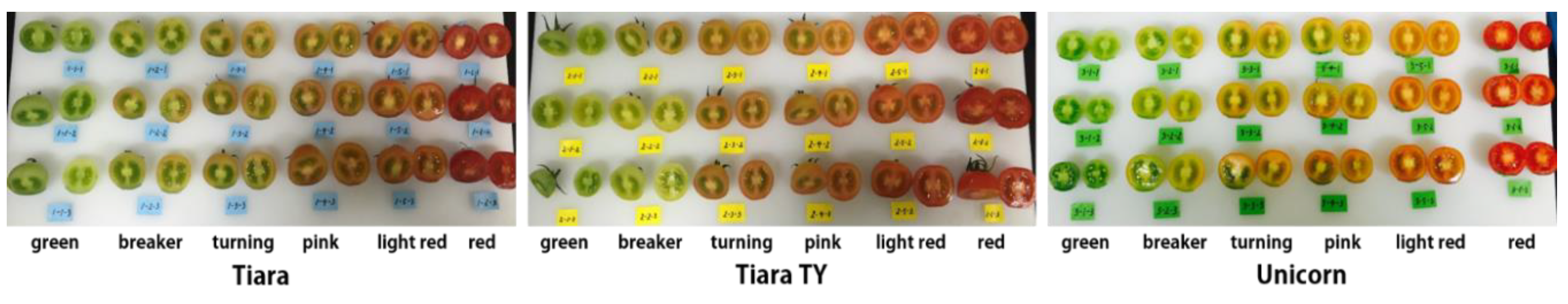
2.1. Materials
2.2. MRI System and Pulse Sequence
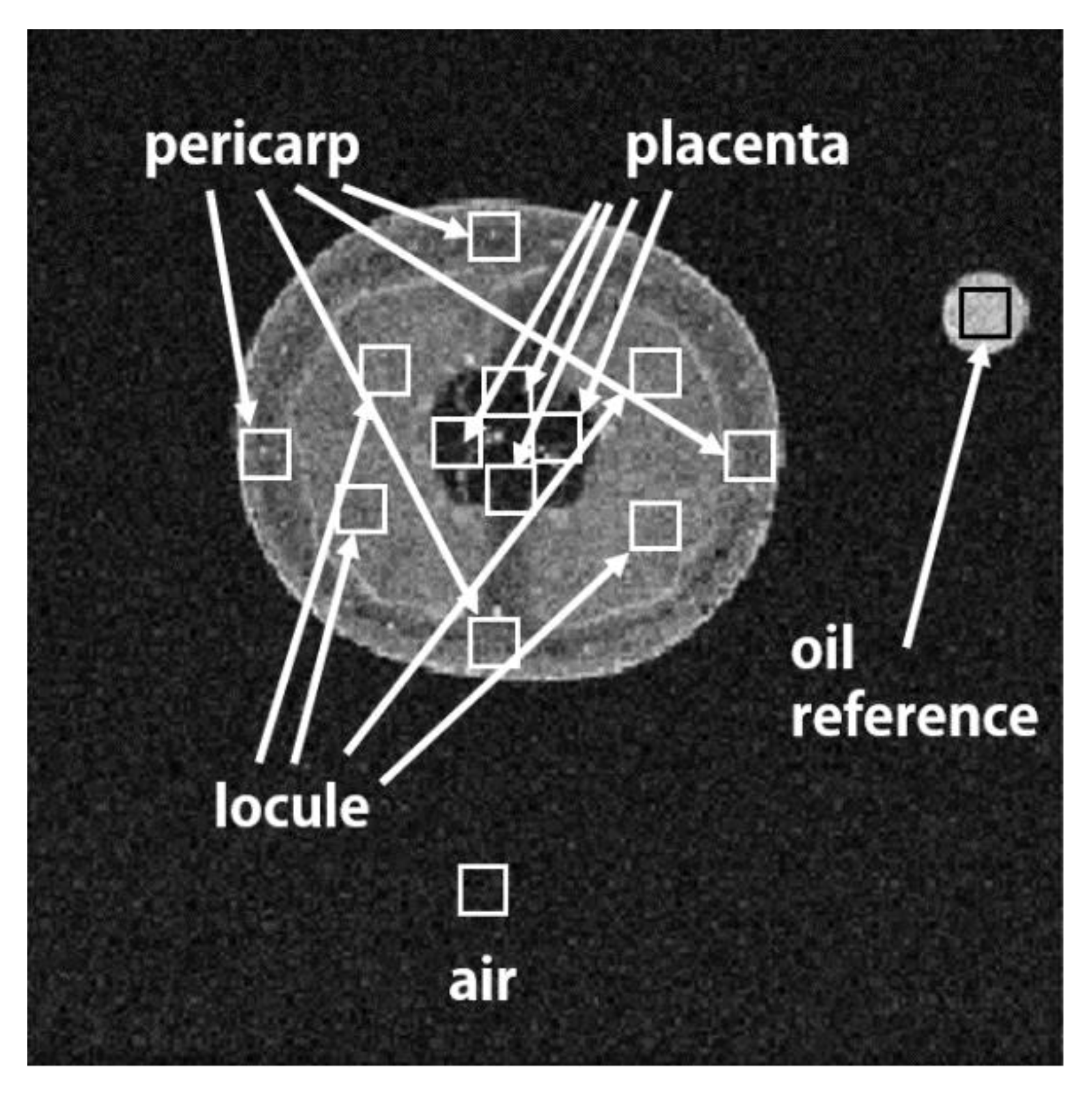
2.3. Characteristics of Magnetic Resonance Images
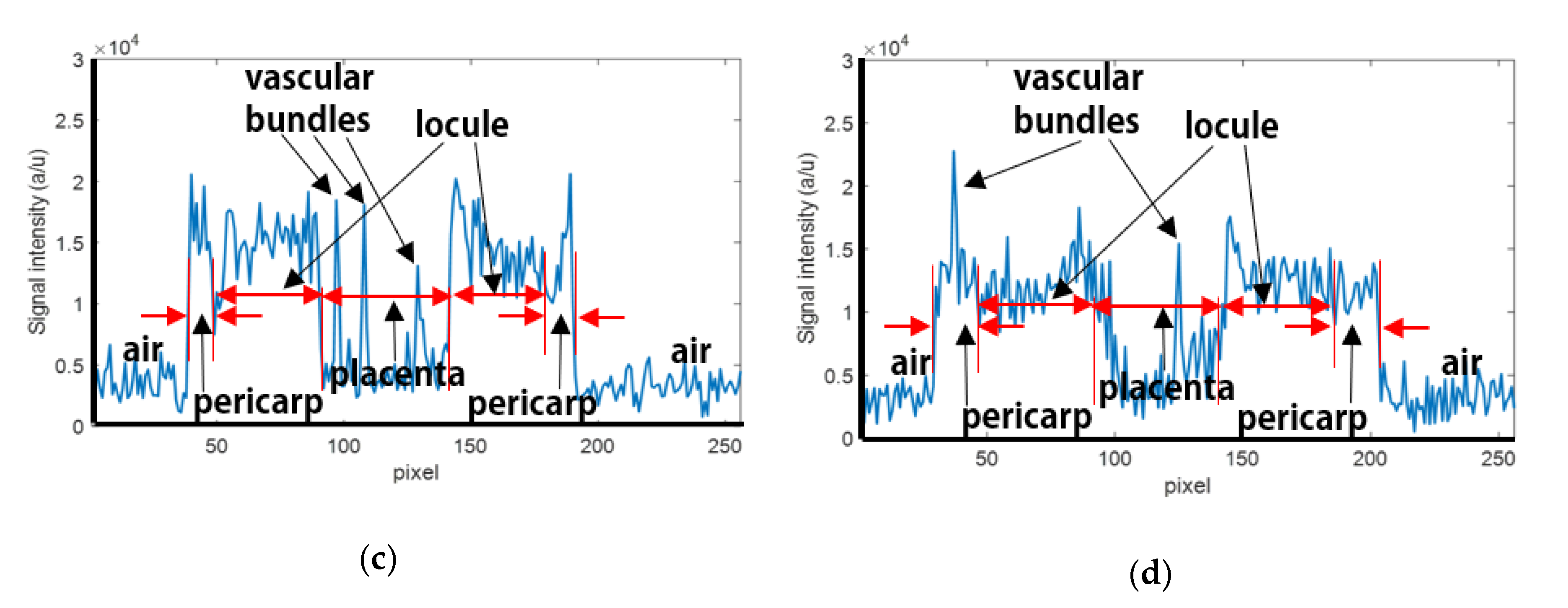
2.4. Data Analysis
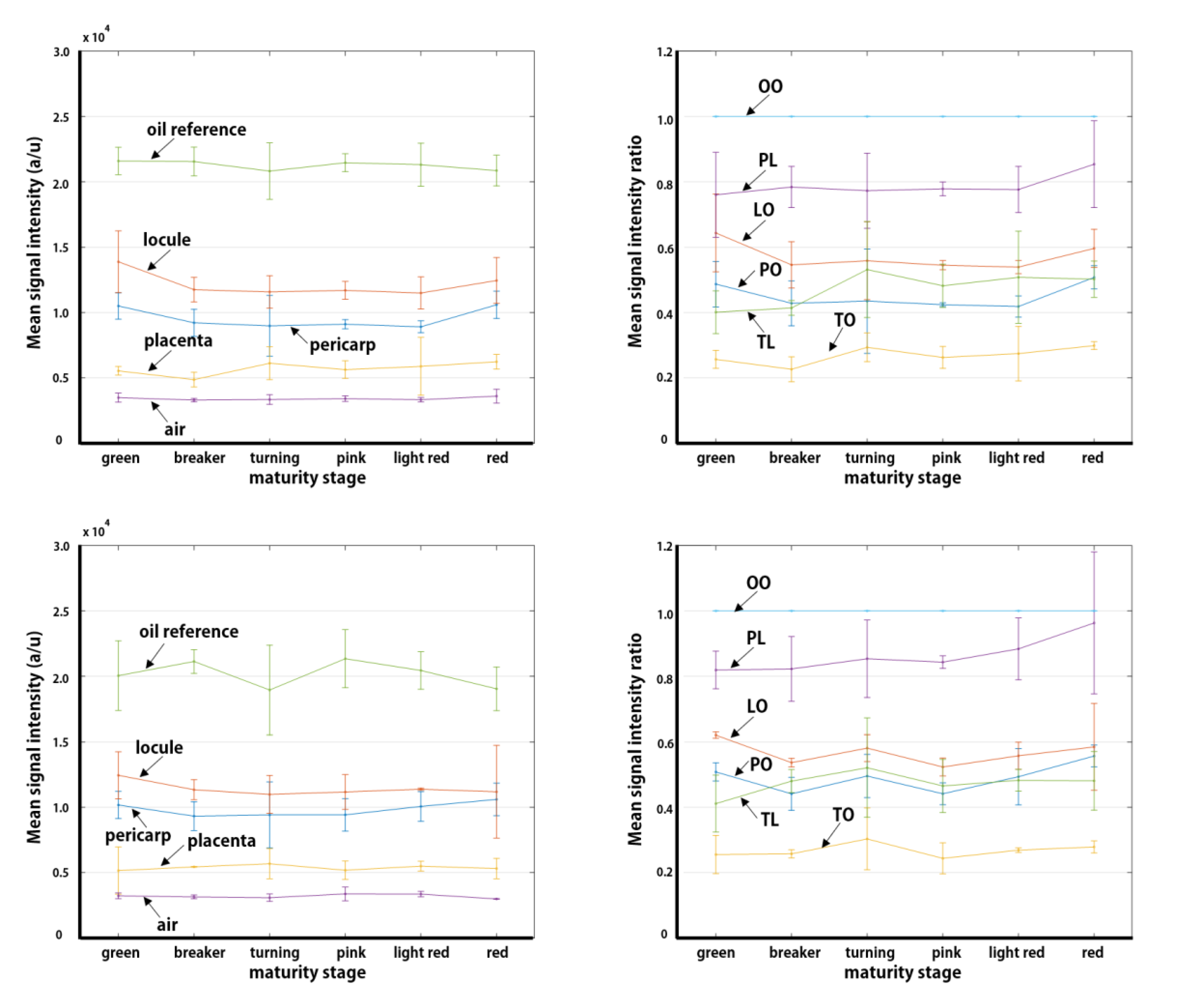
3. Results
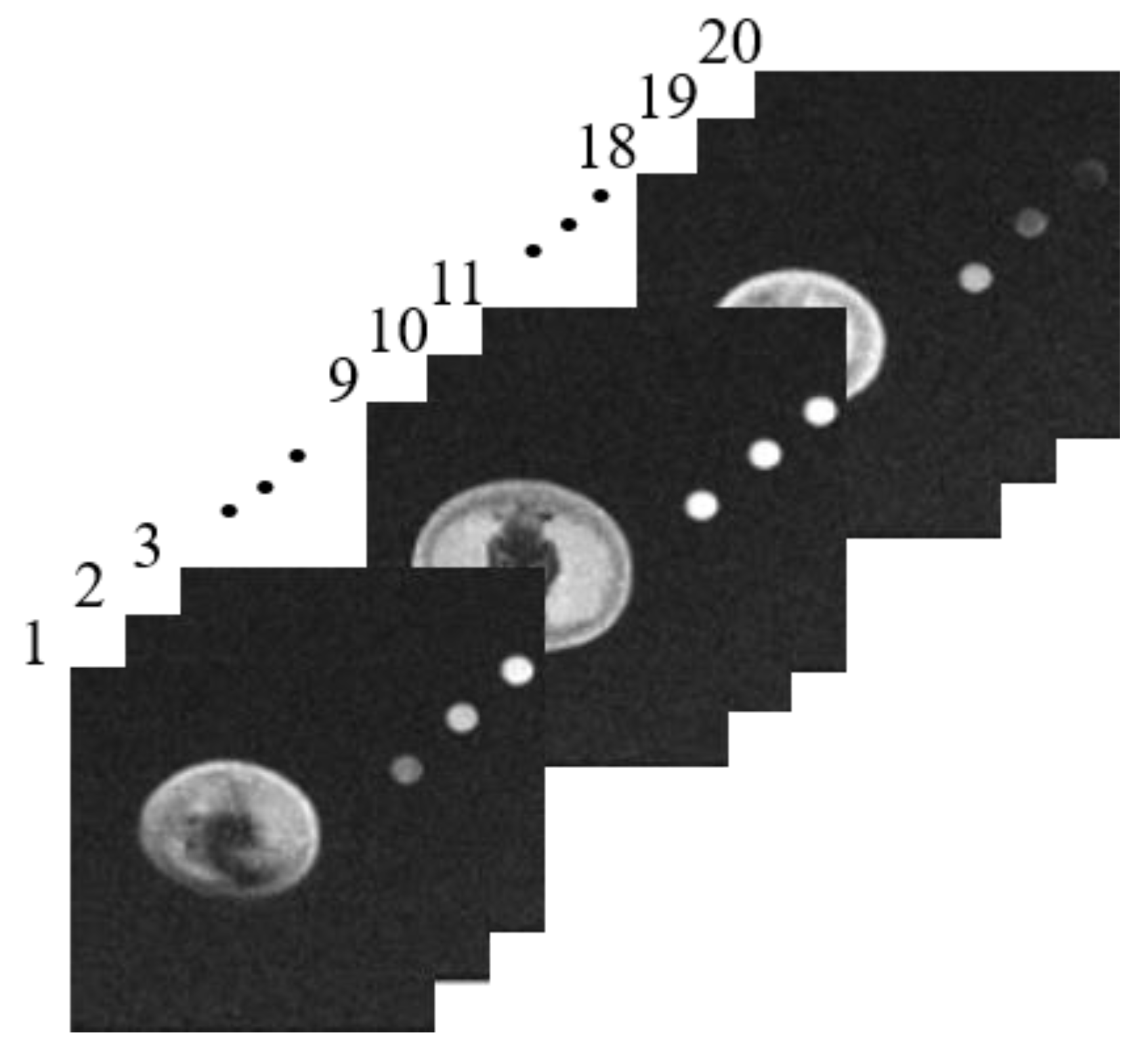
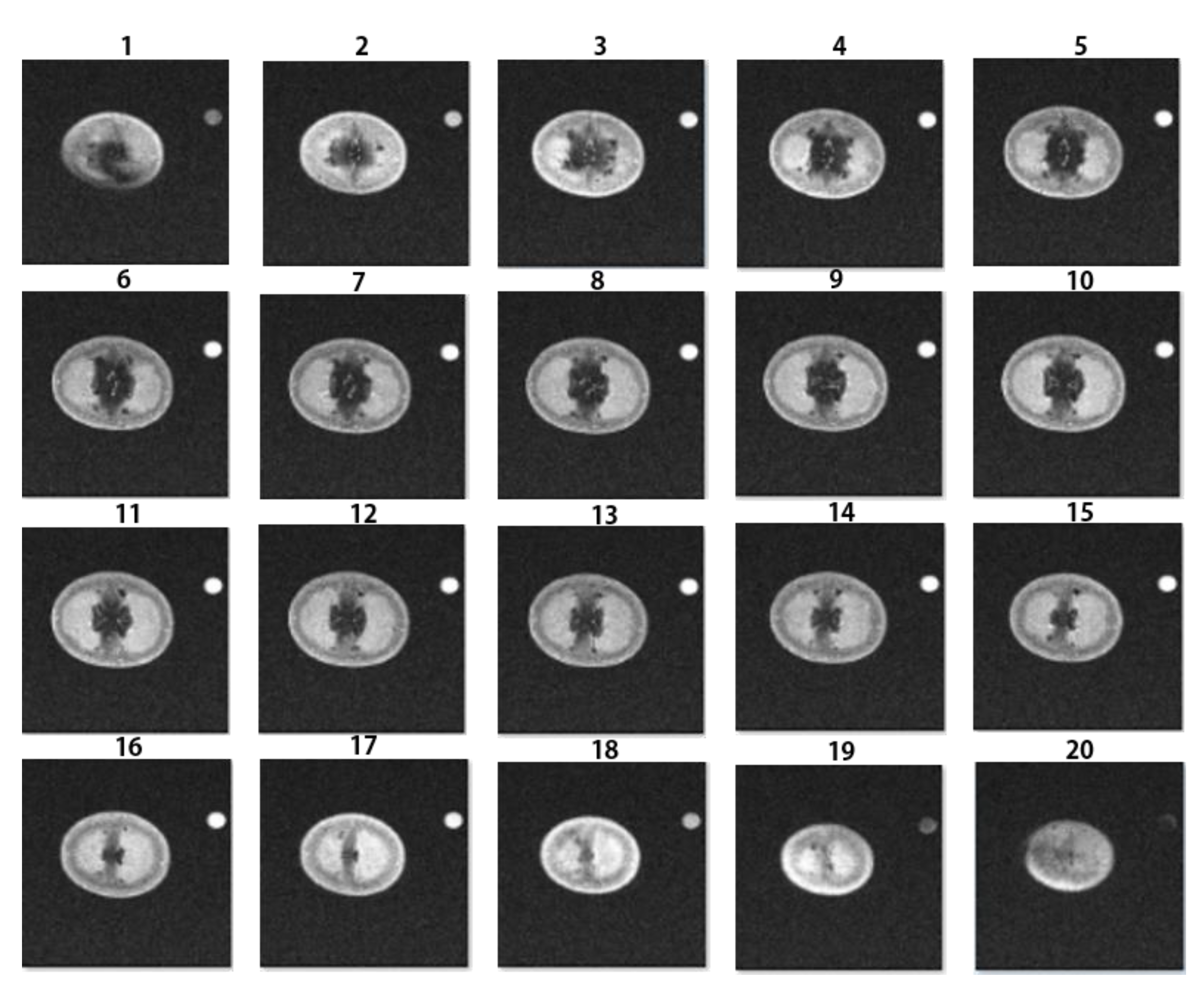
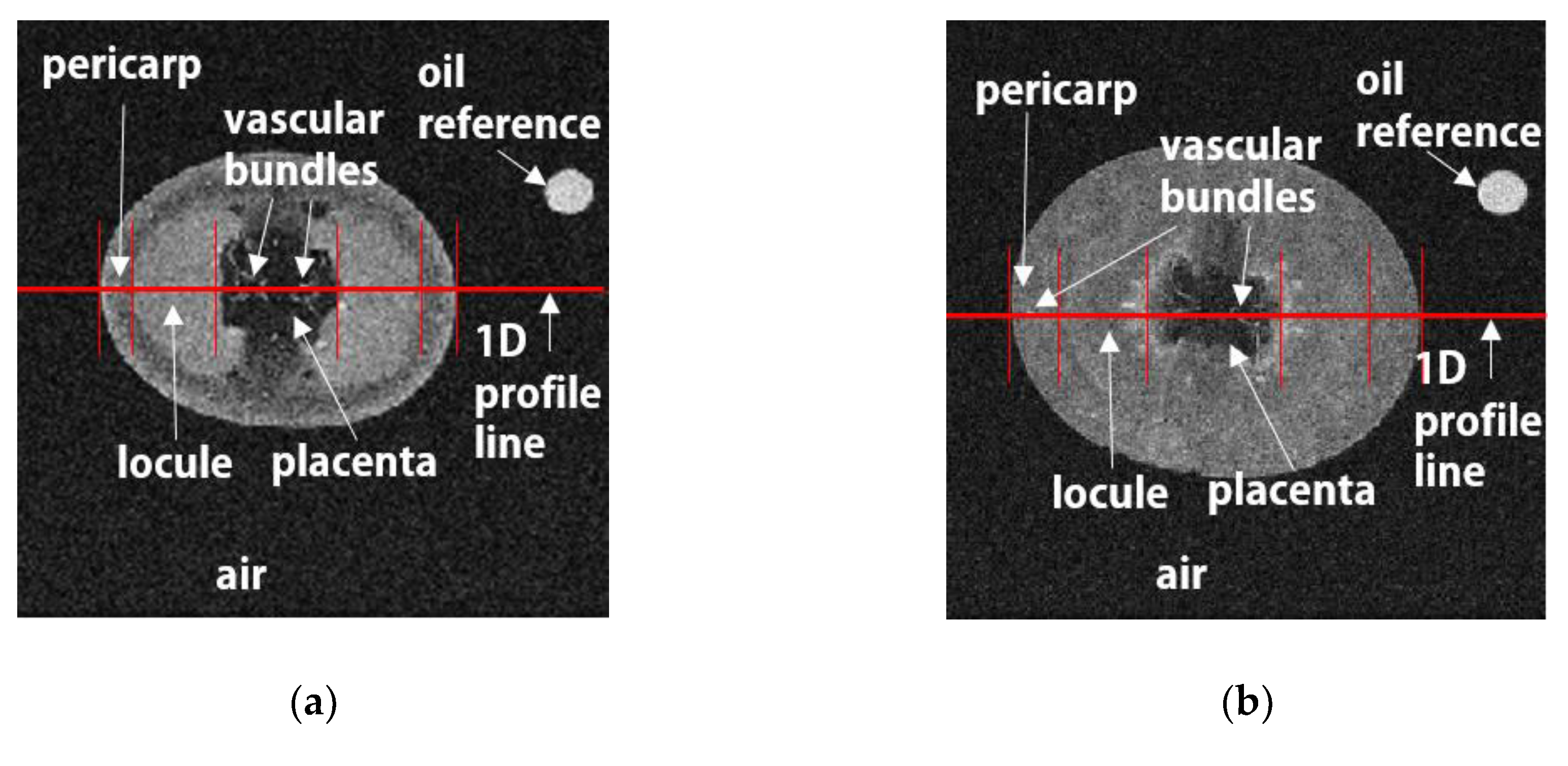
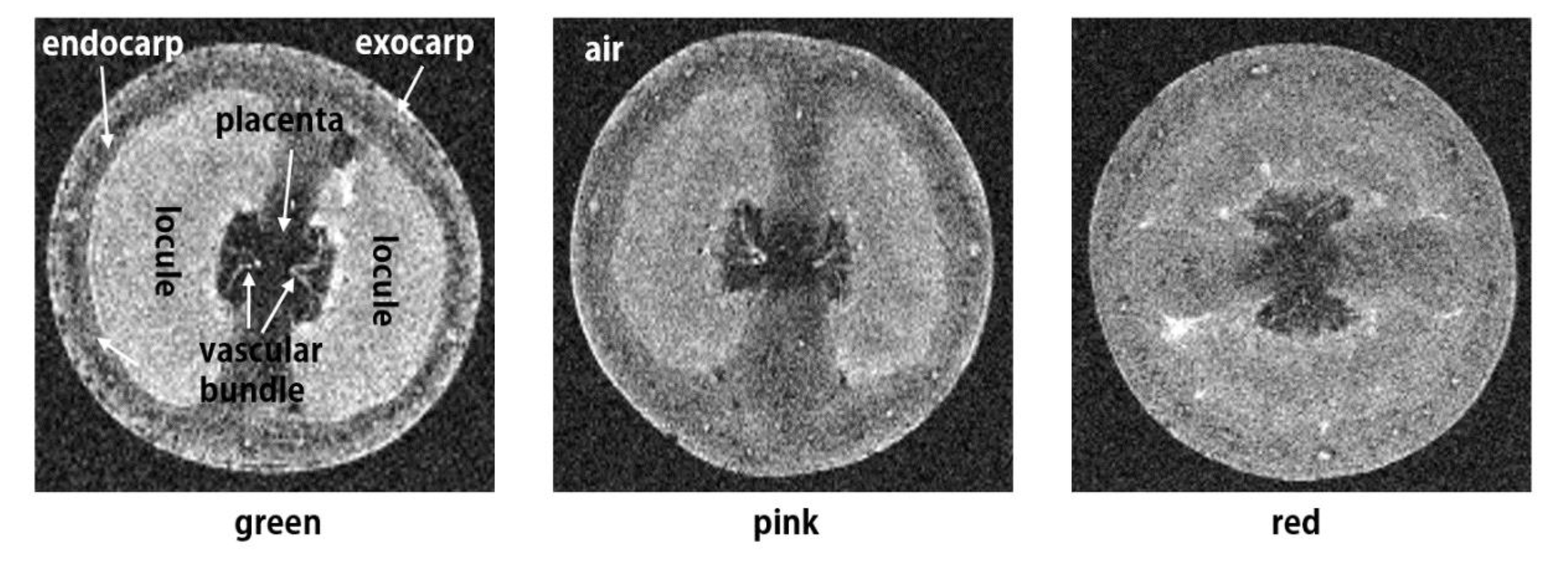
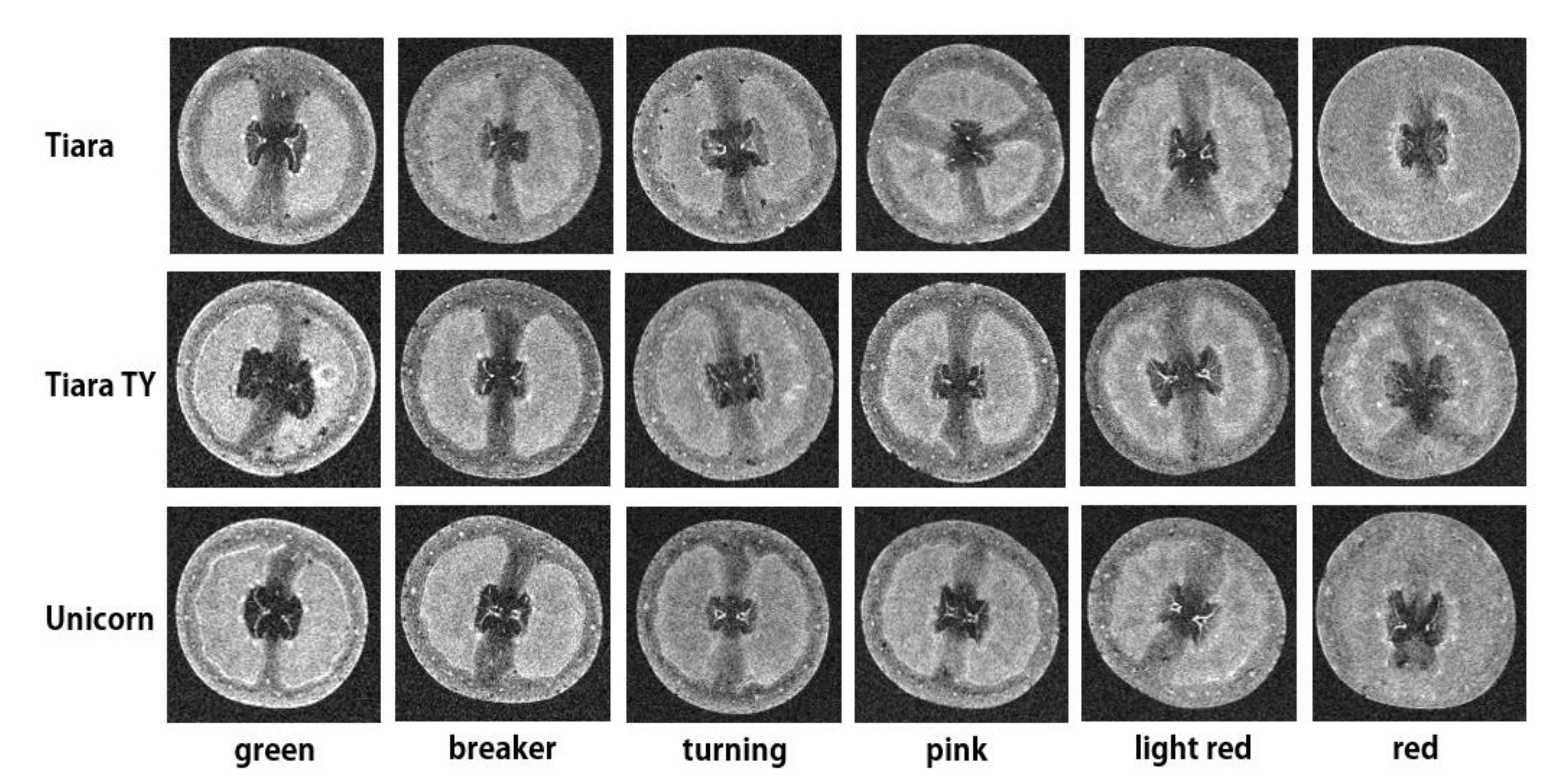
3.1. Qualitative MR Image Analysis
