First-Principles Study on the Photoelectric Properties of CsGeI3 under Hydrostatic Pressure
Abstract
1. Introduction
2. Calculation Method
3. Results and Discussion
3.1. Crystal Structure
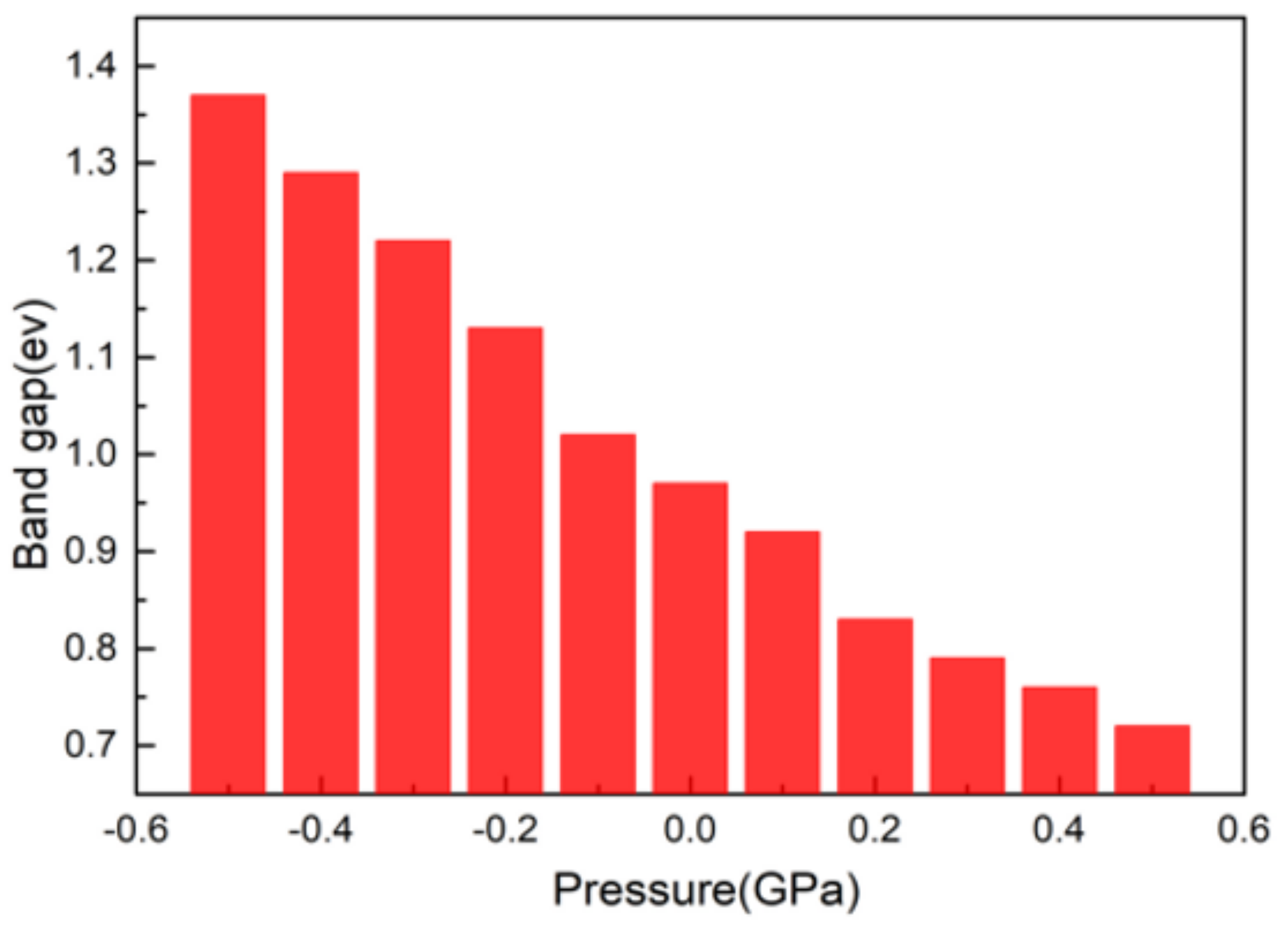
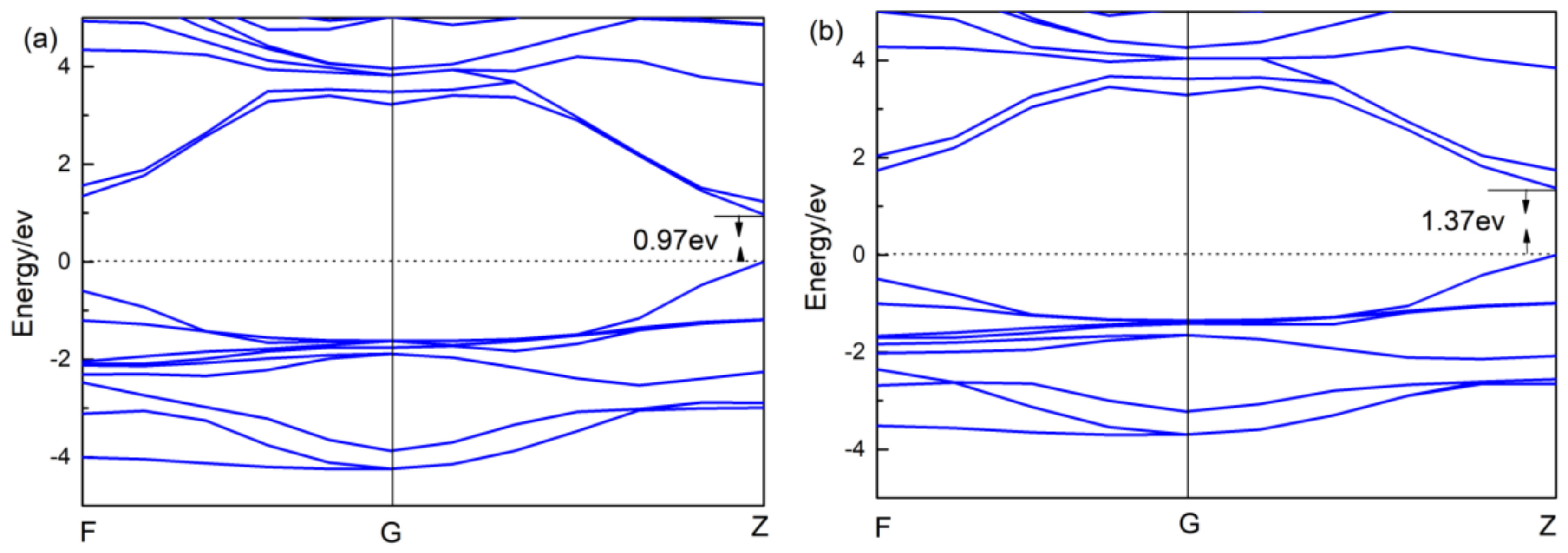
