NO Separation Characteristics in Integrated Electromigration Membrane Reactor
Abstract
1. Introduction
2. Experiments
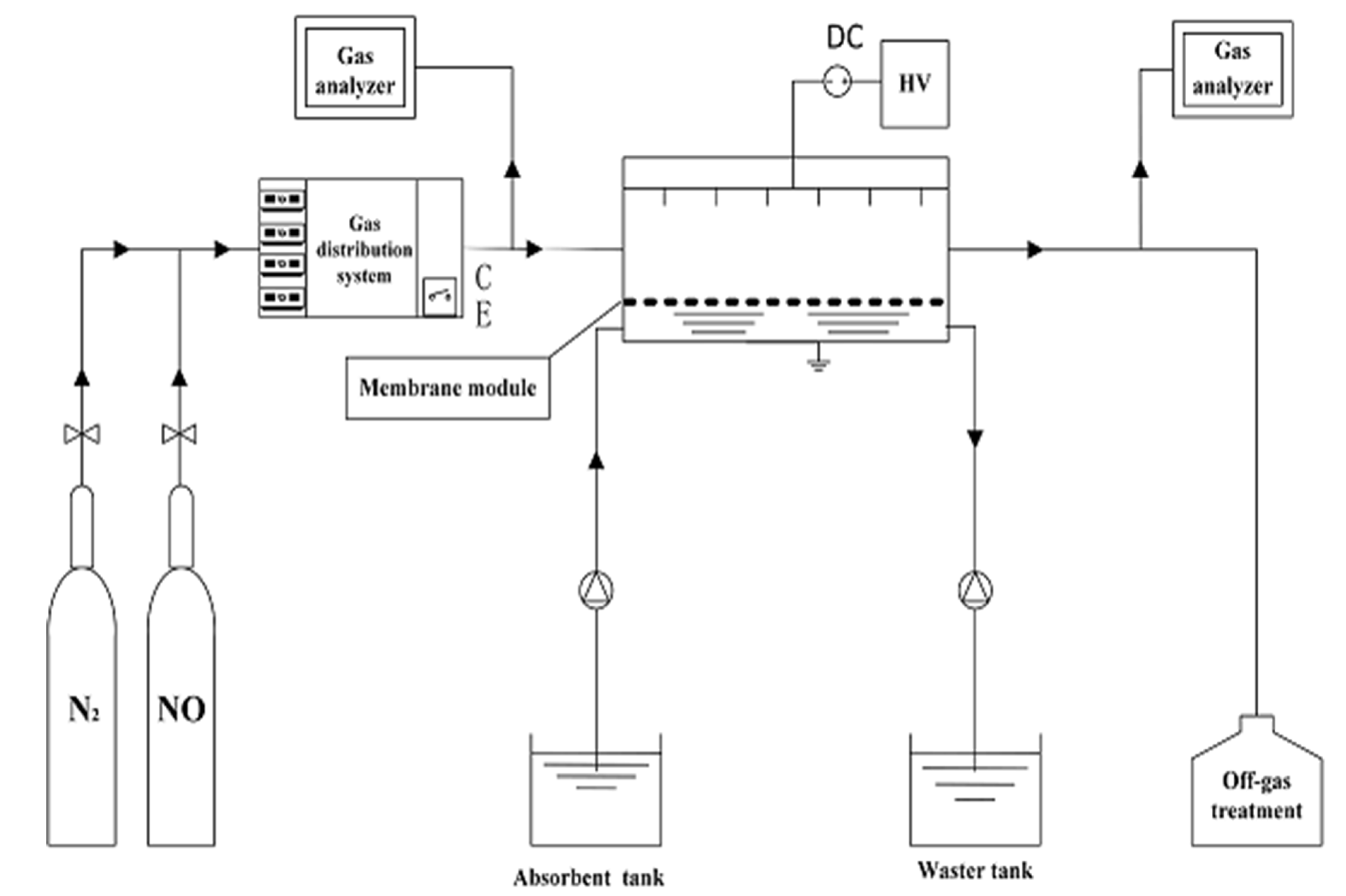
2.1. Experimental Methods
2.2. Process Description and Calculation
- (1)
