Phase Transformation, Optical and Emission Performance of Zinc Silicate Glass-Ceramics Phosphor Derived from the ZnO–B2O3–SLS Glass System
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Density and Linear Shrinkage Analysis
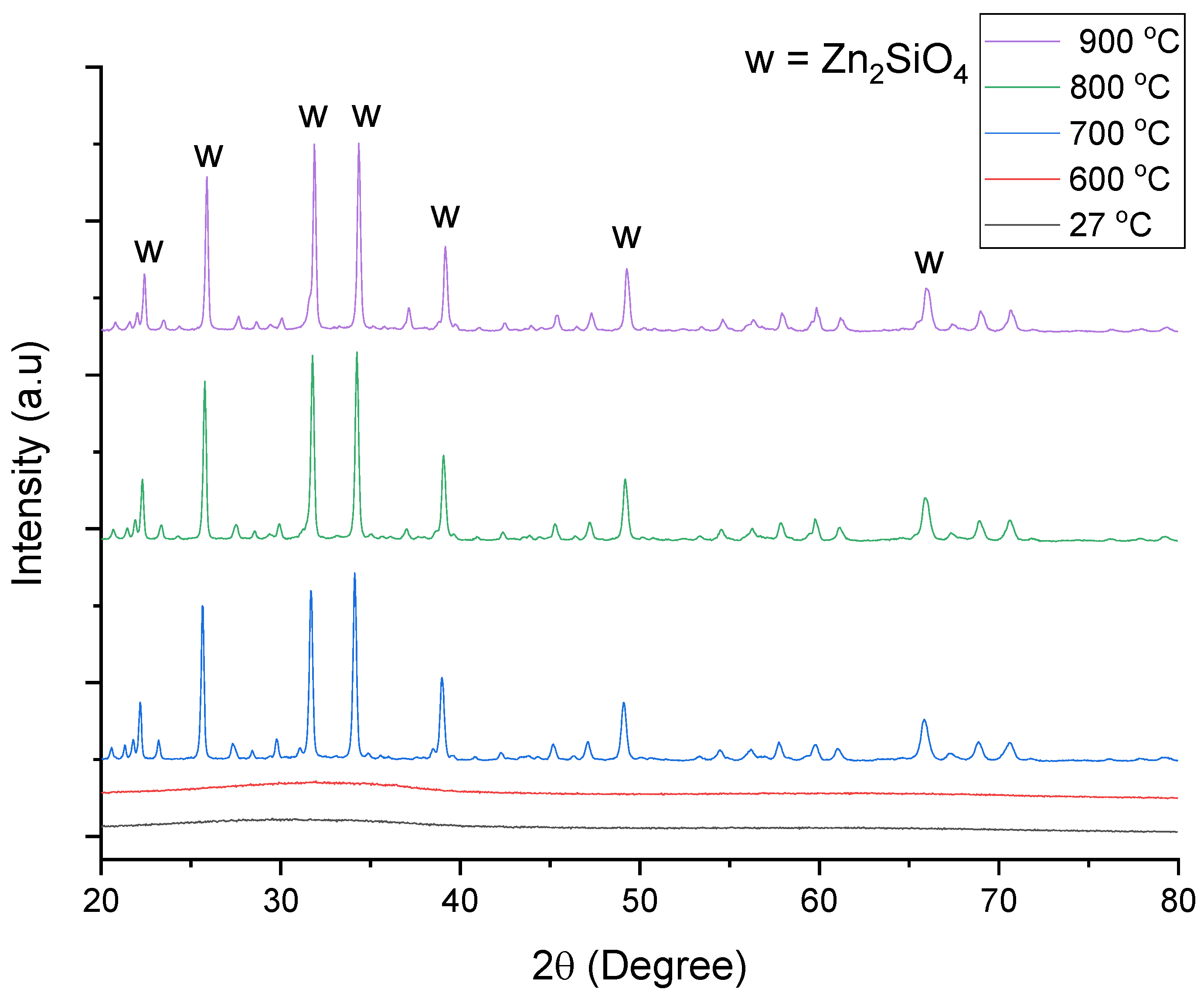
3.2. X-ray Diffraction (XRD) Analysis
3.3. Fourier Transform Infrared Radiation (FTIR) Analysis
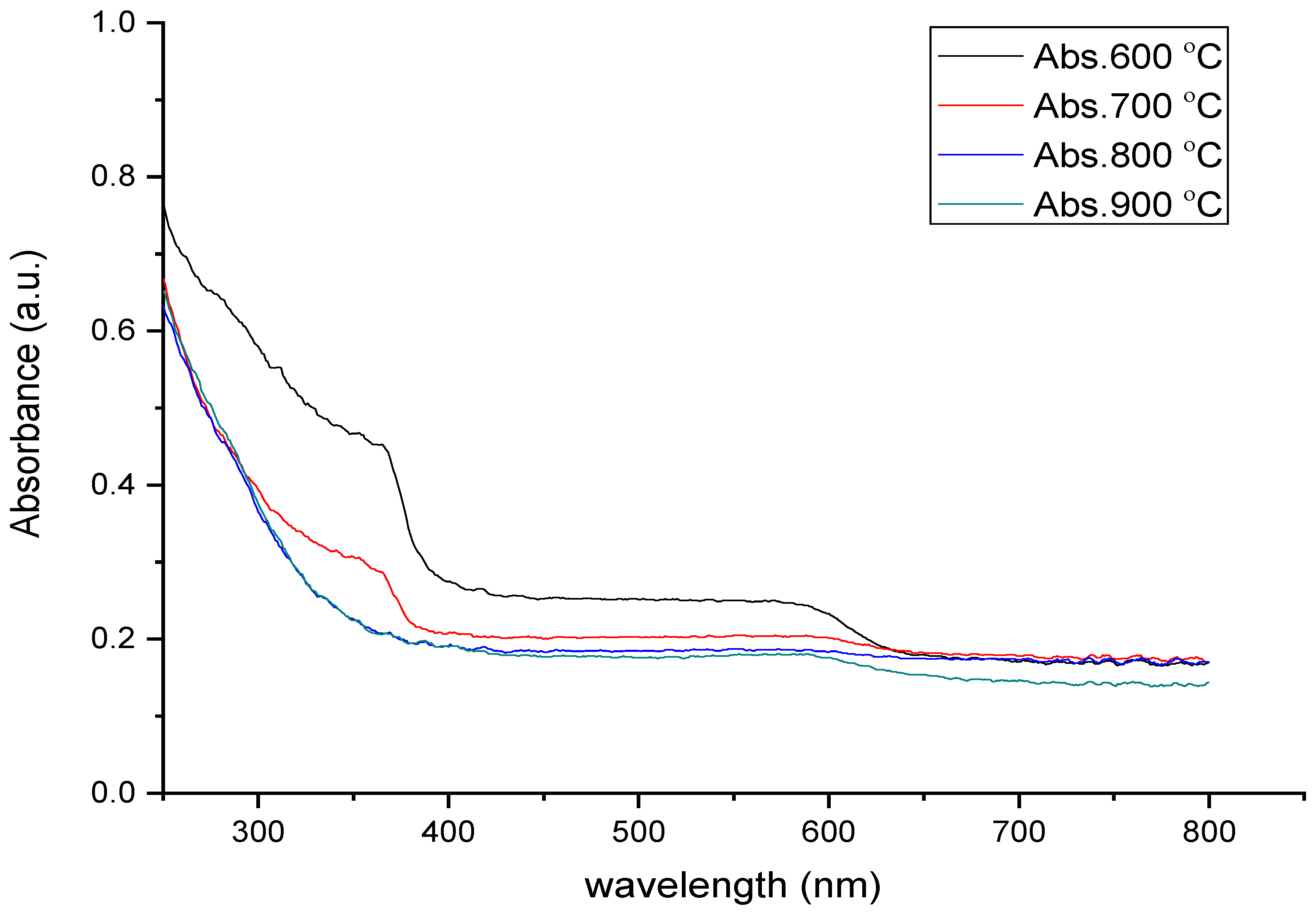
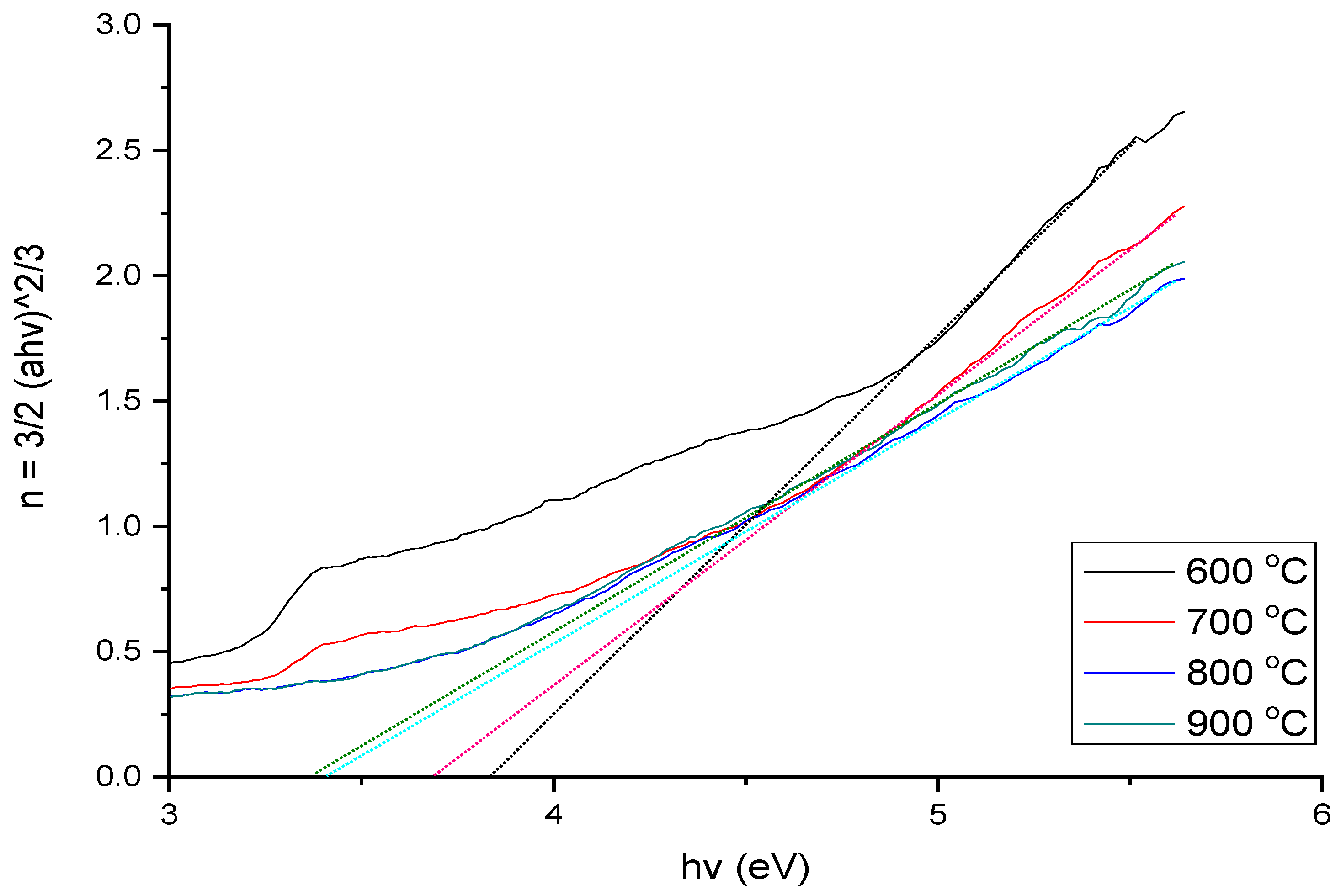
3.4. UV–Vis Analysis
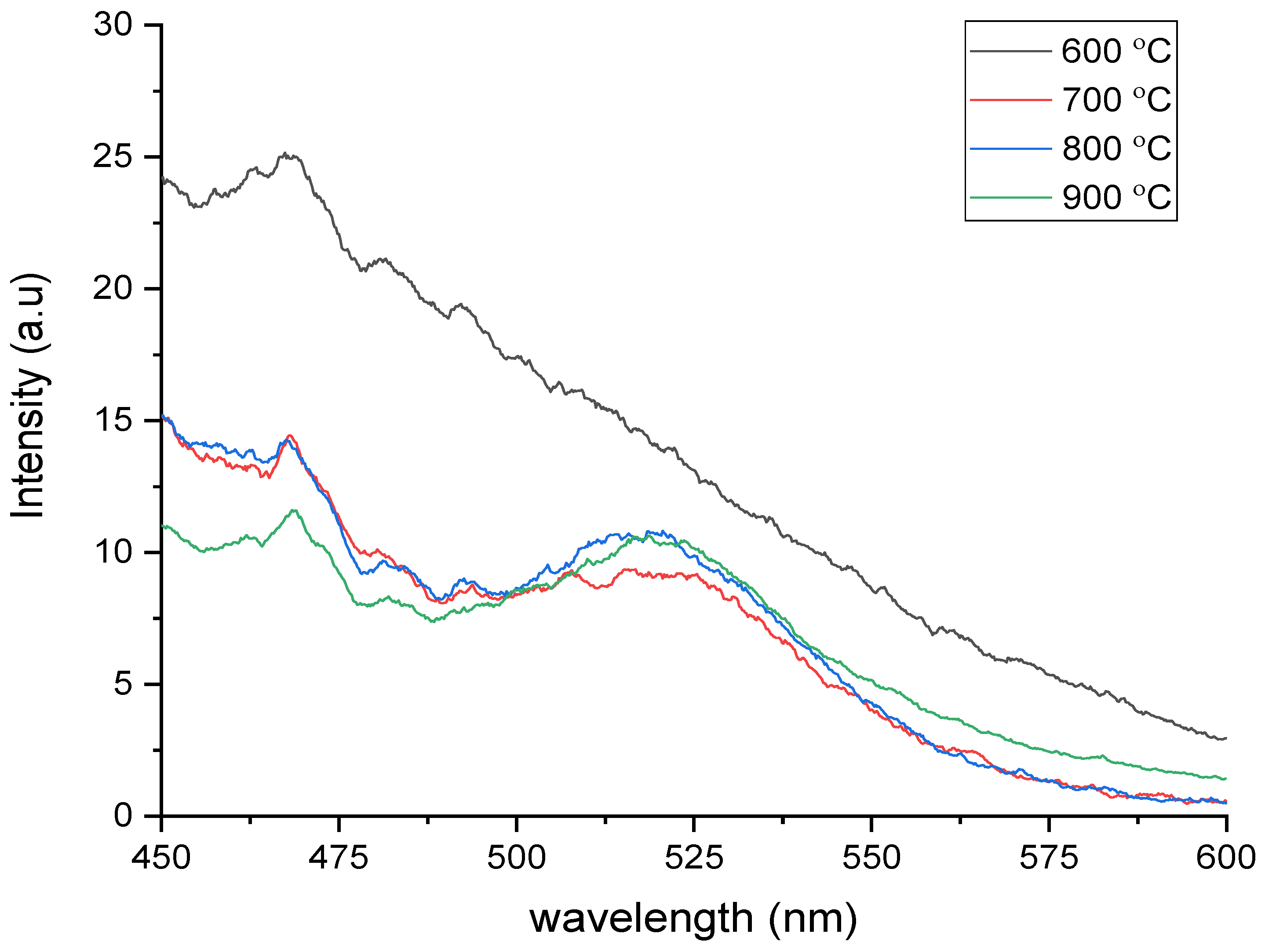
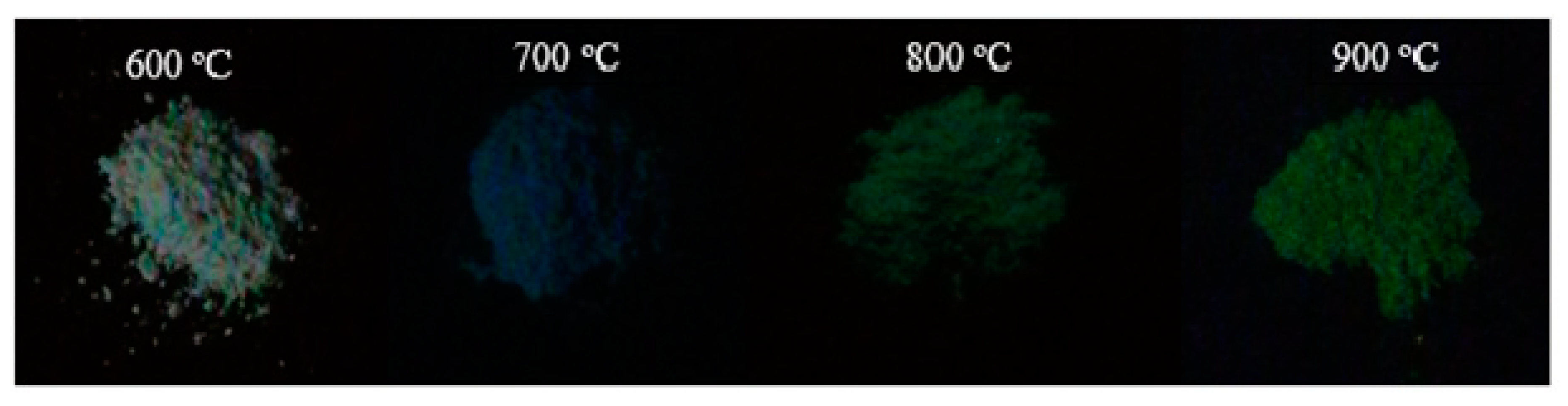
3.5. Photoluminescence Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ehrt, D.; Flügel, S. Properties of zinc silicate glasses and melts. Mat. Sci. Eng. A 2011, 1, 312. [Google Scholar]
- Tarafder, A.; Molla, A.R.; Mukhopadhyay, S.; Karmakar, B. Fabrication and enhanced photoluminescence properties of Sm3+-doped ZnO–Al2O3–B2O3–SiO2 glass derived willemite glass-ceramic nanocomposites. Opt. Mater. 2014, 36, 1463–1470. [Google Scholar] [CrossRef]
- Xu, G.Q.; Xu, H.T.; Zheng, Z.X.; Wu, Y.C. Preparation and characterization of Zn2SiO4: Mn phosphors with hydrothermal methods. J. Lumin. 2010, 130, 1717–1720. [Google Scholar] [CrossRef]