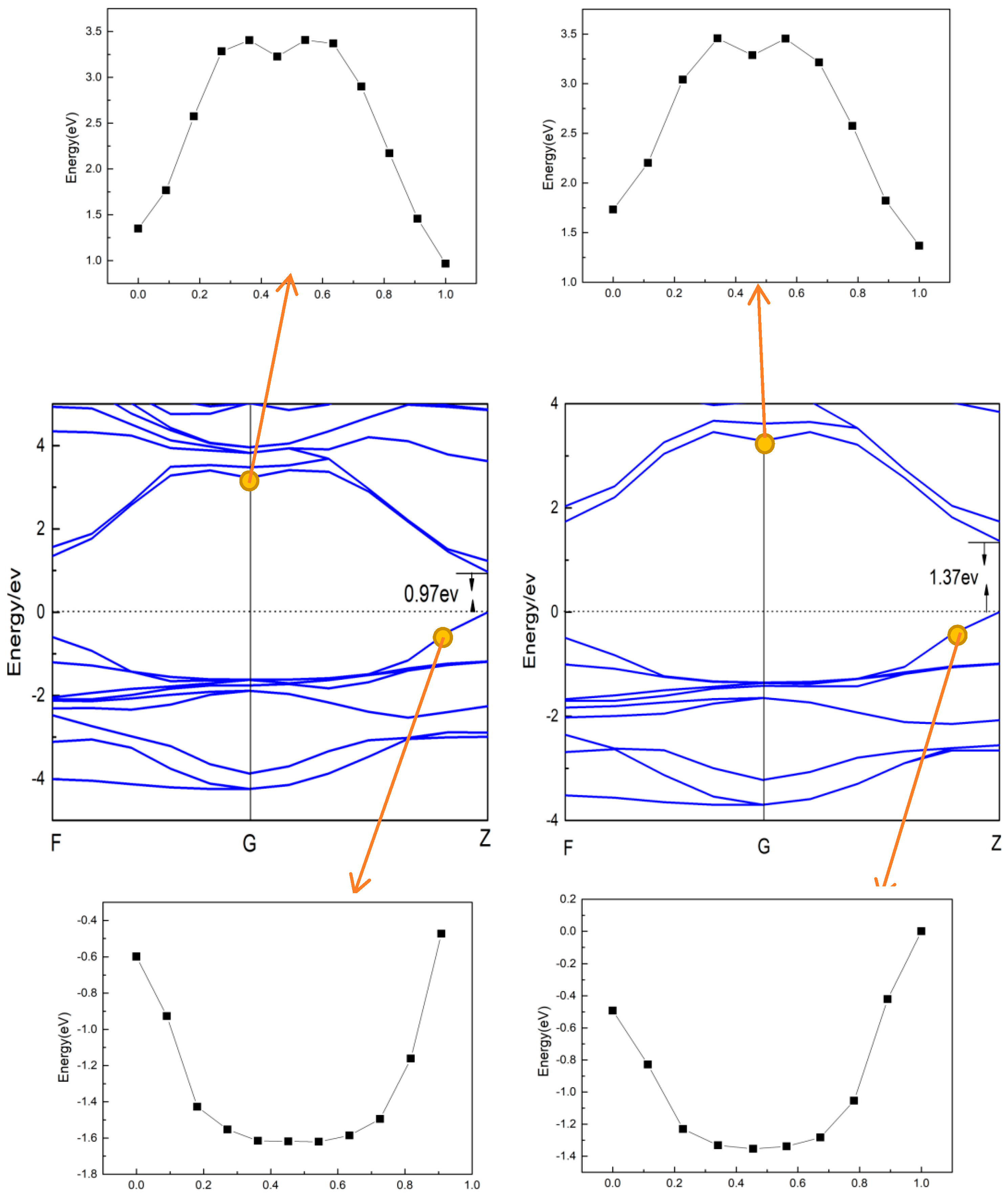
3.2. Electronic Structure
3.3. Optical Properties
3.4. Carrier Transport Properties
3.5. Elastic Properties
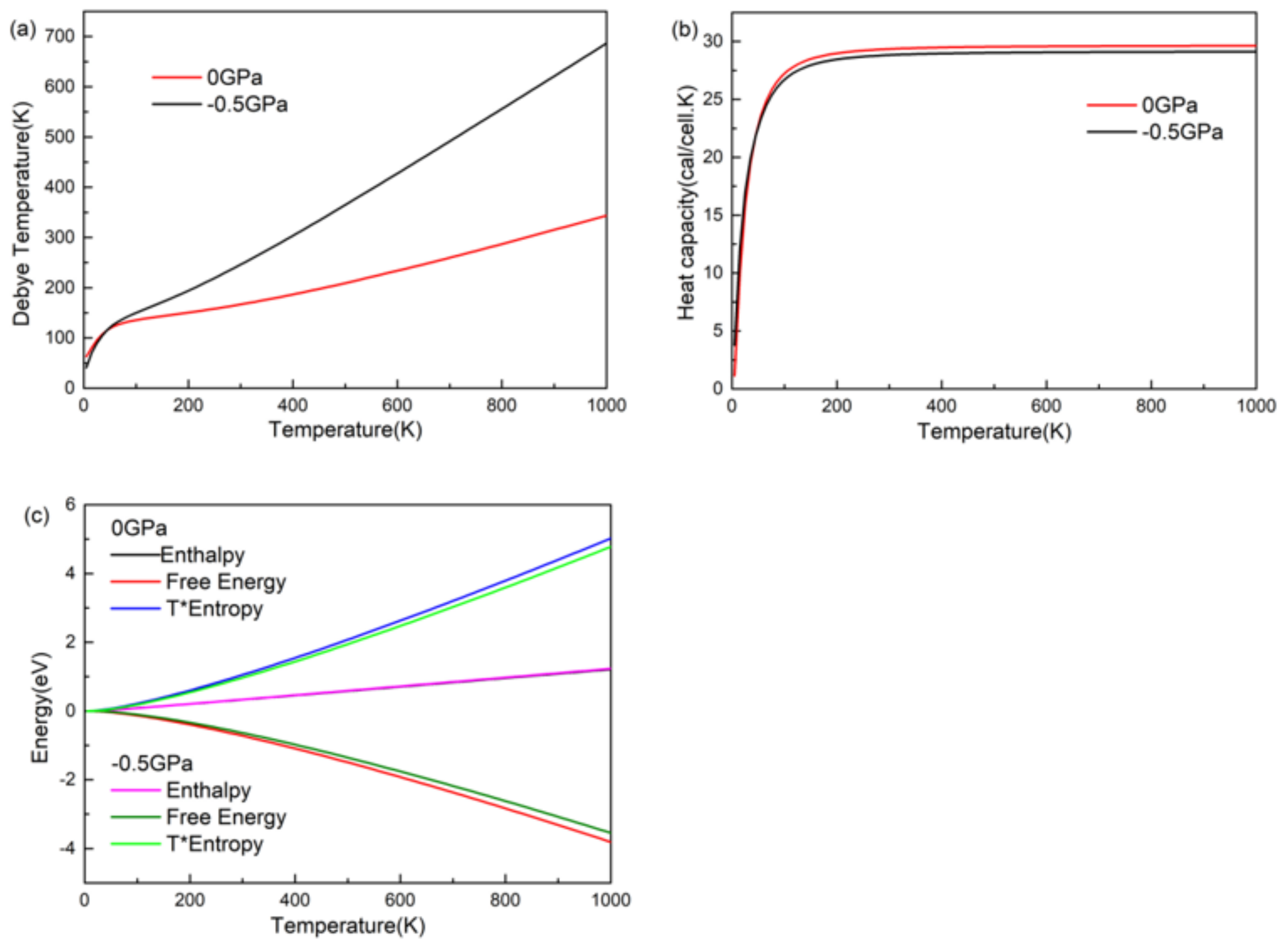
3.6. Thermodynamic Properties
4. Conclusions
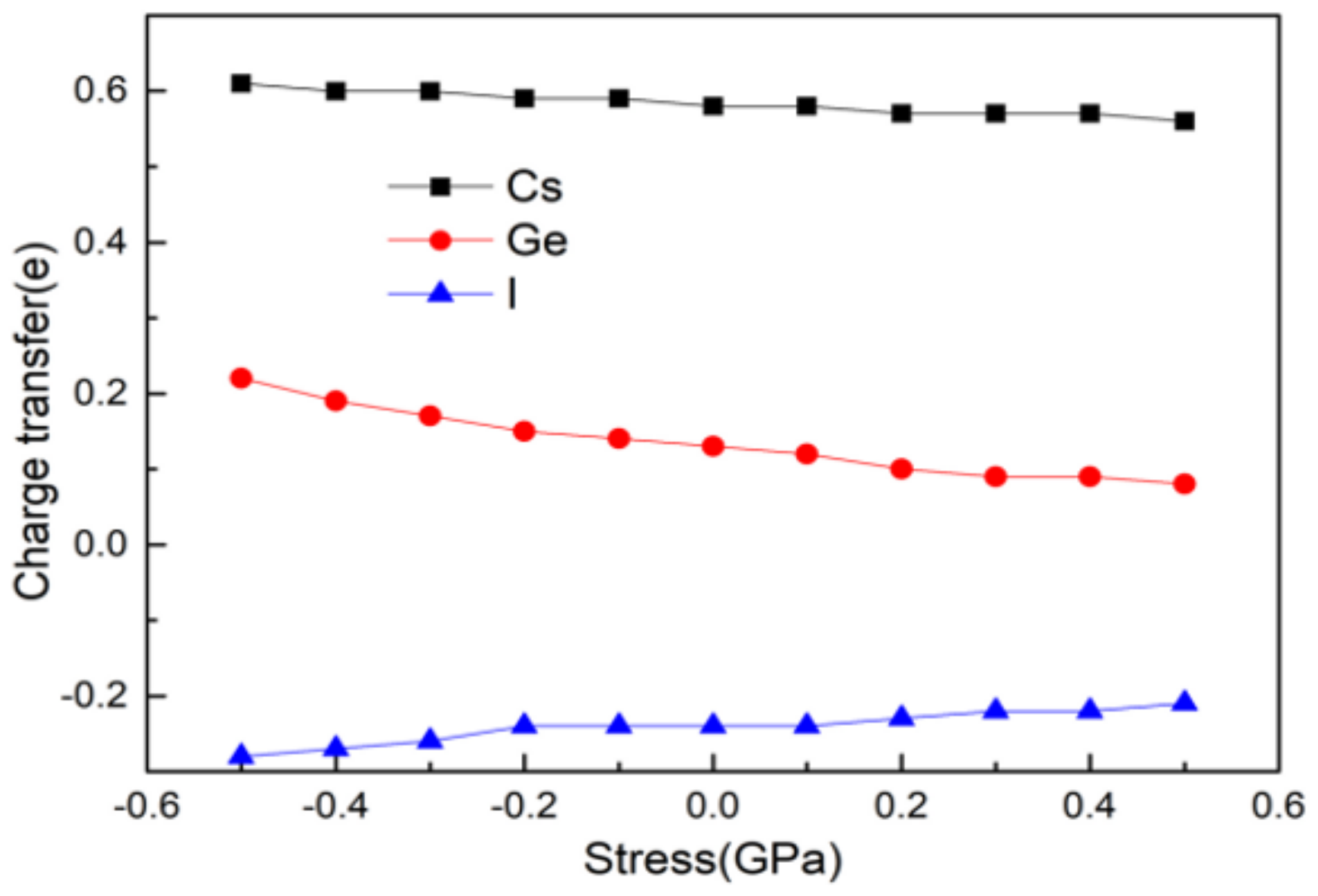
- CsGeI3 is still a direct band gap semiconductor. The band structure and DOS of CsGeI3 hardly changed after applied pressure, but the conduction band became a little flatter. When the pressure changed from −0.5 GPa to 0.5 GPa, the charge transfer number of Cs+, Ge2+, and I- decreased.