- Formation of NO negative ions:
- (2)
- Separation of NO from simulated mixture gas:
- (3)
- Absorption of NO in the absorption solution:
3. Results and Discussion
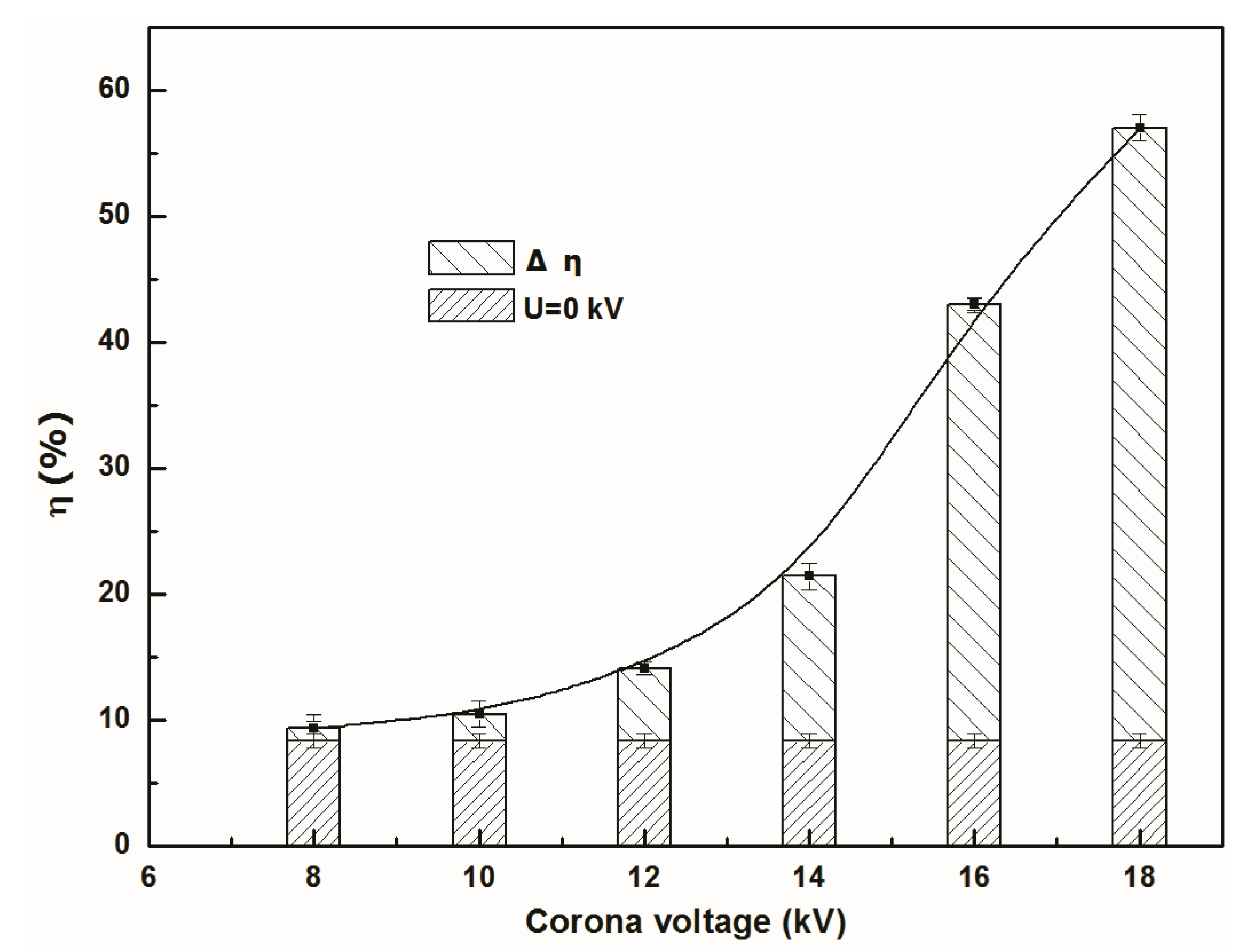
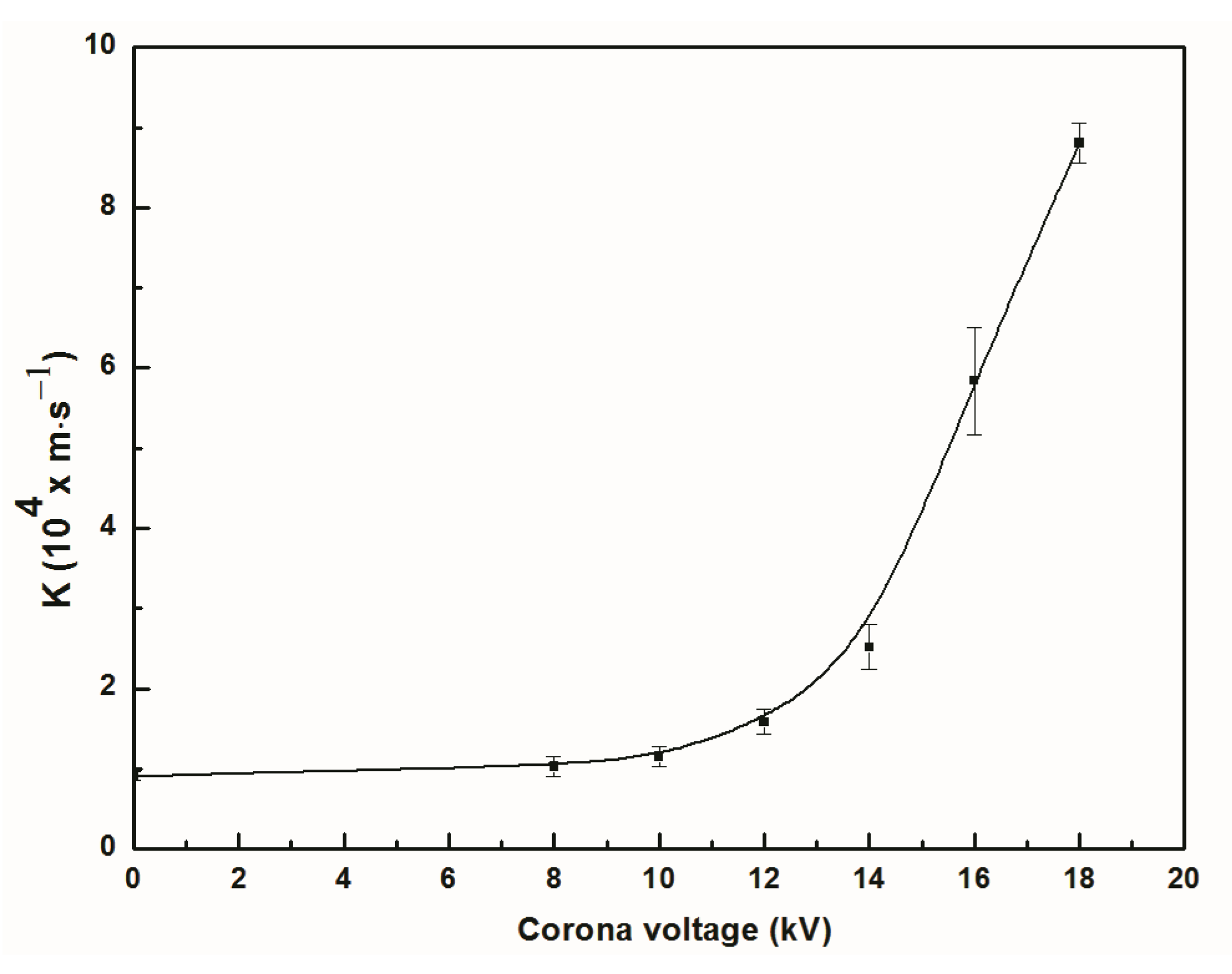
