Layer-by-Layer Deposition of Copper and Phosphorus Compounds to Develop Flame-Retardant Polyamide 6/Montmorillonite Hybrid Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing Methods
2.3. Ionic Solutions Preparation
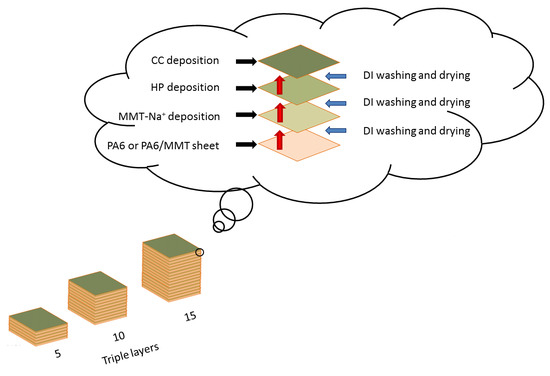
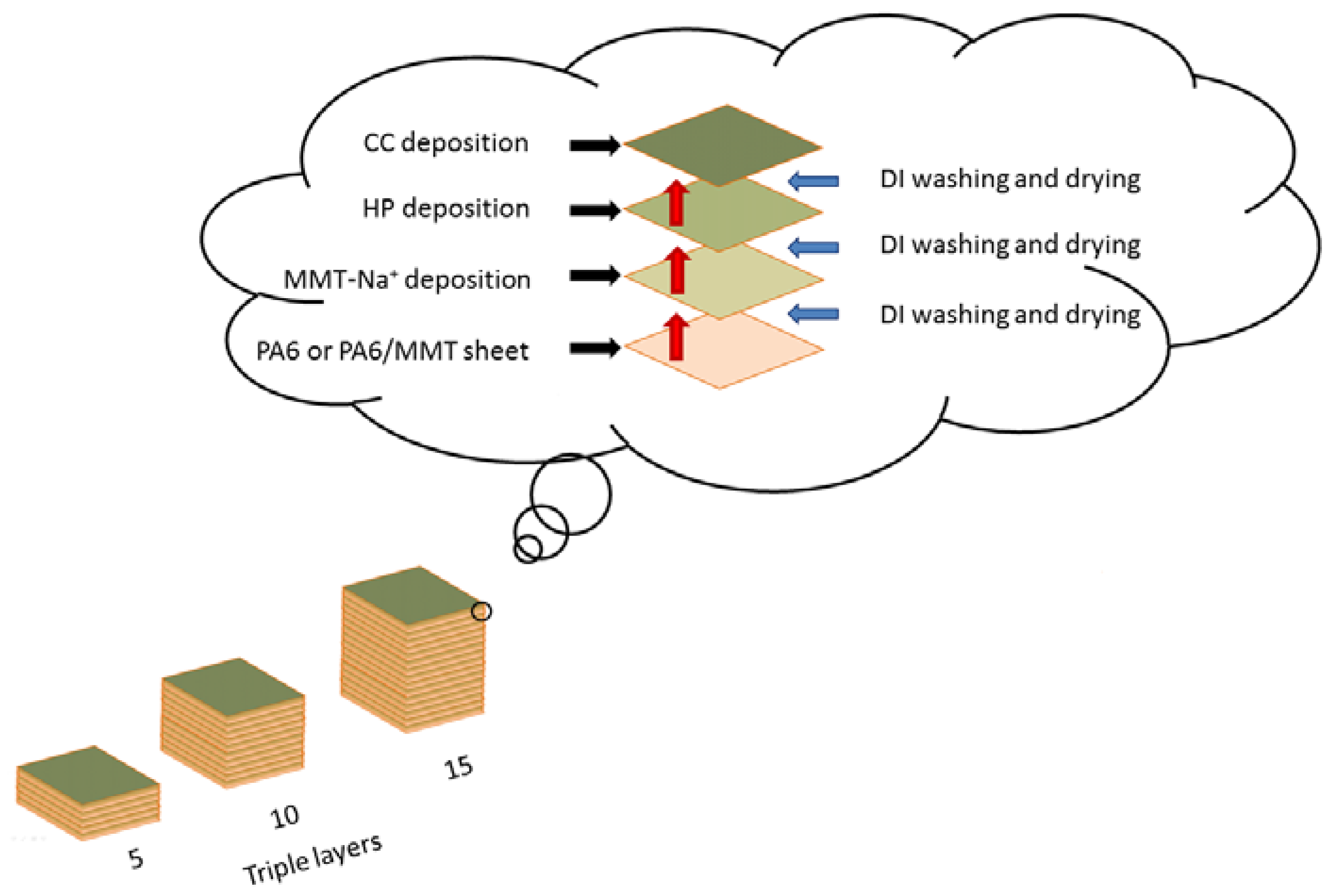
2.4. Layer-by-Layer Deposition (LbL)