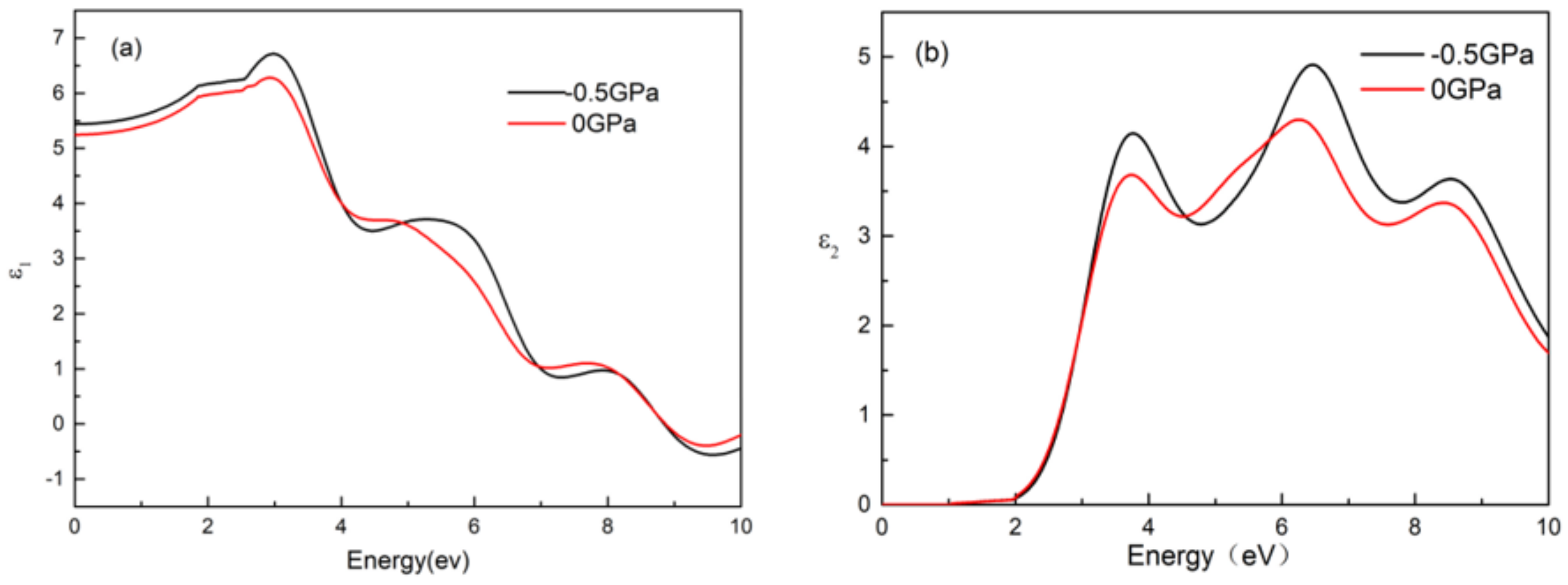
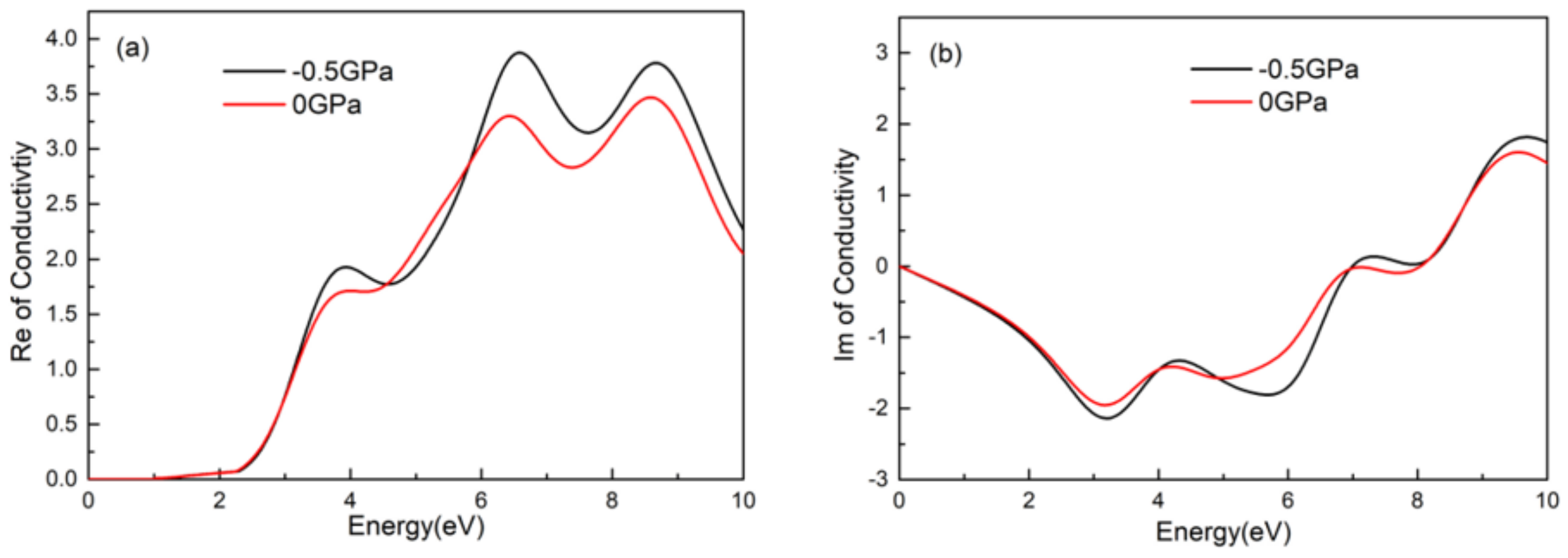
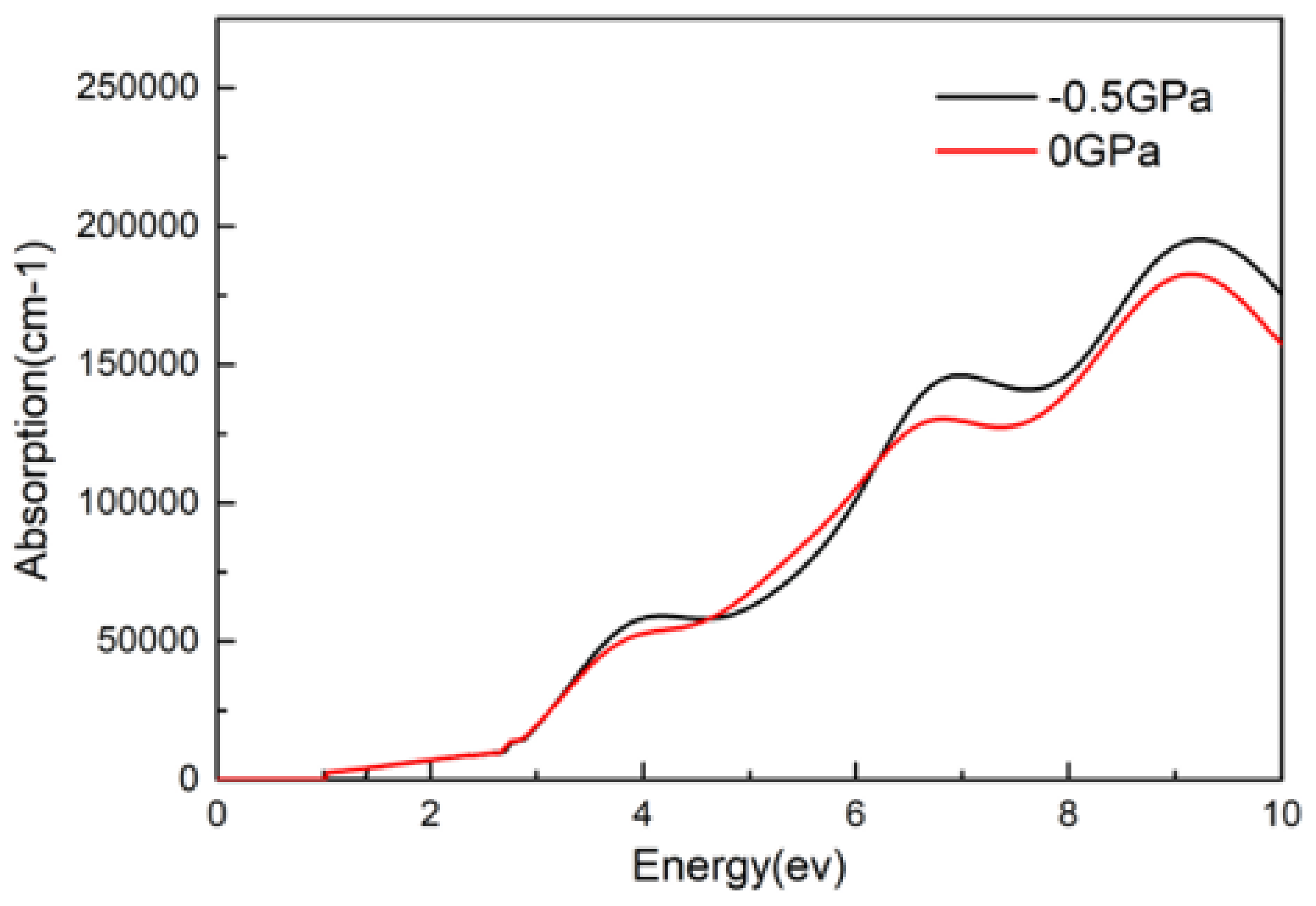
- The dielectric, conductivity, and absorption coefficient of CsGeI3 under pressure of −0.5 GPa were higher than that without pressure. Three peaks appeared simultaneously in the imaginary part of the dielectric function, the real part of the conductivity, and the absorption coefficient, located around 4.0 eV, 6.5 eV, and 8.5 eV. In either visible light or an ultraviolet region, the absorption peak was slightly larger after applied pressure than that without applied pressure, and the absorption light had a blue shift phenomenon under pressure.
- Both the effective mass and exciton binding energy were reduced after the application of pressure, indicating that photogenic carriers were more likely to be generated after pressure, and that carriers had better migration efficiency, which was conducive to improving the photoelectric conversion efficiency.
- Though multiple calculations of the Born–Huang stability criterion, the tolerance factor T, and phonon spectrum with or without virtual frequency, it was found that CsGeI3 was stable under both pressure conditions. We also calculated the elastic modulus of both pressure conditions and found that they were both soft, ductile, and anisotropic, and the values of B, B/G, and A decreased after applying pressure.