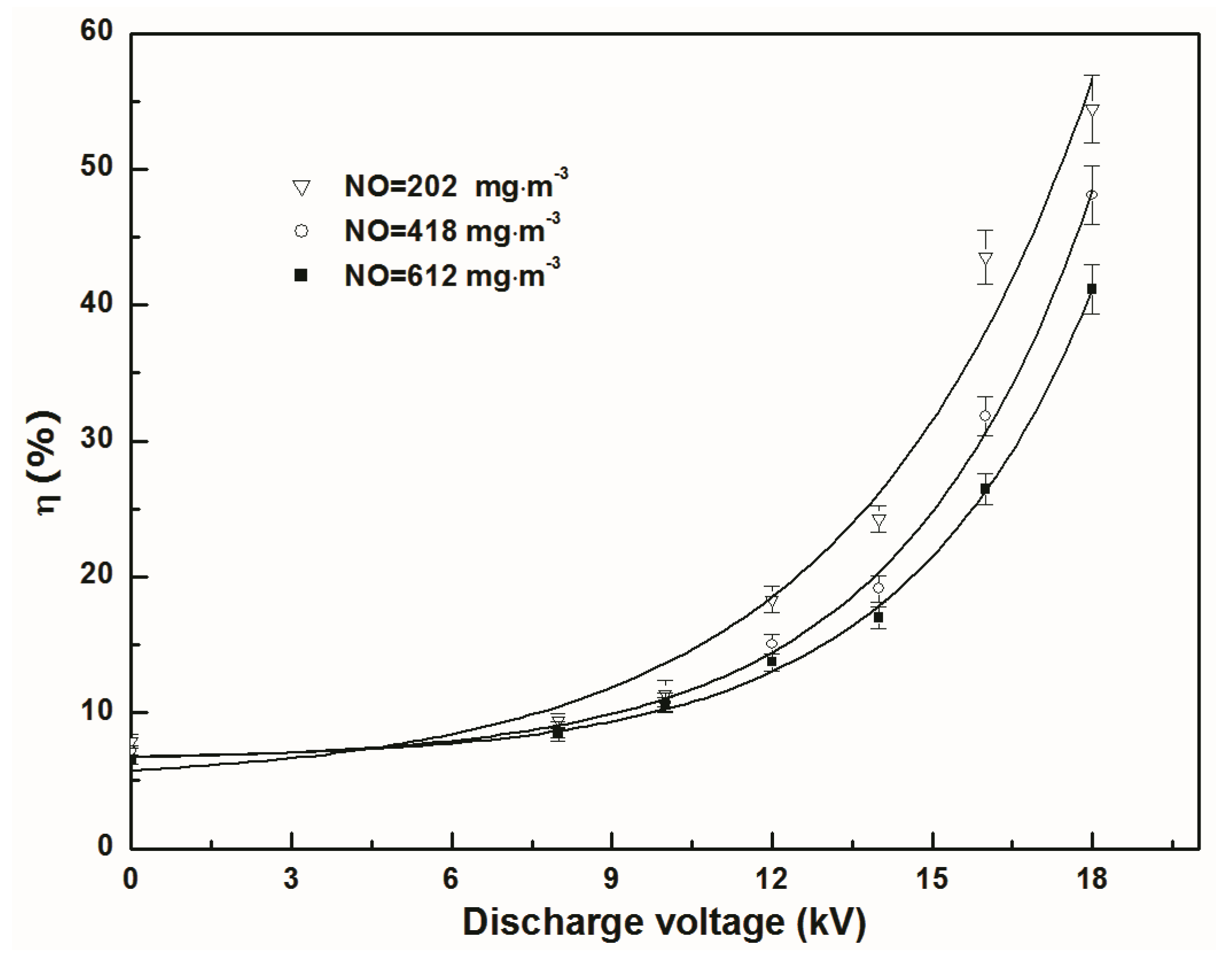
3.1. Effect of Discharge Voltage on Separation Efficiency and Total Mass Transfer Coefficient
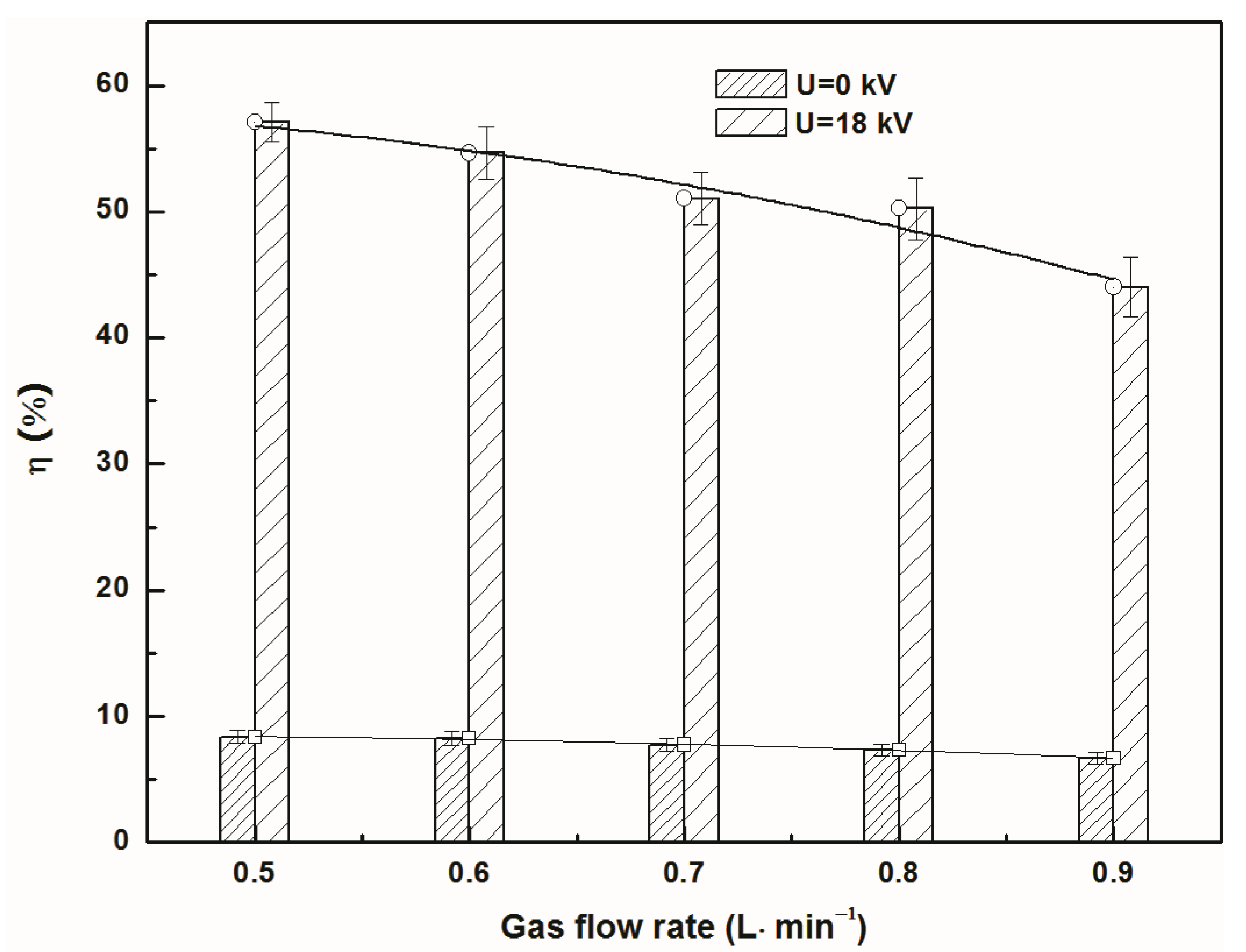
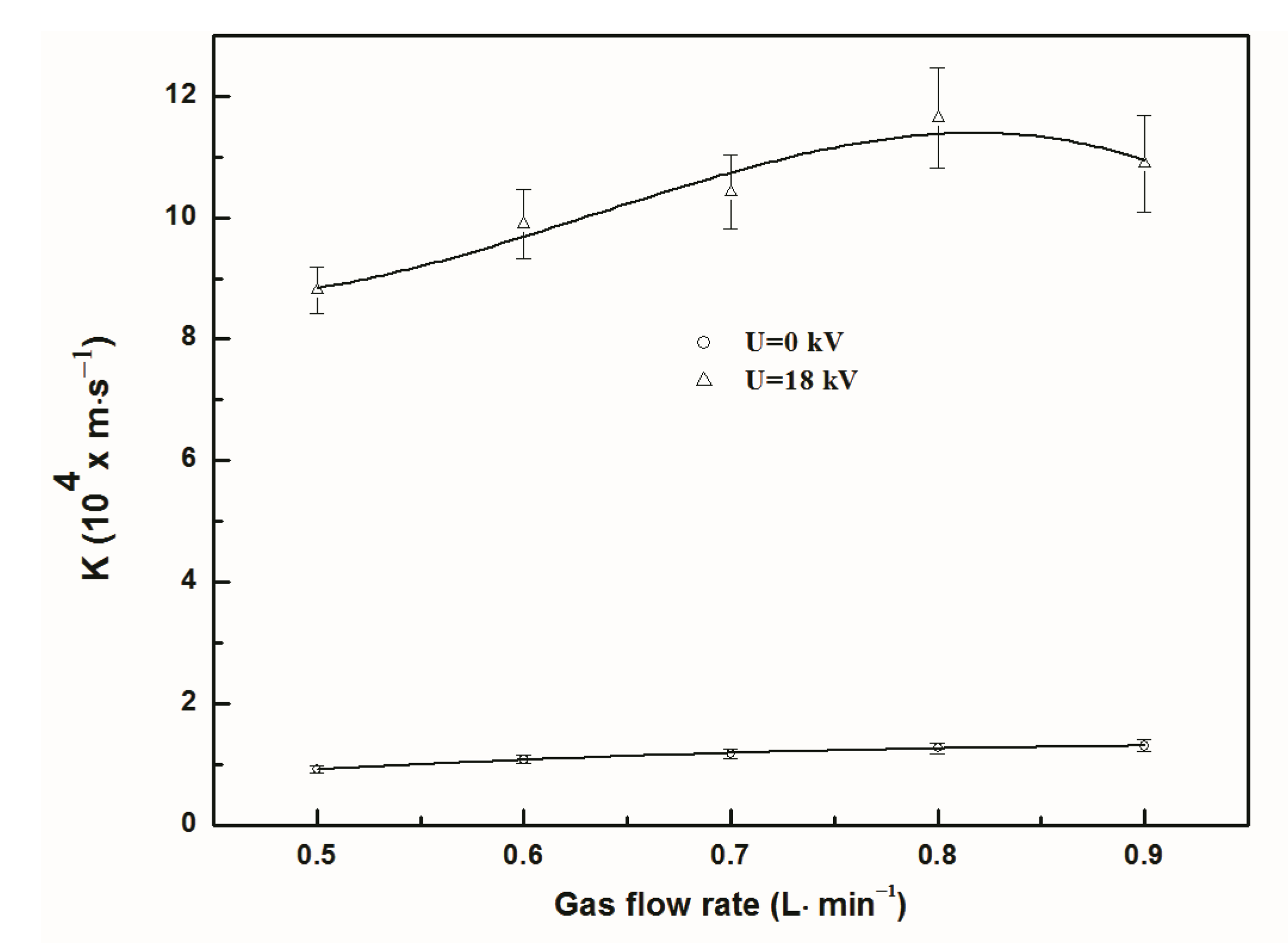