2.5. Thermogravimetric Analysis
2.6. Flammability Testing
2.7. Scanning Electron Microscopy (SEM)
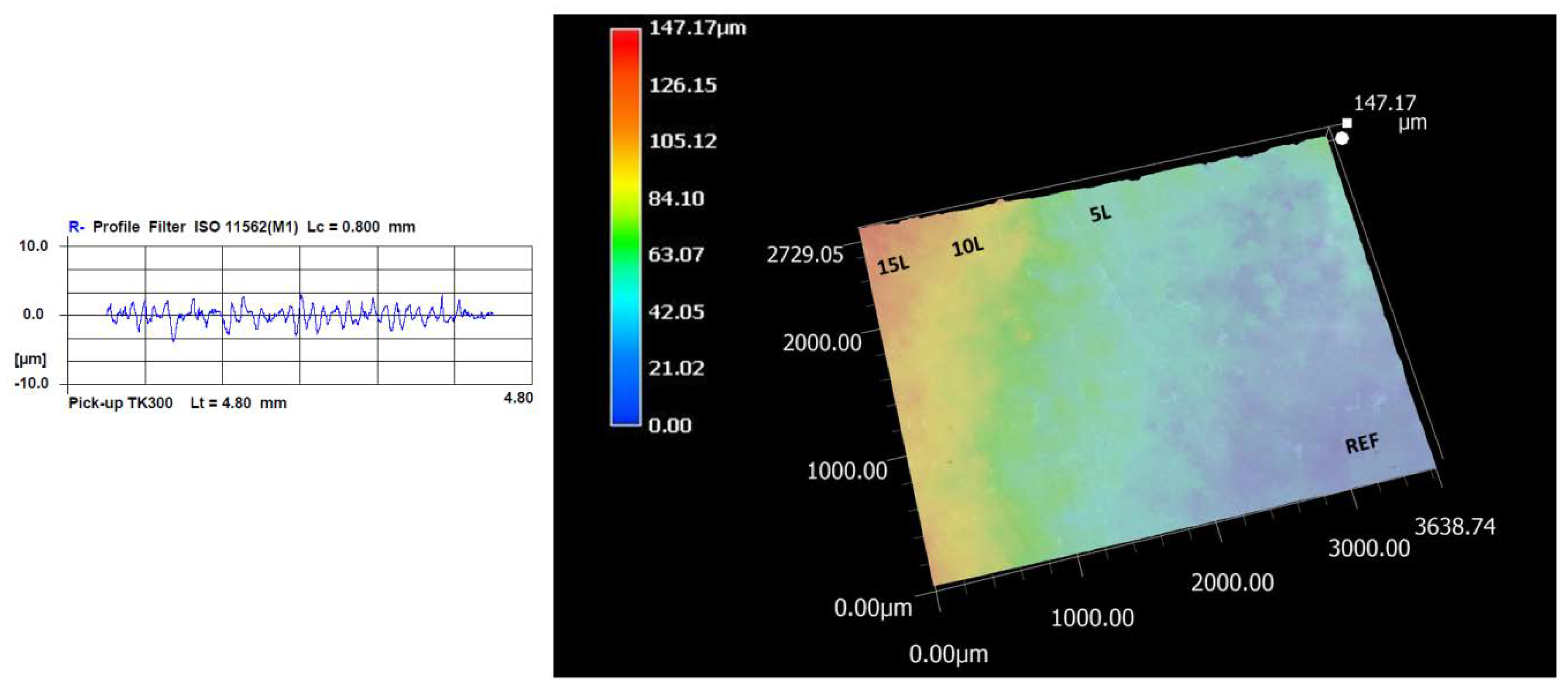
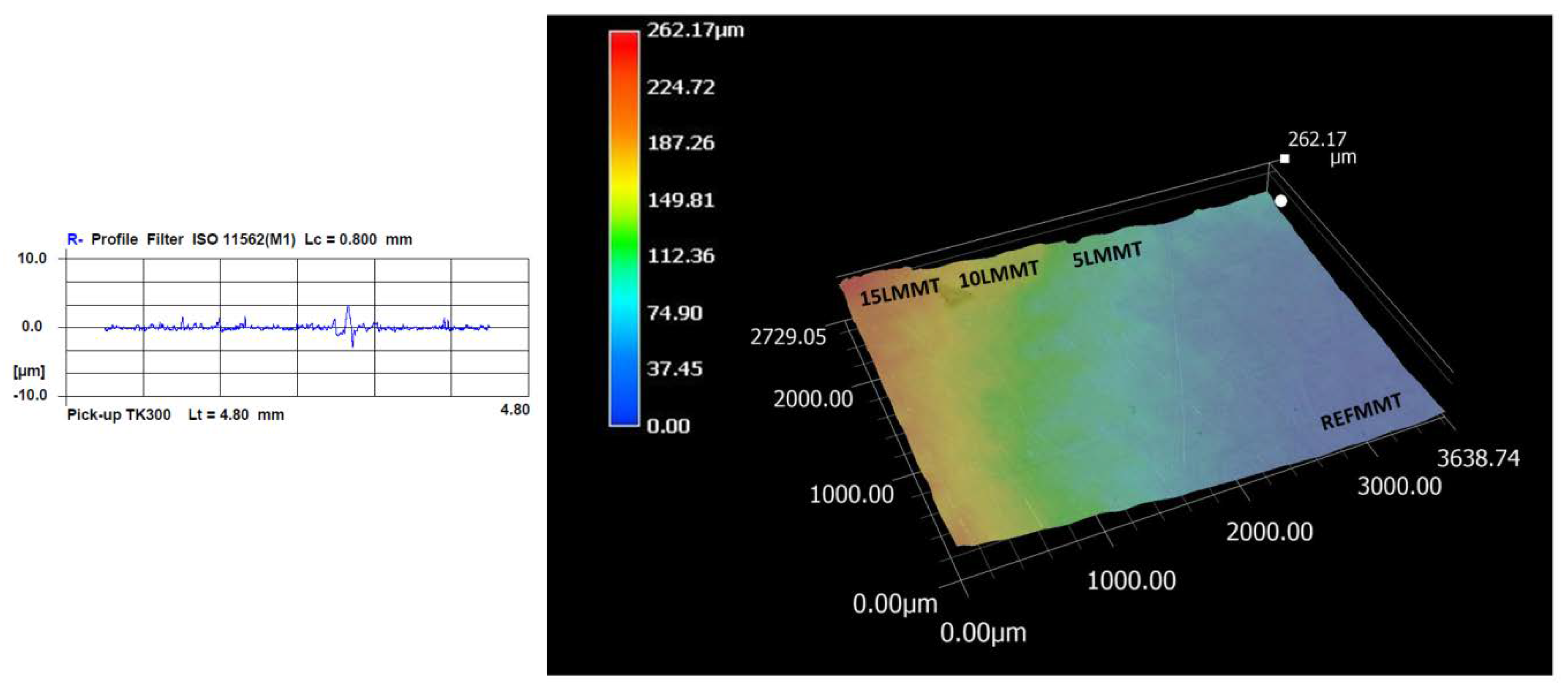
2.8. Surface Roughness and 3D Scanning
3. Results and Discussion
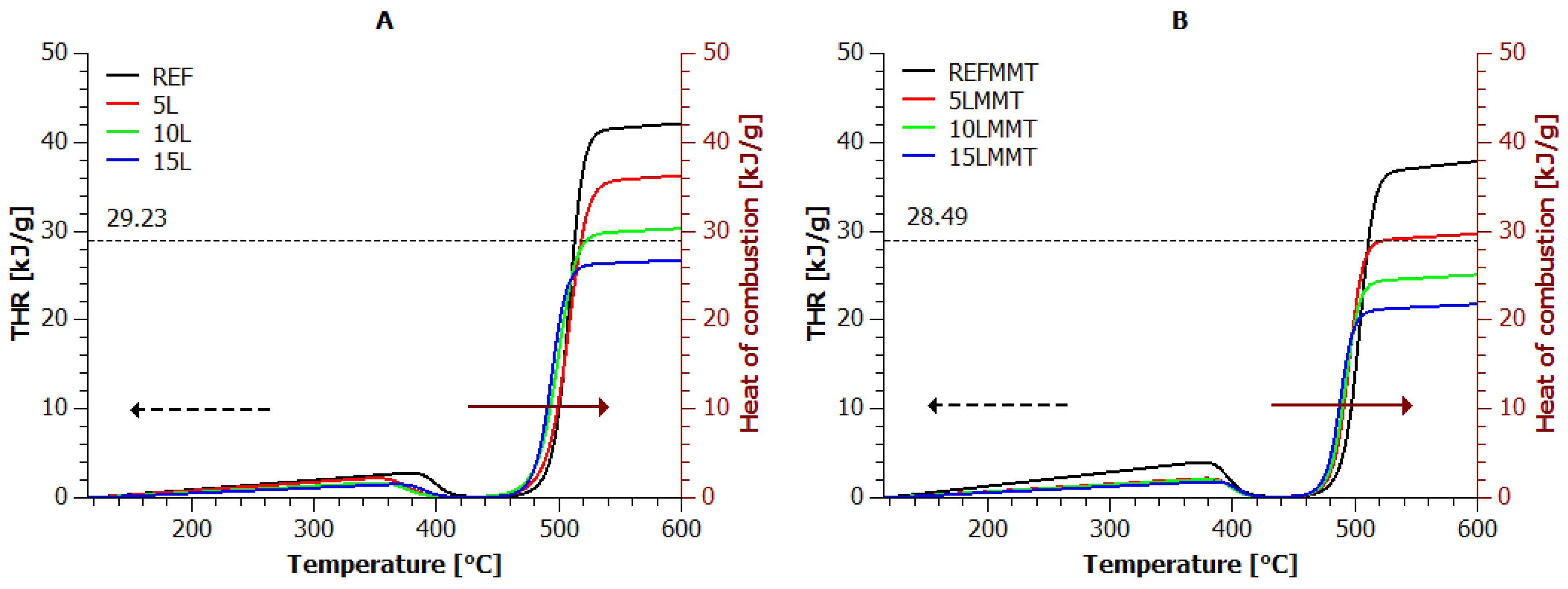
3.1. Thermogravimetric Analysis
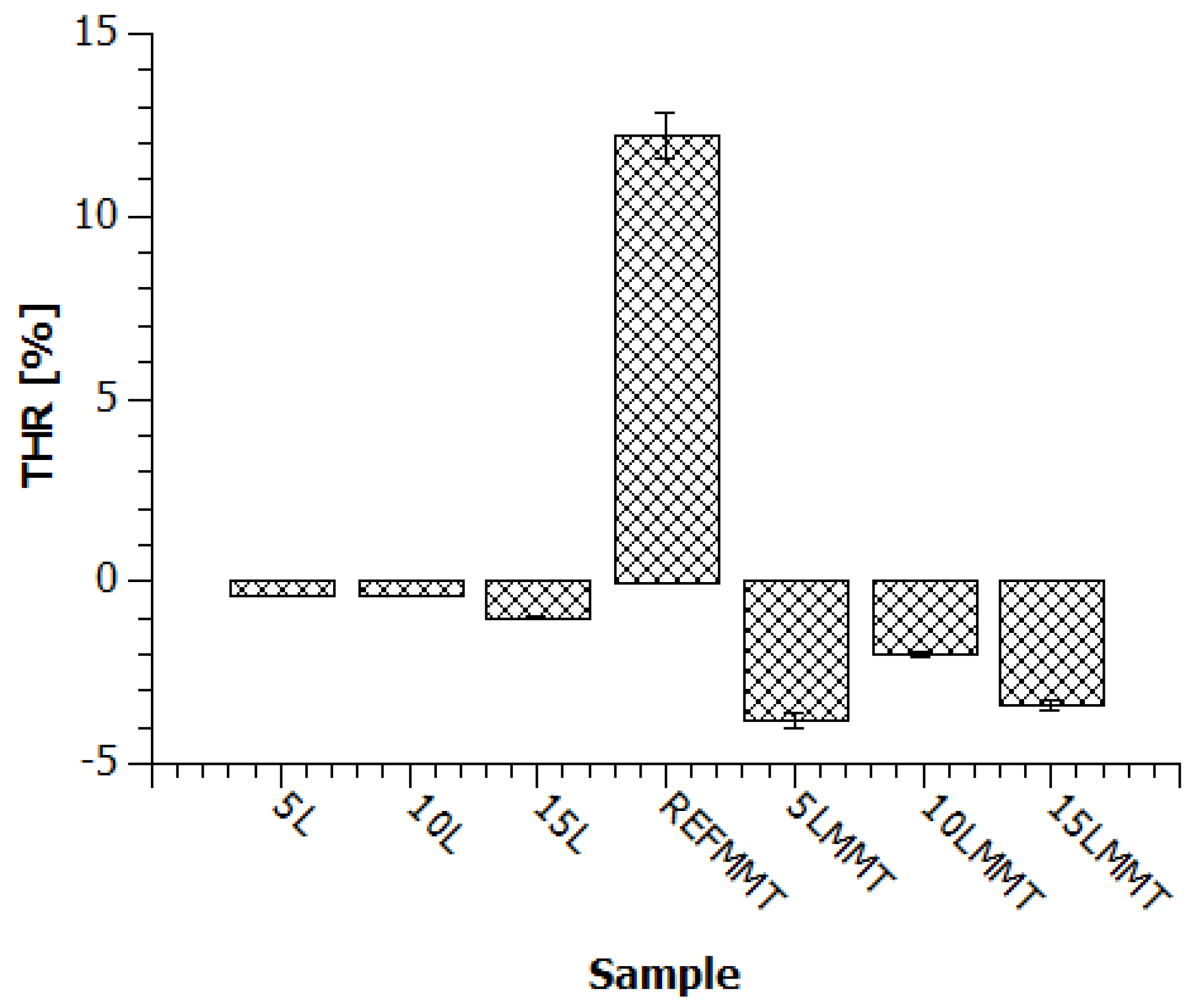