- It was found that the Debye temperature and heat capacity increased with the increase of thermodynamic temperature, and the Debye temperature increased rapidly after pressure, while the heat capacity increased slowly and eventually stabilized. Through the calculation of enthalpy, entropy, and Gibbs free energy of CsGeI3, it was found that the Gibbs free energy of CsGeI3 decreases faster with the increase of temperature without pressure, which indicates that CsGeI3 has higher stability without pressure.
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Katan, C.; Mercier, N.; Even, J. Quantum and dielectric confinement effects in lower-dimensional hybrid perovskite semiconductors. Chem. Rev. 2019, 119, 3140–3192. [Google Scholar] [CrossRef]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.Y.; Lee, Y.G.; Kim, G.; Shin, H.W.; Seok, S.I.; Lee, J.; Seo, J. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 2018, 3, 682–689. [Google Scholar] [CrossRef]
- Ehli, C.; Oelsner, C.; Guldi, D.M.; Mateo-Alonso, A.; Prato, M.; Schmidt, C.; Backes, C.; Hauke, F.; Hirsch, A. Manipulating single-wall carbon nanotubes by chemical doping and charge transfer with perylene dyes. Nat. Chem. 2009, 1, 243–249. [Google Scholar] [CrossRef]
- Piao, Y.M.; Meany, B.; Powell, L.R.; Valley, N.; Kwon, H.; Schatz, G.C.; Wang, Y.H. Brightening of carbon nanotube photoluminescence through the incorporation of sp(3) defects. Nat. Chem. 2013, 5, 840–845. [Google Scholar] [CrossRef]
- Williams, S.T.; Rajagopal, A.; Chueh, C.C.; Jen, A.K.Y. Current challenges and prospective research for upscaling hybrid perovskite photovoltaics. J. Phys. Chem. Lett. 2016, 7, 811–819. [Google Scholar] [CrossRef]
- Boix, P.P.; Agarwala, S.; Koh, T.M.; Mathews, N.; Mhaisalkar, S.G. Perovskite solar cells: Beyond methylammonium lead iodide. J. Phys. Chem. Lett. 2015, 6, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Sun, S.J.; Cheetham, A.K. Solid-state principles applied to organic-inorganic perovskites: New tricks for an old dog. Chem. Sci. 2014, 5, 4712–4715. [Google Scholar] [CrossRef]
- Sutton, R.J.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Giustino, F.; Snaith, H.J. Cubic or orthorhombic? Revealing the crystal structure of metastable black-phase CsPbl(3) by theory and experiment. Acs. Energy Lett. 2018, 3, 1787–1794. [Google Scholar] [CrossRef]
- Wang, K.; Jin, Z.W.; Liang, L.; Bian, H.; Bai, D.L.; Wang, H.R.; Zhang, J.R.; Wang, Q.; Liu, S.Z. All-inorganic cesium lead iodide perovskite solar cells with stabilized efficiency beyond 15%. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Sanehira, E.M.; Marshall, A.R.; Christians, J.A.; Harvey, S.P.; Ciesielski, P.N.; Wheeler, L.M.; Schulz, P.; Lin, L.Y.; Beard, M.C.; Luther, J.M. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 2017, 3, eaao4204. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A. Density-functional energy gaps of solids demystified. Eur. Phys. J. B 2018, 91, 108. [Google Scholar] [CrossRef]