- An, J.S.; Noh, J.H.; Cho, I.S.; Roh, H.S.; Kim, J.Y.; Han, H.S.; Hong, K.S. Tailoring the morphology and structure of nanosized Zn2SiO4: Mn2+ phosphors using the hydrothermal method and their luminescence properties. J. Phys. Chem. C 2010, 114, 10330–10335. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. A simple sonochemical approach for synthesis and characterization of Zn2SiO4 nanostructures. Ultrason. Sonochem. 2016, 29, 226–235. [Google Scholar] [CrossRef]
- Babu, B.C.; Buddhudu, S. Dielectric Properties of Willemite Zn2SiO4 Nano Powders by Sol-gel Method. Phys. Procedia 2013, 49, 128–136. [Google Scholar] [CrossRef]
- Kang, Y.C.; Park, S. Zn2SiO4: Mn phosphor particles prepared by spray pyrolysis using a filter expansion aerosol generator. Mater. Res. Bull. 2000, 35, 1143–1151. [Google Scholar] [CrossRef]
- Su, K.; Tilley, T.D.; Sailor, M.J. Molecular and polymer precursor routes to manganese-doped zinc orthosilicate phosphors. J. Am. Chem. Soc. 1996, 118, 3459–3468. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Dey, C.; Karmakar, B. Thermal, Structural, and Enhanced Photoluminescence Properties of Eu3+-doped Transparent Willemite Glass–Ceramic Nanocomposites. J. Am. Ceram. Soc. 2013, 96, 2424–2431. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Ab Aziz, S.H.; Kamari, H.M.; Wahab, Z.A.; Effendy, N.; Alibe, I.M. Comprehensive study on compositional dependence of optical band gap in zinc soda lime silica glass system for optoelectronic applications. J. Non-Cryst. Solids 2016, 449, 107–112. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Wah, L.C.; Sidek, H.A.A.; Halimah, M.K.; Wahab, Z.A.; Azmi, B.Z. Elastic moduli prediction and correlation in soda lime silicate glasses containing ZnO. Int. J. Phys. Sci. 2011, 6, 1404–1410. [Google Scholar]
- Yanbo, Q.; Ning, D.; Mingying, P.; Lüyun, Y.; Danping, C.; Jianrong, Q.; Chongshan, Z.; Akai, T. Spectroscopic properties of Nd3+-doped high silica glass prepared by sintering porous glass. J. Rare Earth. 2006, 24, 765–770. [Google Scholar] [CrossRef]
- Chimalawong, P.; Kirdsiri, K.; Kaewkhao, J.; Limsuwan, P. Investigation on the physical and optical properties of Dy3+ doped soda-lime-silicate glasses. Procedia Eng. 2012, 32, 690–698. [Google Scholar] [CrossRef]
- Hamnabard, Z.; Khalkhali, Z.; Qazvini, S.S.A.; Baghshahi, S.; Maghsoudipour, A. Preparation, heat treatment and photoluminescence properties of V-doped ZnO–SiO2–B2O3 glasses. J. Lumin. 2012, 132, 1126–1132. [Google Scholar] [CrossRef]
- Qazvini, S.S.A.; Hamnabard, Z.; Khalkhali, Z.; Baghshahi, S.; Maghsoudipour, A. Photoluminescence and microstructural properties of SiO2–ZnO–B2O3 system containing TiO2 and V2O5. Ceram. Int. 2012, 38, 1663–1670. [Google Scholar] [CrossRef]
- Li, H.C.; Wang, D.G.; Hu, J.H.; Chen, C.Z. Effect of the partial substitution of K2O, MgO, B2O3 for CaO on crystallization, structure and properties of Na2O–CaO–SiO2–P2O5 system glass-ceramics. Mater. Lett. 2013, 106, 373–376. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The atomic arrangement in glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Azuraida, A.; Halimah, M.K.; Sidek, A.A.; Azurahanim, C.A.C.; Iskandar, S.M.; Ishak, M.; Nurazlin, A. Comparative studies of bismuth and barium boro-tellurite glass system: Structural and optical properties. Chalcogenide Lett. 2015, 12, 497–503. [Google Scholar]
- Lakshminarayana, G.; Yang, R.; Mao, M.; Zhang, Y.; Qiu, J. Spectral analysis of optical centres formed in Bi−, Bi/Yb−, Pb−, Pb/Yb−, Sb−, Sb/Yb− and Sn−, Sn/Yb-co-doped germanate glasses. J. Phys. D Appl. Phys. 2009, 42, 145108. [Google Scholar] [CrossRef]
- Loiko, P.; Dymshits, O.; Volokitina, A.; Alekseeva, I.; Shemchuk, D.; Tsenter, M.; Bachina, A.; Khubetsov, A.; Vilejshikova, E.; Petrov, P.; et al. Structural transformations and optical properties of glass-ceramics based on ZnO, β- and α-Zn2SiO4 nanocrystals and doped with Er2O3 and Yb2O3: Part, I. The role of heat-treatment. J. Lumin. 2018, 202, 47–56. [Google Scholar] [CrossRef]
- Chen, Q.; Ferraris, M.; Milanese, D.; Menke, Y.; Monchiero, E.; Perrone, G. Novel Er-doped PbO and B2O3 based glasses: Investigation of quantum efficiency and non-radiative transition probability for 1.5 μm broadband emission fluorescence. J. Non-Cryst. Solids 2003, 324, 12–20. [Google Scholar] [CrossRef]