3.2. Limiting Oxygen Index (LOI)
3.3. Vertical (VFT) and Horizontal (HFT) Flammability Tests (UL-94)
- The material does not burn visibly, and the flame front does not reach the measuring line beginning (25 mm); and/or
- The flame front reaches the entire range of the measuring section (100 mm) at the same time not exceeding the measurement end line; and/or
- The flame front exceeds the measuring range, and the combustion speed for the samples with a thickness of 3–13 mm is not greater than 40 mm/min [34].
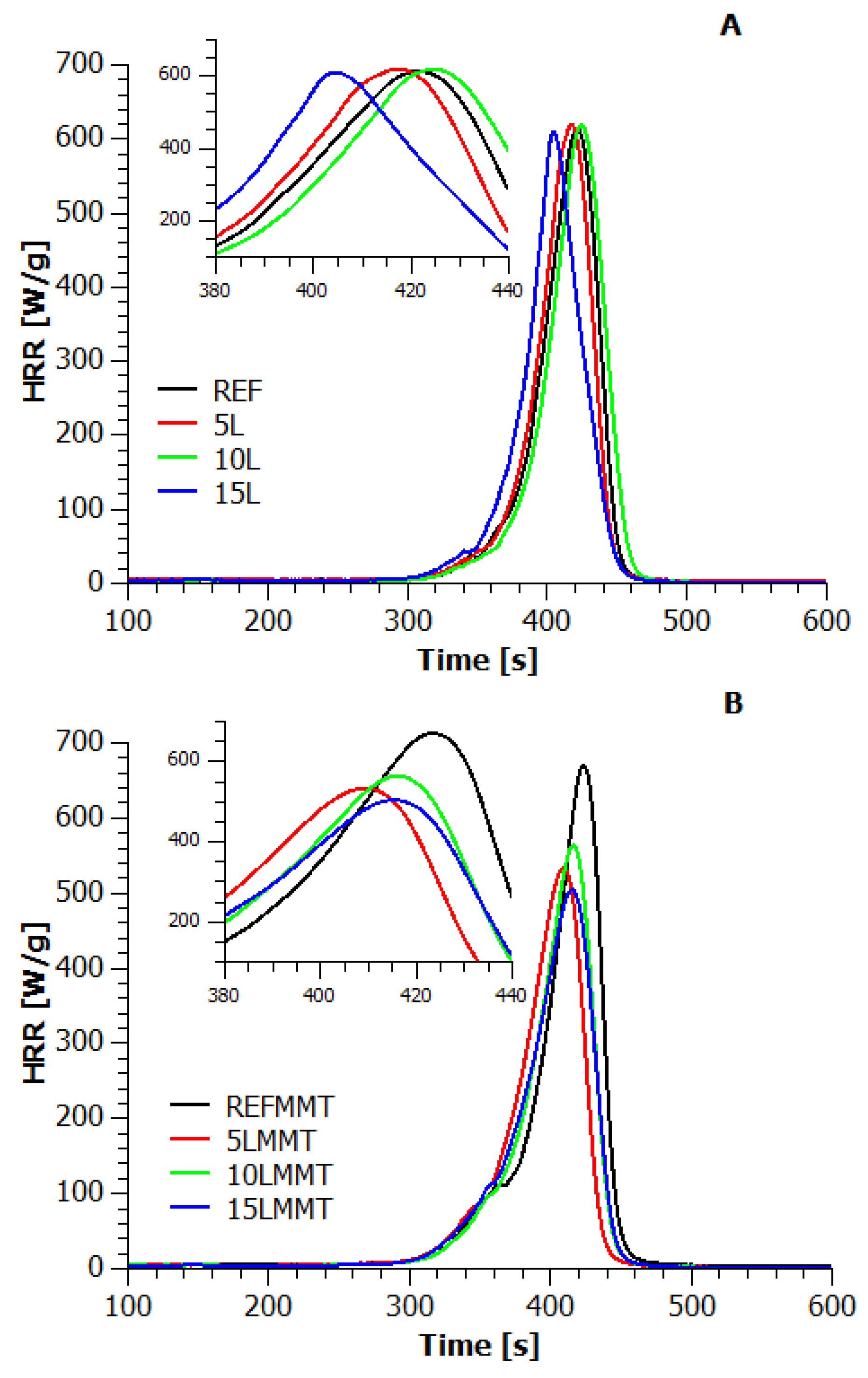
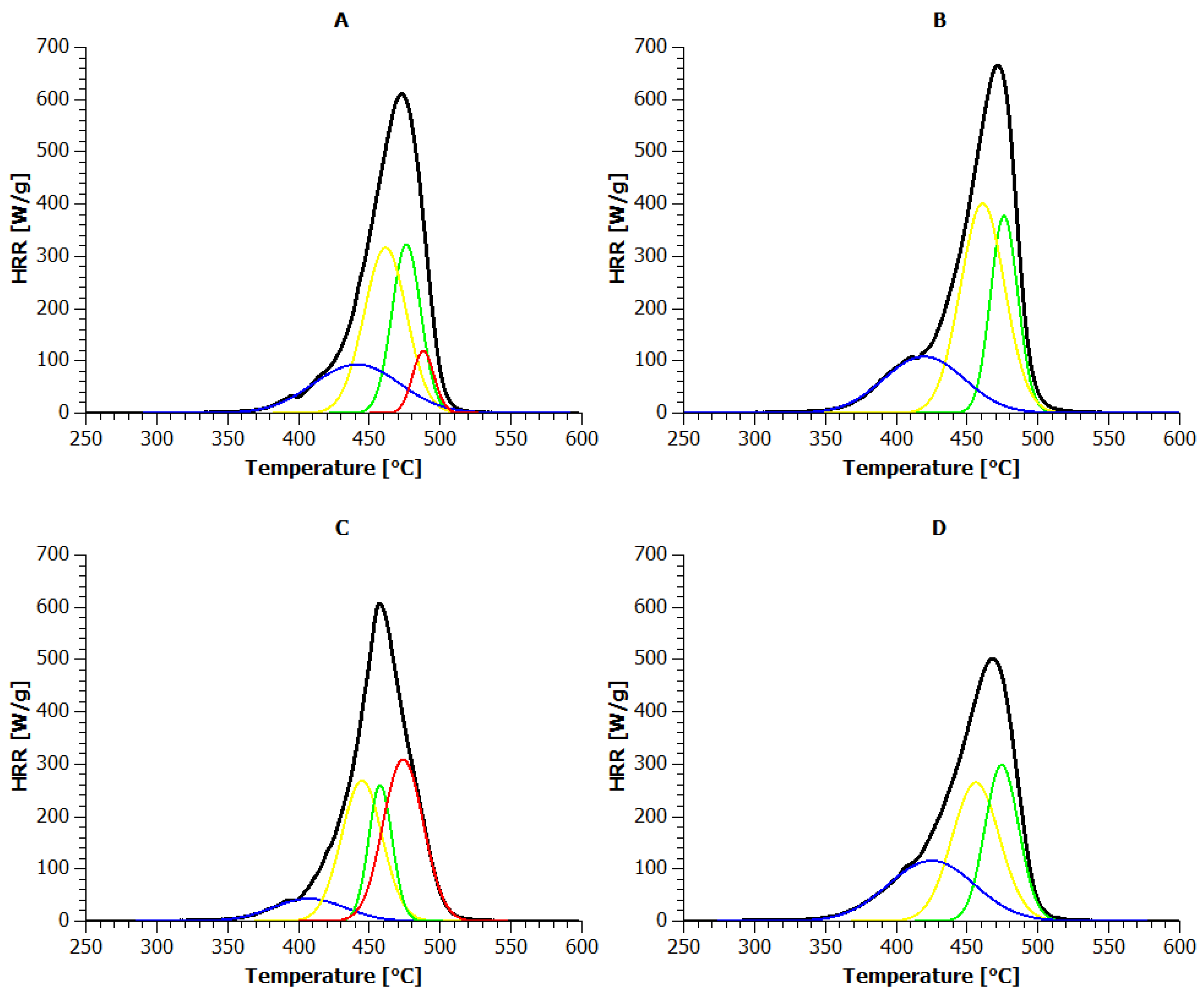
3.4. Microscale Combustion Calorimetry (MCC)
3.5. TG vs. MCC
- the reaction rate of weight loss, s−1;
- the weight derivative over time;
- the initial mass of the sample, kg;
- the final mass of the sample, kg;
- the reaction rate of heat release, s−1;
- the specific heat release rate, W/g;
- the heat of combustion per unit mass of the sample, J/g;
- the total heat of combustion per unit mass of the sample, J/g.