3.2. Quantitative MR Image Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.R.; Yasmin, J.; Lee, W.H.; Mo, C.; Cho, B.K. Imaging Technologies for Nondestructive Measurement of Internal Properties of Agricultural Products: A Review. J. Biosyst. Eng. 2017, 42, 199–216. [Google Scholar] [CrossRef]
- Lim, J.G.; Kim, C.S.; Lee, S.J.; Kim, S.M. Internal Quality Evaluation and Age Identification of Fresh Korean Ginseng using Magnetic Resonance Imaging. J. KSAM 2003, 28, 157–166. [Google Scholar]
- Magwaza, L.S.; Opara, U.L. Investigation non-destructive quantification and characterization of pomegranate fruit internal structure using X-ray computed tomography. Postharvest Biol. Technol. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Patel, K.K.; Khan, M.A.; Kar, A. Recent development in applications of MRI techniques for foods and agricultural produce-an overview. J. Food Sci. Technol. 2015, 52, 1–26. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Guyer, D.E.; Pease, A. Postharvest noninvasive assessment of undesirable fibrous tissue in fresh processing carrots using computer tomography images. J. Food Eng. 2016, 190, 154–166. [Google Scholar] [CrossRef]
- Joyce, D.C.; Hockings, P.D.; Mazucco, R.A.; Shorter, A.J. 1H-Nuclear magnetic resonance imaging of ripening ‘Kensington Pride’ mango fruit. Funct. Plant Biol. 2002, 29, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Milczarek, R.; McCarthy, M.J. Fast Detection of Seeds and Freeze Damage of Mandarins using Magnetic Resonance Imaging. Mod. Phys. Lett. B 2008, 22, 941–946. [Google Scholar] [CrossRef]