3.2. Effect of Gas Flow Rate on Separation Efficiency and Total Mass Transfer Coefficient
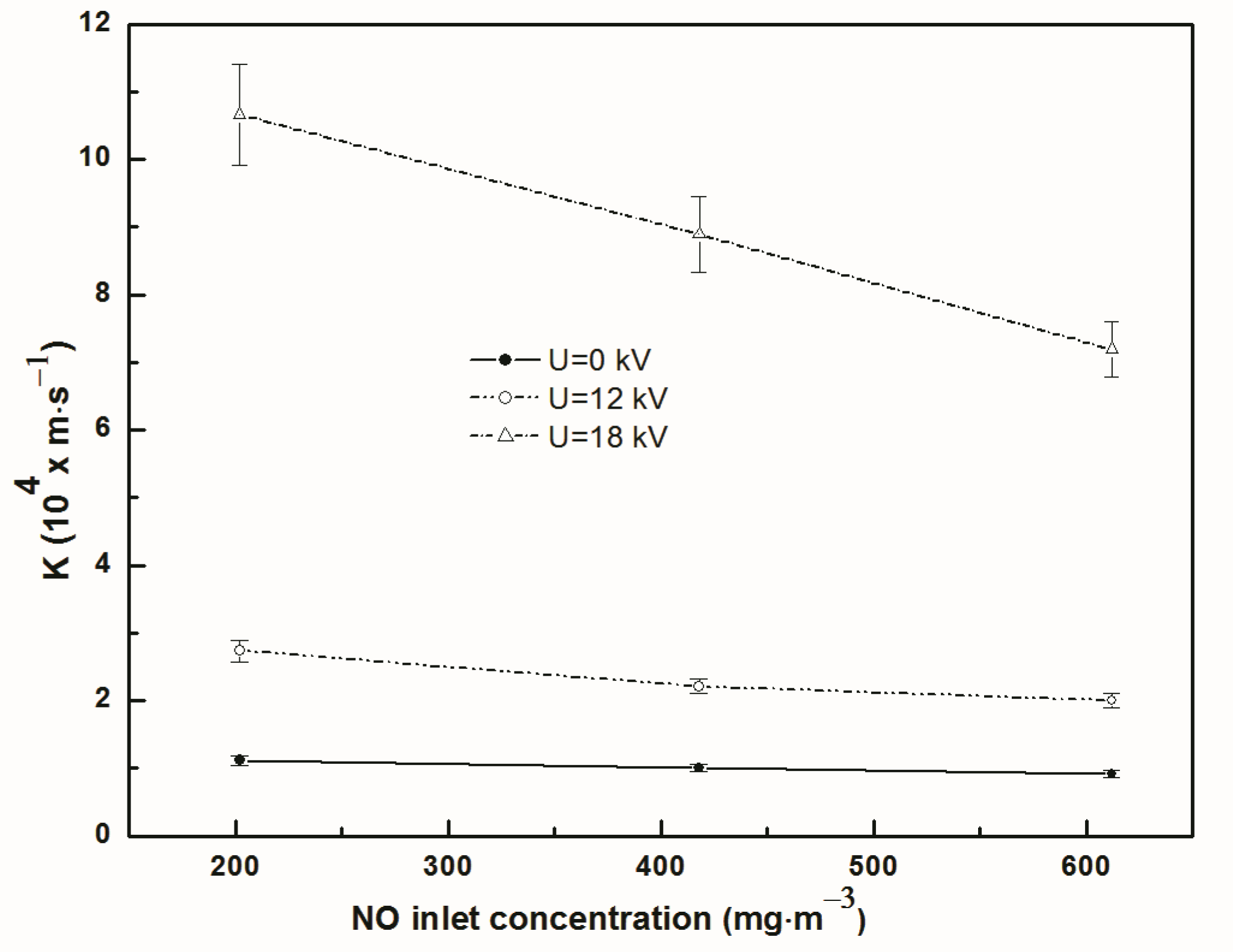
3.3. Effect of Inlet Concentration on Separation Efficiency and Total Mass Transfer Coefficient
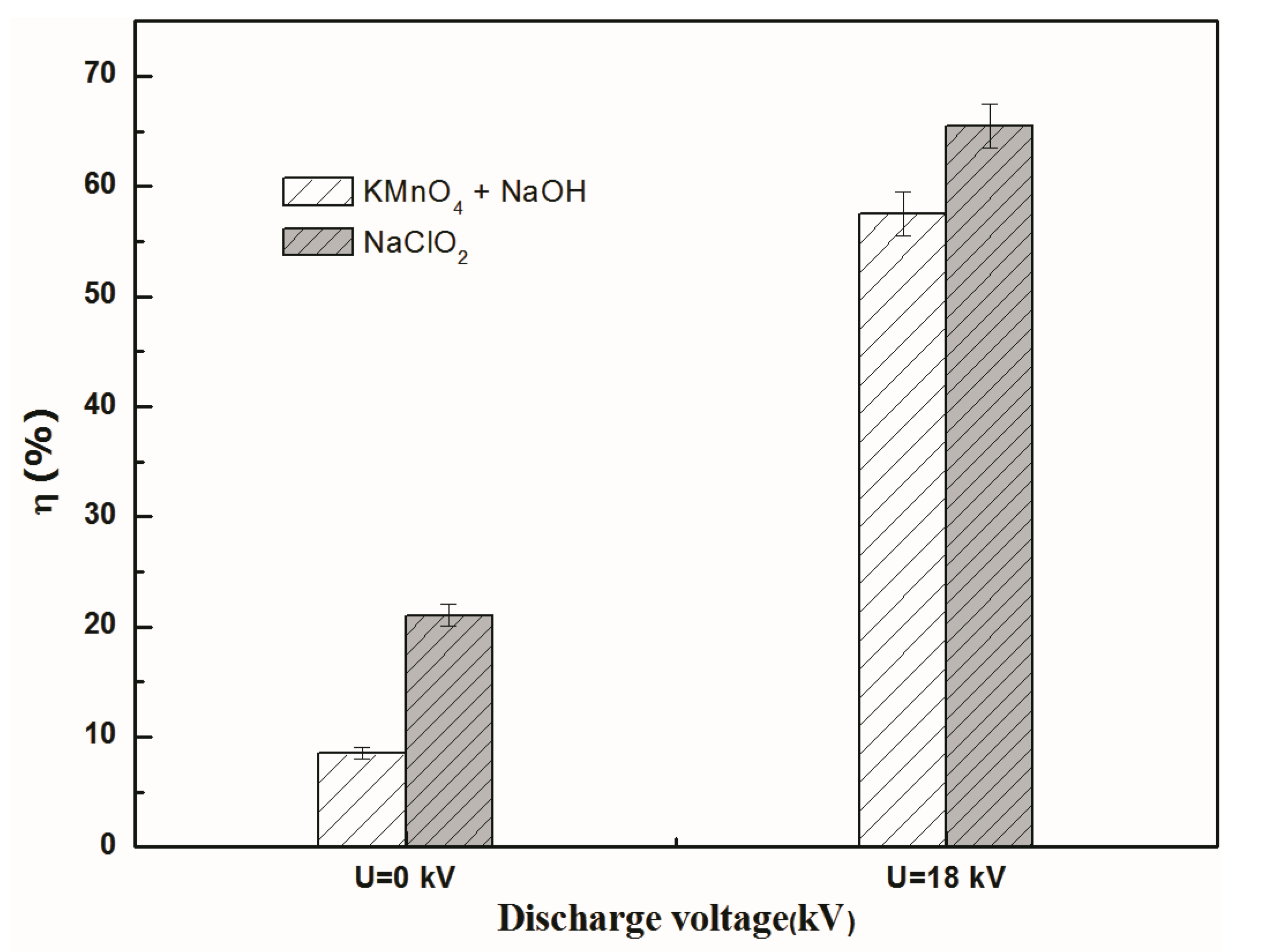
3.4. Effect of Absorbent on no Separation Efficiency and Total Mass Transfer Coefficient
4. Conclusions
- (1)
- The integrated electromigration membrane separation method can effectively separate NO from NO-N2 mixture gas. At the discharge voltage of 18kV, the separation efficiency of NO in the reactor was about 57%. It increased by 48.7%, and the total mass transfer coefficient of NO was raised 9.7 times compared with the one in membrane absorption process without discharge.