3.6. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, K.; Li, Y.; Tao, L.; Xiao, R. Preparation and characterization of polyamide 6 fibre based on a phosphorus-containing flame retardant. RSC Adv. 2018, 8, 9261–9271. [Google Scholar] [CrossRef]
- Majka, T.M.; Leszczyńska, A.; Kandola, B.K.; Pornwannachai, W.; Pielichowski, K. Modification of organo-montmorillonite with disodium H-phosphonate to develop flame retarded polyamide 6 nanocomposites. Appl. Clay Sci. 2017, 139, 28–39. [Google Scholar] [CrossRef]
- He, W.-T.; Liao, S.-T.; Xiang, Y.-S.; Long, L.-J.; Qin, S.-H.; Yu, J. Structure and Properties Study of PA6 Nanocomposites Flame Retarded by Aluminium Salt of Diisobutylphosphinic Acid and Different Organic Montmorillonites. Polymers 2018, 10, 312. [Google Scholar] [CrossRef]
- Horrocks, R.; Sitpalan, A.; Zhou, C.; Kandola, B.K. Flame retardant polyamide fibres: The challenge of minimising flame retardant additive contents with added nanoclays. Polymers 2016, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, H.; Xiang, Y.; Long, L.; Qin, S.; Yu, J. Enhancement of flame retardancy and mechanical properties of polyamide 6 by incorporating an aluminum salt of diisobutylphosphinic combined with organoclay. Polym. Degrad. Stab. 2017, 144, 442–453. [Google Scholar] [CrossRef]
- Liu, T.; Wang, R.; Dong, Z.-F.; Zhu, Z.-G.; Zhang, X.-Q.; Liu, J.-G. Role of caged bicyclic pentaerythritol phosphate alcohol in flame retardancy of PA6 and mechanism study. J. Appl. Polym. Sci. 2018, 135, 46236. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, L.; Fischer, A.; Wang, L.; Drummer, D.; Wu, W. The effect of hBN on the flame retardancy and thermal stability of P-N flame retardant PA6. J. Macromol. Sci. Part A 2018, 55, 17–23. [Google Scholar] [CrossRef]
- Yuan, L.; Feng, S.; Hu, Y.; Fan, Y. Effect of char sulfonic acid and ammonium polyphosphate on flame retardancy and thermal properties of epoxy resin and polyamide composites. J. Fire Sci. 2017, 35, 521–534. [Google Scholar] [CrossRef]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials 2017, 10, 784. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The potential of metal oxalates as novel flame retardants and synergists for engineering polymers. Polym. Degrad. Stab. 2014, 110, 290–297. [Google Scholar] [CrossRef]
- Li, M.; Cui, H.; Li, Q.; Zhang, Q. Thermally conductive and flame—Retardant polyamide 6 composites. J. Reinf. Plast. Compos. 2016, 35, 435–444. [Google Scholar] [CrossRef]
- Zope, I.S.; Dasari, A.; Guan, F.; Yu, Z.Z. Influence of metal ions on thermo-oxidative stability and combustion response of polyamide 6/clay nanocomposites. Polymer 2016, 92, 102–113. [Google Scholar] [CrossRef]
- Zhan, Z.; Xu, M.; Li, B. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Kadir, A.; Abdelghani, L.; Vincent, B.; Maude, J.; Serge, B.; Valérie, T.; David, R. Polyallylamine-montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stab. 2013, 98, 627–634. [Google Scholar]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Malucelli, G. Surface-Engineered Fire Protective Coatings for Fabrics through Sol-Gel and Layer-by-Layer Methods: An Overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Intumescent coating of (polyallylamine-polyphosphates) deposited on polyamide fabrics via layer-by-layer technique. Polym. Degrad. Stab. 2014, 106, 158–164. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Ruch, D. Layer-by-layer deposition of a TiO2-filled intumescent coating and its effect on the flame retardancy of polyamide and polyester fabrics. Colloids Surf. A Pchysicochem. Eng. Asp. 2015, 469, 1–10. [Google Scholar] [CrossRef]
- Mosurkal, R.; Muller, W.S. Layer-By-Layer Assembly of Halogen-Free Polymeric Materials on Nylon/Cotton. Fire Mater. 2014, 40, 206–2018. [Google Scholar]
- Kundu, C.; Wang, W.; Zhou, S.; Wang, X.; Sheng, H.; Pan, Y.; Song, L.; Hu, Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr. Polym. 2017, 166, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kundu, C.K.; Wang, X.; Song, L.; Hu, Y. Borate cross-linked layer-by-layer assembly of green polyelectrolytes on polyamide 66 fabrics for flame-retardant treatment. Prog. Org. Coat. 2018, 121, 173–181. [Google Scholar] [CrossRef]
- Majka, T.M.; Cokot, M.; Pielichowski, K. Studies on the thermal properties and flammability of polyamide 6 nanocomposites surface-modified via layer-by-layer deposition of chitosan and montmorillonite. J. Anal. Calorim. 2018, 131, 405–416. [Google Scholar] [CrossRef]
- Abbaszadeh, M.; Krizak, D.; Kundu, S. Layer-by-layer assembly of graphene oxide nanoplatelets embedded desalination membranes with improved chlorine resistance. Desalination 2019, 470, 114116. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kundu, C.K.; Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Hybrid coatings for durable flame retardant and hydrophilic treatment of Polyamide 6.6 fabrics. Prog. Org. Coat. 2020, 144, 105640. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Song, L.; Hu, Y. Chitosan-based flame retardant coatings for polyamide 66 textiles: One-pot deposition versus layer-by-layer assembly. Int. J. Biol. Macromol. 2020, 143, 1–10. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Liu, L.X.; Song, L.; Hu, Y. Few layer deposition and sol-gel finishing of organic-inorganic compounds for improved flame retardant and hydrophilic properties of polyamide 66 textiles: A hybrid approach. Prog. Org. Coat. 2019, 129, 318–326. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Fouquet, T.; Jimenez, M.; Bourbigot, S.; Ruch, D. Mechanistic investigation of a flame retardant coating made by layer-by-layer assembly. RSC Adv. 2014, 4, 43326–43334. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, L.; Yang, Y.; Zhang, S.; Huang, Y.; Bielawski, C.W.; Geng, J. Core-Shell Structured Polyamide 66 Nanofibers with Enhanced Flame Retardancy. ACS Omega 2017, 2, 2665–2671. [Google Scholar] [CrossRef]
- Wu, H.; Krifa, M.; Koo, J.H. Flame retardant polyamide 6/nanoclay/intumescent nanocomposite fibers through electrospinning. Text. Res. J. 2014, 84, 1106–1118. [Google Scholar] [CrossRef]
- Katsoulis, C.; Kandare, E.; Kandola, B.K. The effect of nanoparticles on structural morphology, thermal and flammability properties of two epoxy resins with different functionalities. Polym. Degrad. Stab. 2011, 96, 529–540. [Google Scholar] [CrossRef]
- Castrovinci, A.; Camino, G. Fire-Retardant Mechanisms in Polymer Nano-Composite Materials. In Multifunctional Barriers for Flexible Structure: Textile, Leather and Paper, 1st ed.; Camino, G., Duquesne, S., Magniez, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 97, pp. 87–108. [Google Scholar]
- Zhang, W.; He, X.; Song, R.; Jiao, Q.; Yang, R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stab. 2014, 109, 209–217. [Google Scholar] [CrossRef]
- Technical Committee: ISO/TC 61/SC 4 Burning Behavior, ISO 1210: 1992 Plastics—Determination of the Burning Behaviour of Horizontal and Vertical Specimens in Contact with a Small-Flame Ignition Source, ICS: 13.220.40 Lgnitability and Burning Behaviour of Materials and Products 83.080.01 Plastics in General. Available online: www.iso.org (accessed on 11 February 2018).