- Rashid, S.S.A.; Ab Aziz, S.H.; Matori, K.A.; Zaid, M.H.M.; Mohamed, N. Comprehensive study on effect of sintering temperature on the physical, structural and optical properties of Er3+ doped ZnO-GSLS glasses. Results Phys. 2017, 7, 2224–2231. [Google Scholar] [CrossRef]
- Kullberg, A.T.G.; Lopes, A.A.S.; Veiga, J.P.B.; Lima, M.M.R.A.; Monteiro, R.C.C. Formation and crystallization of zinc borosilicate glasses: Influence of the ZnO/B2O3 ratio. J. Non-Cryst. Solids 2016, 441, 79–85. [Google Scholar] [CrossRef]
- Khalkhali, Z.; Hamnabard, Z.; Qazvini, S.S.A.; Baghshahi, S.; Maghsoudipour, A. Preparation phase formation and photoluminescence properties of ZnO–SiO2–B2O3 glasses with different ZnO/B2O3 ratios. Opt. Mater. 2012, 34, 850–855. [Google Scholar] [CrossRef]
- Iordanova, R.; Dimitrov, V.; Dimitriev, Y.; Klissurski, D. Glass formation and structure of glasses in the V2O5 MoO3 Bi2O system. J. Non-Cryst. Solids 1994, 180, 58–65. [Google Scholar] [CrossRef]
- Torres, F.J.; de Sola, E.R.; Alarcón, J. Mechanism of crystallization of fast fired mullite-based glass–ceramic glazes for floor-tiles. J. Non-Cryst. Solids 2006, 352, 2159–2165. [Google Scholar] [CrossRef]
- Effendy, N.; Abdul Wahab, Z.; Kamari, H.M.; Matori, K.A.; Aziz, S.H.A.; Zaid, M.H.M. Structural and optical properties of Er3+-doped willemite glass-ceramics from waste materials. Optik 2016, 127, 11698–11705. [Google Scholar] [CrossRef]