- (2)
- The electromigration of NO negative ions can enhance NO separation and mass transfer. “When the applied voltage is higher onset voltage, higher discharge voltage has greater separation efficiency and total mass transfer coefficient of NO under experimental” condition.
- (3)
- Regardless of discharge or not, the separation efficiency of NO continuously declined with the increase of gas flow rate and inlet concentration of NO. The total mass transfer coefficient of NO increased first and then reduced with the increase of gas flow rate, while it decreased with the increase of NO inlet concentration.
Author Contributions
Funding
Conflicts of Interest
References
- Hao, R.; Wang, X.; Zhao, X.; Xu, M.; Zhao, Y.; Mao, X.; Yuan, B.; Zhang, Y.; Gao, K. A novel integrated method of vapor oxidation with dual absorption for simultaneous removal of SO2 and NO: Feasibility and prospect. Chem. Eng. J. 2018, 333, 583–593. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Zhao, L.; Wang, Y.; Pan, J.; Wang, Q.; Zhang, J. Removal of NO in flue gas using vacuum ultraviolet light/ultrasound/chlorine in a VUV-US coupled reactor. Fuel Process. Technol. 2018, 169, 226–235. [Google Scholar] [CrossRef]
- Sun, Y.; Zwolińska, E.; Chmielewski, A.G. Abatement technologies for high concentrations of NOx and SO2 removal from exhaust gases: A review. Crit. Rev. Environ. Sci. Technol. 2015, 46, 119–142. [Google Scholar] [CrossRef]
- Krzyzynska, R.; Hutson, N.D. The importance of the location of sodium chlorite application in a multipollutant flue gas cleaning system. J. Air Waste Manag. Assoc. 2012, 62, 707–716. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Wang, Y.; Xu, W.; Yang, W.; Pan, Z.; Wang, Q. Simultaneous absorption–oxidation of nitric oxide and sulfur dioxide using ammonium persulfate synergistically activated by UV-light and heat. Chem. Eng. Res. Des. 2018, 130, 321–333. [Google Scholar] [CrossRef]
- Hao, R.; Mao, X.; Wang, Z.; Zhao, Y.; Wang, T.; Sun, Z.; Yuan, B.; Li, Y. A novel method of ultraviolet/NaClO2-NH4OH for NO removal: Mechanism and kinetics. J. Hazard. Mater. 2019, 368, 234–242. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Xu, W.Q.; Li, Y.; Han, Y.; Sun, Q. Simultaneous removal of SO2 and NOx from flue gas by wet scrubbing using a urea solution. Environ. Technol. 2018, 40, 2620–2632. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Trubyanov, M.M.; Yanbikov, N.R.; Vorotyntsev, A.V.; Drozdov, P.N.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive experimental study of membrane cascades type of “continuous membrane column” for gases high-purification. J. Membr. Sci. 2019, 572, 92–101. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E. Microporous hollow fibers for gas absorption: I. Mass transfer in the liquid. J. Membr. Sci. 1985, 23, 321–332. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E. Microporous hollow fibers for gas absorption: II. Mass transfer across the membrane. J. Membr. Sci. 1985, 23, 333–345. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K.; Al-Marzouqi, M. H2S absorption at high pressure using hollow fibre membrane contactors. Chem. Eng. Process. Process. Intensif. 2014, 83, 33–42. [Google Scholar] [CrossRef]
- Sun, X.; Meng, F.; Yang, F. Application of seawater to enhance SO2 removal from simulated flue gas through hollow fiber membrane contactor. J. Membr. Sci. 2008, 312, 6–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Rezakazemi, M.; Chen, F.; Luis, P.; van der Bruggen, B. Effect of flow and module configuration on SO2 absorption by using membrane contactors. Global NEST J. 2018, 19, 716–725. [Google Scholar]
- Kartohardjono, S.; Stephanie, S.; Dixon, A.O.; Saksono, N. Utilization of super hydrophobic membrane contactor for NOx Absorption. In IOP Conference Series: Earth Environment Science, Proceedings of the 2nd International Tropical Renewable Energy Conference (i-TREC), Bali, Indonesia, 3–4 October 2017; IOP Publishing: Bristol, UK, 2018; Volume 105, pp. 1315–1755. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X. Removal of NO research in a polypropylene hollow fiber membrane contactor. In Advances in Engineering Research, Proceedings of the 2017 6th International Conference on Energy, Environment and Sustainable Development (ICEESD 2017), Zhuhai, China, 11–12 March 2017; Atlantis Press: Paris, France, 2017; Volume 129, pp. 1015–1022. [Google Scholar] [CrossRef]