- Singla, P.; Mehta, R.; Upadhyay, S.N. Clay Modification by the Use of Organic Cations. Green Sustain. Chem. 2012, 2, 21–25. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; König, A.; Deglmann, P.; Uske, K.; Minges, C. BASF SE, Flame-Retardant Polyamides with Pale Color. U.S. Patent 20140080949A1, 20 March 2014. [Google Scholar]
- Faraji, S.; Rahim, A.A.; Mohamed, N.; Sipaut, C.S. A study of electroless copper-phosphorus coatings with the addition of silicon carbide (SiC) and graphite (Cg) particles. Surf. Coat. Technol. 2011, 206, 1259–1268. [Google Scholar] [CrossRef]
- Yi, D.; Yang, H.; Zhao, M.; Huang, L.; Camino, G.; Frache, A.; Yanga, R. A novel, low surface charge density, anionically modified montmorillonite for polymer nanocomposites. RSC. Adv. 2017, 7, 5980–5988. [Google Scholar] [CrossRef]
- Berkowicz, D.; Majka, T.M.; Żukowski, W. The pyrolysis and combustion of polyoxymethylene in a fluidised bed with the possibility of incorporating CO2. Energy Convers. Manag. 2020, 214, 112888. [Google Scholar] [CrossRef]
- Whitehouse, D. Surface and their measurements. In Kogan Page Science Paper Edition; Butterworth-Heinemann: London, UK, 2006. [Google Scholar]
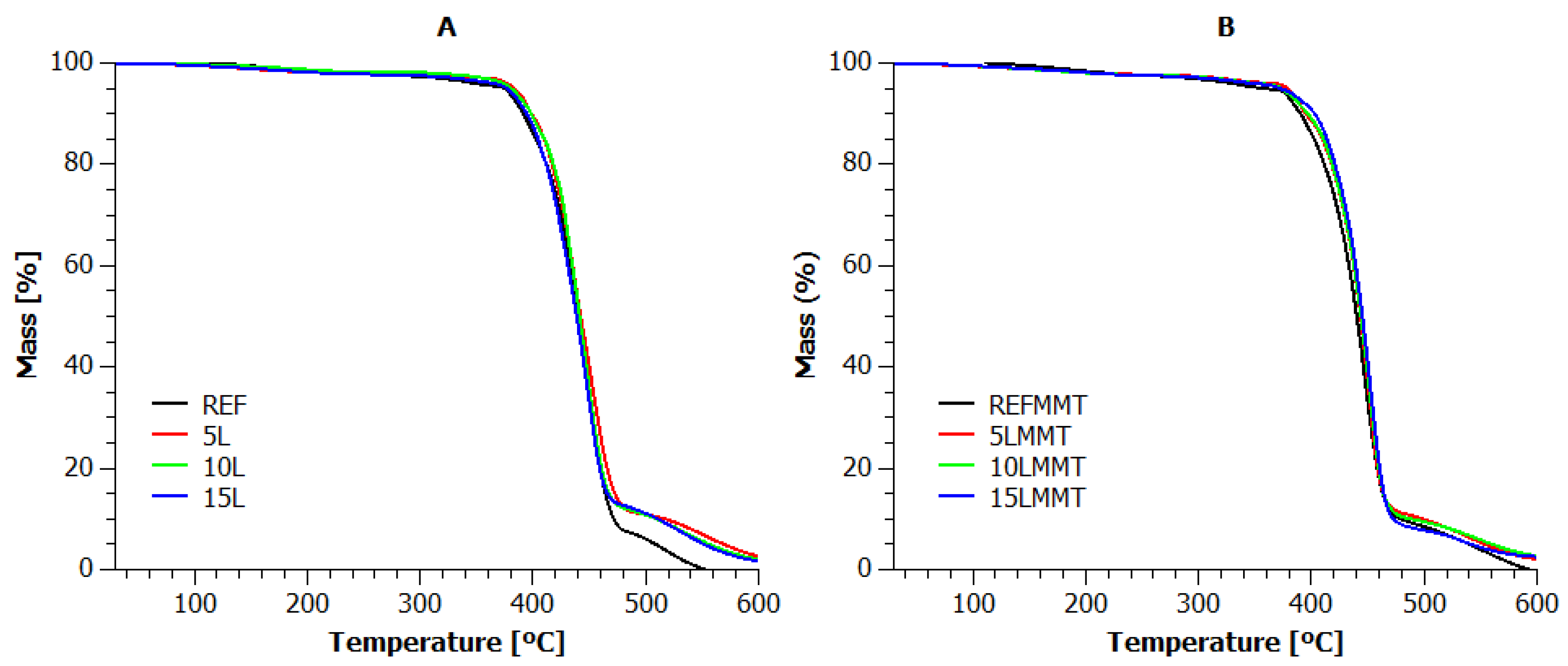



| Sample | T5% (°C) | T10% (°C) | T20% (°C) | T50% (°C) | Tmax (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|
| REF | 374 | 391 | 412 | 440 | 445 | 0.9 |
| 5L | 383 | 399 | 417 | 442 | 454 | 2.4 |
| 10L | 381 | 399 | 418 | 442 | 449 | 1.8 |
| 15L | 377 | 395 | 412 | 439 | 450 | 1.5 |
| REFMMT | 336 | 384 | 409 | 438 | 445 | 1.8 |
| 5LMMT | 379 | 395 | 418 | 444 | 454 | 2.0 |
| 10LMMT | 373 | 397 | 418 | 444 | 460 | 2.6 |
| 15LMMT | 372 | 403 | 421 | 445 | 453 | 2.5 |
| Sample | LOI (% Oxygen) | ∆LOI (% Oxygen) |
|---|---|---|
| REF | 19.8 | - |
| 5L | 19.6 | −0.2 |
| 10L | 19.4 | −0.4 |
| 15L | 19.3 | −0.5 |
| REFMMT | 19.7 | - |
| 5LMMT | 19.9 | 0.2 |
| 10LMMT | 19.9 | 0.2 |
| 15LMMT | 20.1 | 0.4 |
| Sample | Burning Time (s) | Length of the Burnt Sample (mm) | Burning Rate (mm/min) | Number of Drops | Type of Standard Class |
|---|---|---|---|---|---|
| REF | 441 ± 2 | 100 | 14 ± 1 | 280 | I |
| 5L | - | Did not reach the starting line * | - | - | II |
| 10L | 264 ± 2 | 60 | 14 ± 1 | 151 | III |
| 15L | 271 ± 2 | 100 | 22 ± 1 | 179 | I |
| REFMMT | 430 ± 2 | 100 | 13 ± 1 | 66 | IV |
| 5LMMT | 255 ± 2 | 100 | 24 ± 1 | 19 | V |
| 10LMMT | 266 ± 2 | 100 | 23 ± 1 | 11 | V |
| 15LMMT | 275 ± 2 | 100 | 22 ± 1 | 26 | V |
| Sample | Burning Time (s) | Length of the Burnt Sample (mm) | Burning Rate (mm/min) | Number of Drops | Type of Standard Class |