- Lukic, S.R.; Petrovic, D.M.; Dramicanin, M.D.; Mitric, M.; Dacanin, L. Optical and structural properties of Zn2SiO4: Mn2+ green phosphor nanoparticles obtained by a polymer-assisted sol-gel method. Scripta Mater. 2008, 58, 655–658. [Google Scholar] [CrossRef]
- Chao, P.T.; Shen, P.; Lin, C.C. Thermal cycle etching of willemite (0001): Effects of surface premelting, dislocation outcrops and polygonization. Mater. Sci. Eng. A 2002, 335, 191–197. [Google Scholar] [CrossRef]
- Kullberg, A.T.; Lopes, A.A.; Veiga, J.P.; Monteiro, R.C. Crystal growth in zinc borosilicate glasses. J. Cryst. Growth 2017, 457, 239–243. [Google Scholar] [CrossRef]
- Sabri, M.G.M.; Azmi, B.Z.; Rizwan, Z.; Halimah, M.K.; Hashim, M.; Zaid, M.H.M. Effect of temperature treatment on the optical characterization of ZnO-Bi2O3-TiO2 varistor ceramics. Int. J. Phys. Sci. 2011, 6, 1388–1394. [Google Scholar]
- Lee, C.S.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Influence of zinc oxide on the physical, structural and optical band gap of zinc silicate glass system from waste rice husk ash. Optik 2017, 136, 129–135. [Google Scholar] [CrossRef]
- Omri, K.; El Ghoul, J.; Alyamani, A.; Barthou, C.; El Mir, L. Luminescence properties of green emission of SiO2/Zn2SiO4: Mn nanocomposite prepared by sol-gel method. Physica E 2013, 53, 48–54. [Google Scholar] [CrossRef]