- Ming, W.M.; Shi, H.L.; Du, M.H. Large dielectric constant, high acceptor density, and deep electron traps in perovskite solar cell material CsGeI3. J. Mater. Chem. A 2016, 4, 13852–13858. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.Y.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Ou, T.J.; Yan, J.J.; Xiao, C.H.; Shen, W.S.; Liu, C.L.; Liu, X.Z.; Han, Y.H.; Ma, Y.Z.; Gao, C.X. Visible light response, electrical transport, and amorphization in compressed organolead iodine perovskites. Nanoscale 2016, 8, 11426–11431. [Google Scholar] [CrossRef]
- Jaffe, A.; Lin, Y.; Umeyama, D.; Beavers, C.; Voss, J.; Mao, W.; Karunadasa, H. Hybrid perovskites under pressure: Accessing new properties through lattice compression. Abstr. Pap. Am. Chem. S. 2017, 253. [Google Scholar]
- Schwarz, U.; Wagner, F.; Syassen, K.; Hillebrecht, H. Effect of pressure on the optical-absorption edges of CsGeBr_ {3} and CsGeCl_ {3}. Phys. Rev. B Condens. Matter 1996, 53, 12545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Thiele, G.; Seo, D.-K.; Whangbo, M.-H.; Hillebrecht, H. Pressure-induced changes in the structure and band gap of CsGeX3 (X = CI, Br) studied by electronic. Inorg. Chem. 1998, 37, 407–410. [Google Scholar]
- Jing, H.J.; Sa, R.J.; Xu, G. Tuning electronic and optical properties of CsPbI3 by applying strain: A first-principles theoretical study. Chem. Phys. Lett. 2019, 732, 136642. [Google Scholar] [CrossRef]
- Liu, D.W.; Li, Q.H.; Jing, H.J.; Wu, K.C. Pressure-induced effects in the inorganic halide perovskite CsGeI3. Rsc. Adv. 2019, 9, 3279–3284. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for brillouin-zone integrations. Phys. Rev. B Condens. Matter 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Even, J.; Pedesseau, L.; Jancu, J.M.; Katan, C. Importance of spin-orbit coupling in hybrid organic/inorganic perovskites for photovoltaic applications. J. Phys. Chem. Lett. 2013, 4, 2999–3005. [Google Scholar] [CrossRef]
- Brivio, F.; Butler, K.T.; Walsh, A.; van Schilfgaarde, M. Relativistic quasiparticle self-consistent electronic structure of hybrid halide perovskite photovoltaic absorbers. Phys. Rev. B 2014, 89, 155204. [Google Scholar] [CrossRef]
- Tang, L.C.; Chang, C.S.; Huang, J.Y. Electronic structure and optical properties of rhombohedral CsGeI3 crystal. J. Phys. Condens. Matter 2000, 12, 9129. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid germanium iodide perovskite semiconductors: Active lone pairs, structural distortions, direct and indirect energy gaps, and strong nonlinear optical properties. J. Am. Chem. Soc. 2015, 137, 6804–6819. [Google Scholar] [CrossRef] [PubMed]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Sahin, S.; Ciftci, Y.O.; Colakoglu, K.; Korozlu, N. First principles studies of elastic, electronic and optical properties of chalcopyrite semiconductor ZnSnP2. J. Alloy. Compd. 2012, 529, 1–7. [Google Scholar] [CrossRef]