- Ghobadi, J.; Ramirez, D.; Khoramfar, S.; Jerman, R.; Crane, M.; Hobbs, K. Simultaneous absorption of carbon dioxide and nitrogen dioxide from simulated flue gas stream using gas-liquid membrane contacting system. Int. J. Greenh. Gas Control 2018, 77, 37–45. [Google Scholar] [CrossRef]
- Monfared, M.A.; Sheikhi, M.H.; Kasiri, N.; Mohammadi, T. Experimental investigation of oil-in-water microfiltration assisted by Dielectrophoresis: Operational condition optimization. Chem. Eng. Res. Des. 2018, 137, 421–433. [Google Scholar] [CrossRef]
- Sarkar, B.; Pal, S.; Ghosh, T.B.; De, S.; Dasgupta, S. A study of electric field enhanced ultrafiltration of synthetic fruit juice and optical quantification of gel deposition. J. Membr. Sci. 2008, 311, 112–120. [Google Scholar] [CrossRef]
- Carman, H.S. Low energy electron attachment to clusters of nitric oxide. J. Chem. Phys. 1994, 100, 2629–2636. [Google Scholar] [CrossRef]
- Chu, Y.; Senn, G.; Matejcik, S.; Scheier, P.; Stampfli, P.; Stamatovic, A.; Illenberger, E.; Märk, T.D. Formation of NO following electron attachment to NO clusters. Chem. Phys. Lett. 1998, 289, 521–526. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Hosseinzadeh, M.; Vatani, A.; Mohammadi, T. Mathematical modeling for the simultaneous absorption of CO2 and SO2 using MEA in hollow fiber membrane contactors. Chem. Eng. Process. Process. Intensif. 2017, 111, 35–45. [Google Scholar] [CrossRef]
- Yang, J. Gaseous Discharge; Science Press: Beijing, China, 1983. [Google Scholar]
- Chu, H.; Chien, T.-W.; Li, S. Simultaneous absorption of SO2 and NO from flue gas with KMnO4/NaOH solutions. Sci. Total. Environ. 2001, 275, 127–135. [Google Scholar] [CrossRef]
- Park, H.-W.; Choi, S.; Park, D.-W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator. J. Hazard. Mater. 2015, 285, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Deshwal, B.R.; Jo, H.D.; Kil Choi, W.; Kim, I.W.; Lee, H.K. Absorption of nitrogen dioxide by PVDF hollow fiber membranes in a G–L contactor. Desalination 2009, 243, 52–64. [Google Scholar] [CrossRef]
- Peng, Z.-G.; Lee, S.-H.; Zhou, T.; Shieh, J.-J.; Chung, T.-S. A study on pilot-scale degassing by polypropylene (PP) hollow fiber membrane contactors. Desalination 2008, 234, 316–322. [Google Scholar] [CrossRef]
- Tamon, H.; Sano, N.; Okazaki, M. Influence of oxygen and water vapor on removal of sulfur compounds by electron attachment. AIChE J. 1996, 42, 1481–1486. [Google Scholar] [CrossRef]
- Georghiou, G.E.; Papadakis, A.P.; Morrow, R.; Metaxas, A.C. Numerical modelling of atmospheric pressure gas discharges leading to plasma production. J. Phys. D Appl. Phys. 2005, 38, R303–R328. [Google Scholar] [CrossRef]
- Brogren, C.; Karlsson, H.T.; Bjerle, I. Absorption of NO in an alkaline solution of KMnO4. Chem. Eng. Technol. 1997, 20, 396–402. [Google Scholar] [CrossRef]
- Ye, J.; Shang, J.; Li, Q.; Xu, W.; Liu, J.; Feng, X.; Zhu, T. The use of vacuum ultraviolet irradiation to oxidize SO2 and NOx for simultaneous desulfurization and denitrification. J. Hazard. Mater. 2014, 271, 89–97. [Google Scholar] [CrossRef]
- Fang, P.; Cen, C.-P.; Wang, X.; Tang, Z.-J.; Tang, Z.-X.; Chen, D.-S. Simultaneous removal of SO2, NO and Hg0 by wet scrubbing using urea+KMnO4 solution. Fuel Process. Technol. 2013, 106, 645–653. [Google Scholar] [CrossRef]
- Chien, T.-W.; Chu, H.; Li, Y.-C. Absorption kinetics of nitrogen oxides using sodium chlorite solutions in twin spray columns. Water Air Soil Pollut. 2005, 166, 237–250. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. Absorption kinetics of SO2/NO in KMnO4/NaOH solutions. J. Zhejiang Univ. 2009, 43, 948–952. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shen, G. NO Separation Characteristics in Integrated Electromigration Membrane Reactor. Appl. Sci. 2020, 10, 5071. https://doi.org/10.3390/app10155071
Wang Z, Shen G. NO Separation Characteristics in Integrated Electromigration Membrane Reactor. Applied Sciences. 2020; 10(15):5071. https://doi.org/10.3390/app10155071
Chicago/Turabian StyleWang, Zuwu, and Guifen Shen. 2020. "NO Separation Characteristics in Integrated Electromigration Membrane Reactor" Applied Sciences 10, no. 15: 5071. https://doi.org/10.3390/app10155071
APA StyleWang, Z., & Shen, G. (2020). NO Separation Characteristics in Integrated Electromigration Membrane Reactor. Applied Sciences, 10(15), 5071. https://doi.org/10.3390/app10155071