|---|---|---|---|---|---|
| REF | 40 | 27 | 41 | 22 | FV-2 |
| 5L | 242 | 125 | 31 | 121 | - |
| 10L | 47 | 31 | 40 | 32 | FV-0 |
| 15L | 58 | 44 | 46 | 45 | FV-1 |
| REFMMT | 148 | 125 | 50 | 13 | - |
| 5LMMT | 86 | 125 | 87 | 12 | - |
| 10LMMT | 80 | 125 | 94 | 4 | - |
| 15LMMT | 66 | 125 | 114 | 4 | - |
| Sample | PHRR (W/g) | Temperature to Reach PHRR (°C) | Time to Reach PHRR (s) |
|---|---|---|---|
| REF | 613.6 | 473 | 422 |
| 5L | 619.4 | 468 | 417 |
| 10L | 618.8 | 472 | 424 |
| 15L | 609.6 | 457 | 404 |
| REFMMT | 670.2 | 471 | 423 |
| 5LMMT | 533.3 | 469 | 409 |
| 10LMMT | 564.2 | 470 | 416 |
| 15LMMT | 504.4 | 468 | 415 |
| Sample | Rt (μm) | Rmax (μm) | Rz (μm) | Ra (μm) |
|---|---|---|---|---|
| REF | 5.04 ± 1.59 | 4.54 ± 1.08 | 3.21 ± 0.54 | 0.58 ± 0.23 |
| 5L | 4.54 ± 1.90 | 4.15 ± 1.95 | 2.67 ± 0.86 | 0.36 ± 0.18 |
| 10L | 4.69 ± 1.50 | 4.60 ± 1.46 | 2.34 ± 0.50 | 0.28 ± 0.09 |
| 15L | 4.79 ± 1.59 | 4.38 ± 1.02 | 2.20 ± 0.83 | 0.31 ± 0.37 |
| REFMMT | 3.35 ± 0.66 | 3.15 ± 0.83 | 2.05 ± 0.06 | 0.23 ± 0.02 |
| 5LMMT | 3.95 ± 0.55 | 3.93 ± 2.26 | 2.25 ± 0.55 | 0.23 ± 0.06 |
| 10LMMT | 3.64 ± 0.58 | 3.56 ± 0.47 | 2.43 ± 0.04 | 0.25 ± 0.01 |
| 15LMMT | 3.56 ± 0.59 | 3.56 ± 0.59 | 2.99 ± 0.45 | 0.24 ± 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majka, T.M.; Witek, M.; Radzik, P.; Komisarz, K.; Mitoraj, A.; Pielichowski, K. Layer-by-Layer Deposition of Copper and Phosphorus Compounds to Develop Flame-Retardant Polyamide 6/Montmorillonite Hybrid Composites. Appl. Sci. 2020, 10, 5007. https://doi.org/10.3390/app10145007
Majka TM, Witek M, Radzik P, Komisarz K, Mitoraj A, Pielichowski K. Layer-by-Layer Deposition of Copper and Phosphorus Compounds to Develop Flame-Retardant Polyamide 6/Montmorillonite Hybrid Composites. Applied Sciences. 2020; 10(14):5007. https://doi.org/10.3390/app10145007
Chicago/Turabian StyleMajka, Tomasz M., Monika Witek, Paulina Radzik, Karolina Komisarz, Agnieszka Mitoraj, and Krzysztof Pielichowski. 2020. "Layer-by-Layer Deposition of Copper and Phosphorus Compounds to Develop Flame-Retardant Polyamide 6/Montmorillonite Hybrid Composites" Applied Sciences 10, no. 14: 5007. https://doi.org/10.3390/app10145007
APA StyleMajka, T. M., Witek, M., Radzik, P., Komisarz, K., Mitoraj, A., & Pielichowski, K. (2020). Layer-by-Layer Deposition of Copper and Phosphorus Compounds to Develop Flame-Retardant Polyamide 6/Montmorillonite Hybrid Composites. Applied Sciences, 10(14), 5007. https://doi.org/10.3390/app10145007