- Saha, S.; Sinha, T.P.; Mookerjee, A. Electronic structure, chemical bonding, and optical properties of paraelectric BaTiO3. Phys. Rev. B 2000, 62, 8828. [Google Scholar] [CrossRef]
- Rodina, A.V.; Dietrich, M.; Göldner, A.; Eckey, L.; Meyer, B.K. Free excitons in wurtzite GaN. Phys. Rev. B Condens. Matter 2001, 64, 115204. [Google Scholar] [CrossRef]
- Born, M. The dynamical theory of crystal lattices. Am. J. Phys. 1955, 23. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. DieGesetzeDerKrystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Li, C.H.; Lu, X.G.; Ding, W.Z.; Feng, L.M.; Gao, Y.H.; Guo, Z.G. Formability of ABX(3) (X = F, Cl, Br, I) halide perovskites. Acta Cryst. B 2008, 64, 702–707. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Afsari, M.; Boochani, A.; Hantezadeh, M. Electronic, optical and elastic properties of cubic perovskite CsPbI3: Using first principles study. Opt. Int. J. Light Electron Opt. 2016, 127, 11433–11443. [Google Scholar] [CrossRef]
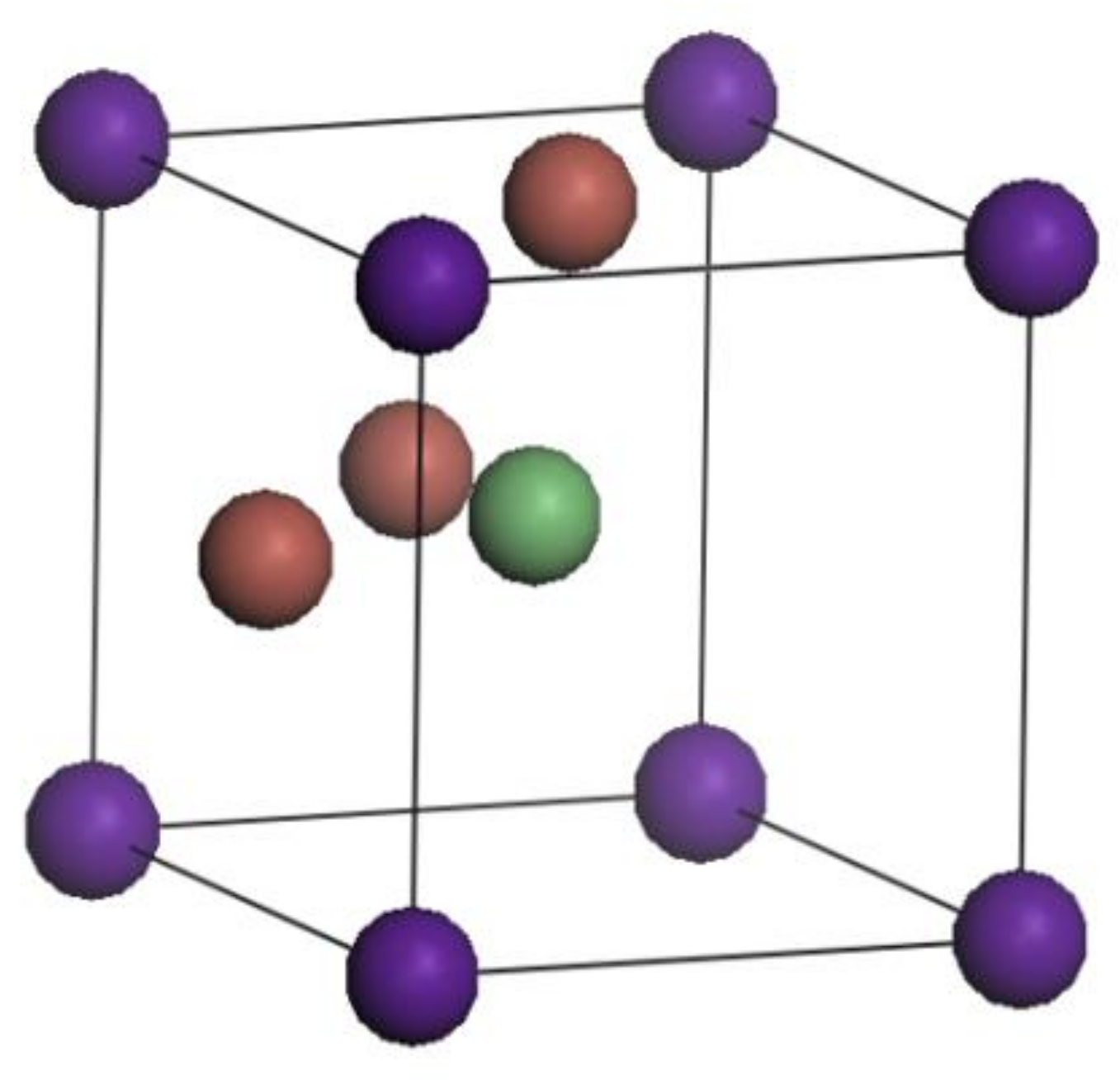
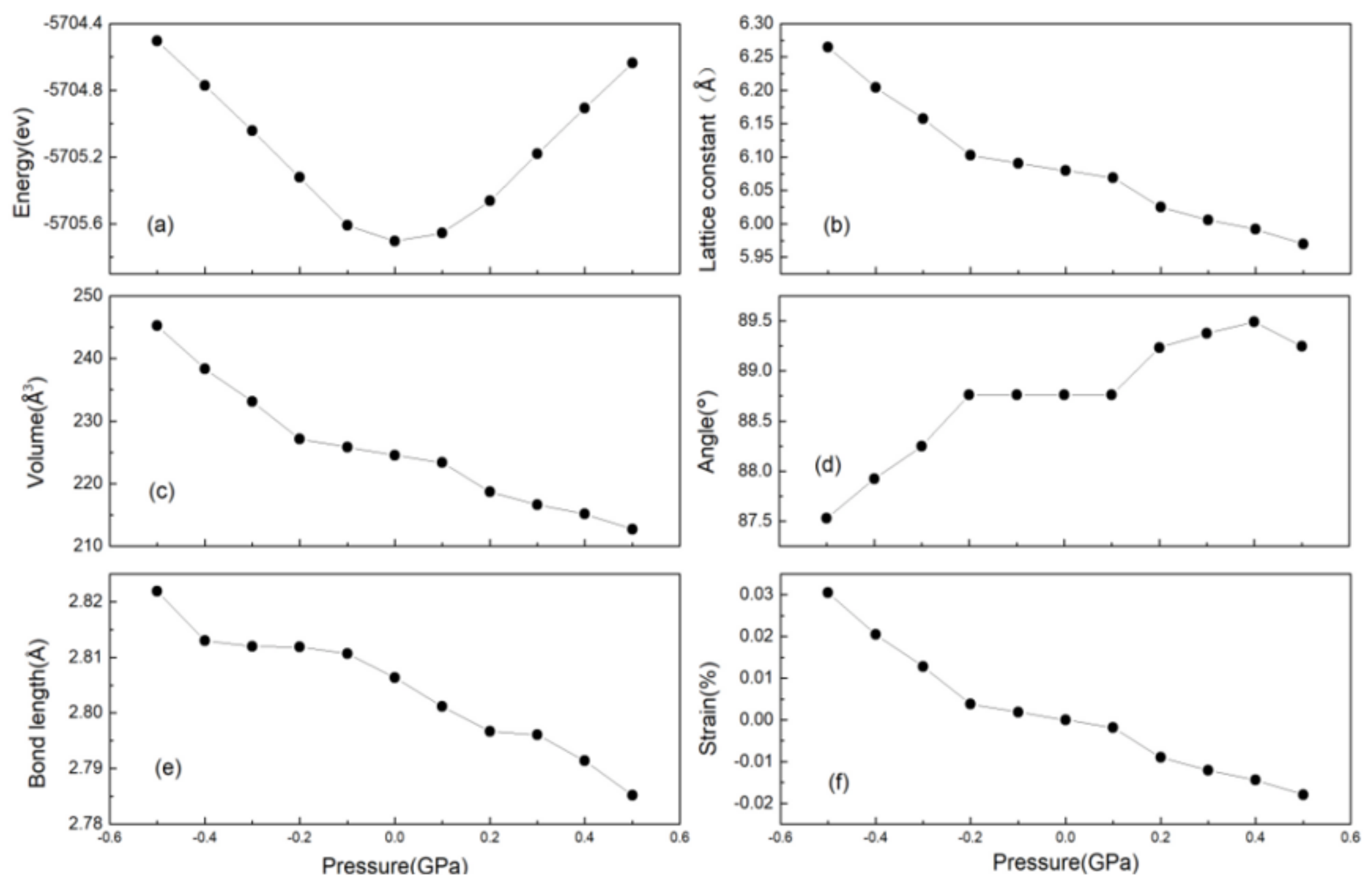


| a = b = c (Å) | α = β = γ (°) | V (Å3) | Space Group | |
|---|---|---|---|---|
| Findit | 5.98 | 88.61 | 213.98 | R-3m |
| GO | 6.08 | 88.76 | 224.55 | R-3m |
| me | mh | μ | εs | Eb (ev) | |
|---|---|---|---|---|---|
| 0 GPa | 0.27 | 0.34 | 0.15 | 5.2 | 0.075 |
| −0.5 GPa | 0.23 | 0.25 | 0.12 | 5.5 | 0.054 |
| C11 | C12 | C13 | C14 | C33 | C44 | B | G | A | |
|---|---|---|---|---|---|---|---|---|---|
| 0 GPa | 60.07 | 48.61 | 32.61 | 22.32 | 74.74 | 23.03 | 46.95 | 10.54 | 4.02 |
| −0.5 GPa | 48.52 | 38.88 | 25.83 | 17.21 | 61.03 | 13.64 | 37.68 | 15.11 | 2.83 |
| Cs+ | Ge2+ | I− | T | |
|---|---|---|---|---|
| R (nm) | 0.167 | 0.073 | 0.22 | 0.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.-K.; Tang, Y.-L.; Diao, X.-F. First-Principles Study on the Photoelectric Properties of CsGeI3 under Hydrostatic Pressure. Appl. Sci. 2020, 10, 5055. https://doi.org/10.3390/app10155055
Gao L-K, Tang Y-L, Diao X-F. First-Principles Study on the Photoelectric Properties of CsGeI3 under Hydrostatic Pressure. Applied Sciences. 2020; 10(15):5055. https://doi.org/10.3390/app10155055
Chicago/Turabian StyleGao, Li-Ke, Yan-Lin Tang, and Xin-Feng Diao. 2020. "First-Principles Study on the Photoelectric Properties of CsGeI3 under Hydrostatic Pressure" Applied Sciences 10, no. 15: 5055. https://doi.org/10.3390/app10155055
APA StyleGao, L.-K., Tang, Y.-L., & Diao, X.-F. (2020). First-Principles Study on the Photoelectric Properties of CsGeI3 under Hydrostatic Pressure. Applied Sciences, 10(15), 5055. https://doi.org/10.3390/app10155055