- Giancaterini, L.; Cantalini, C.; Cittadini, M.; Sturaro, M.; Guglielmi, M.; Martucci, A.; Resmini, A.; Anselmi-Tamburini, U. Au and Pt nanoparticles effects on the optical and electrical gas sensing properties of sol-gel-based ZnO thin-film sensors. IEEE Sens. J. 2015, 15, 1068–1076. [Google Scholar] [CrossRef]
- Ghoul, J.E.; Barthou, C.; Mir, L.E. Synthesis, structural and optical properties of nanocrystalline vanadium doped zinc oxide aerogel. Physica E 2012, 44, 1910–1915. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Norhafizah, M.R.; Nurzilla, M.; Zamratul, M.I.M. Synthesis and optical properties of europium doped zinc silicate prepared using low cost solid state reaction method. J. Mater. Sci.-Mater. Electron. 2016, 27, 1092–1099. [Google Scholar] [CrossRef]
- Syamimi, N.F.; Matori, K.A.; Lim, W.F.; Sidek, H.A.A.; Zaid, M.H.M. Effect of sintering temperature on structural and morphological properties of europium (III) oxide doped willemite. J. Spectroscopy 2014, 2014, 328931. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Wahab, Z.A.; Fen, Y.W.; Alibe, I.M. Synthesis and characterization of low cost willemite based glass–ceramic for opto-electronic applications. J. Mater. Sci.-Mater. Electron. 2016, 27, 11158–11167. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, Z.Q. Fragility of photonic band gaps in inverse-opal photonic crystals. Phys. Rev. B 2000, 62, 1516. [Google Scholar] [CrossRef]