- Taglienti, A.; Massantini, R.; Botondi, R.; Mencarelli, F.; Valentini, M. Postharvest structural changes of Hayward kiwifruit by means of magnetic resonance imaging spectroscopy. Food Chem. 2009, 114, 1583–1589. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Burnet, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Musse, M.; Quellec, S.; Cambert, M.; Devaux, M.F.; Lahaye, M.; Mariette, F. Monitoring the postharvest ripening of tomato fruit using quantitative MRI and NMR Relaxometry. Postharvest Biol. Technol. 2009, 53, 22–35. [Google Scholar] [CrossRef]
- Ciampa, A.; Dell’Abate, M.T.; Masetti, O.; Valentini, M.; Sequi, P. Seasonal chemical-physical changes of PGI Pachino cherry tomatoes detected by magnetic resonance imaging. Food Chem. 2010, 122, 1253–1260. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Measurement and evaluation of tomato maturity using magnetic resonance imaging. Postharvest Biol. Technol. 2012, 67, 37–43. [Google Scholar] [CrossRef]
- Zhang, L.; Barrett, D.M.; McCarthy, M.J. Characterization of the Red Layer and Pericarp of Processing Tomato using Magnetic Resonance Imaging. J. Food Sci. 2013, 78, E50–E55. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Lim, J.; Lee, J.G.; McCarthy, M.J.; Kim, S.M. Investigation of the Maturity Changes of Cherry Tomato Using Magnetic Resonance Imaging. Appl. Sci. 2020, 10, 5188. https://doi.org/10.3390/app10155188
Baek S, Lim J, Lee JG, McCarthy MJ, Kim SM. Investigation of the Maturity Changes of Cherry Tomato Using Magnetic Resonance Imaging. Applied Sciences. 2020; 10(15):5188. https://doi.org/10.3390/app10155188
Chicago/Turabian StyleBaek, Seunghoon, Jongguk Lim, Jun Gu Lee, Michael J. McCarthy, and Seong Min Kim. 2020. "Investigation of the Maturity Changes of Cherry Tomato Using Magnetic Resonance Imaging" Applied Sciences 10, no. 15: 5188. https://doi.org/10.3390/app10155188
APA StyleBaek, S., Lim, J., Lee, J. G., McCarthy, M. J., & Kim, S. M. (2020). Investigation of the Maturity Changes of Cherry Tomato Using Magnetic Resonance Imaging. Applied Sciences, 10(15), 5188. https://doi.org/10.3390/app10155188