- Iskandar, S.M.; Halimah, M.K.; Daud, W.M.; Sidek, H.A.A.; Zaman, M.K. Optical and structural properties of PbO-B2O3-TeO2 glasses. J. Solid State Sci. Technol. 2010, 18, 84–90. [Google Scholar]
- Li, J.; Kuwabara, M. Preparation and luminescent properties of Eu-doped BaTiO3 thin films by sol–gel process. Sci. Technol. Adv. Mat. 2003, 4, 143–148. [Google Scholar] [CrossRef]
- Kam, C.H.; Buddhudu, S. Luminescence enhancement in (Nd3++Ce3+) doped SiO2: Al2O3 sol–gel glasses. Opt. Laser Eng. 2001, 35, 11–17. [Google Scholar] [CrossRef]
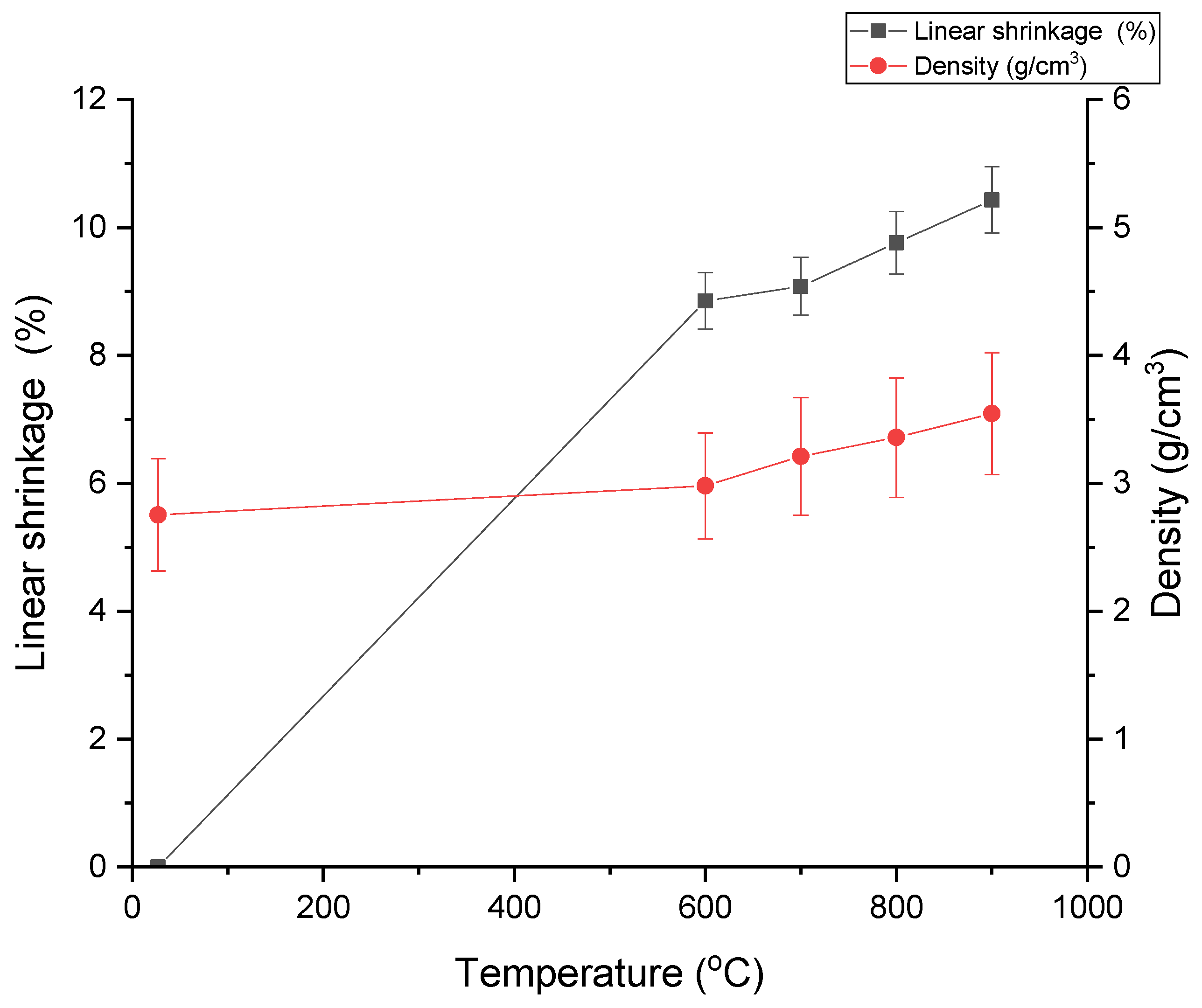

| Temperature (°C) | Density (g/cm3) | Linear Shrinkage (%) |
|---|---|---|
| 27 | 2.754 | 0.00 |
| 600 | 2.980 | 8.85 |
| 700 | 3.211 | 9.08 |
| 800 | 3.358 | 9.76 |
| 900 | 3.545 | 10.43 |
| Wavenumber (cm−1) | Assigned of Vibration Modes |
|---|---|
| 457 | Asymmetric deformation of SiO4 |
| 570 | Symmetric stretching of ZnO4 |
| 611 | Asymmetric stretching of ZnO4 |
| 710 | Si-O bond vibration |
| 866 | Symmetric stretching of SiO4 |
| 900, 930, 977 | Asymmetric stretching of SiO4 |
| Temperature (°C) | Band Gap Energy (eV) |
|---|---|
| 600 | 3.867 |
| 700 | 3.743 |
| 800 | 3.484 |
| 900 | 3.423 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Shofri, M.F.S.; Mohd Zaid, M.H.; Matori, K.A.; Fen, Y.W.; Yaakob, Y.; Jaafar, S.H.; Wahab, S.A.A.; Iwamoto, Y. Phase Transformation, Optical and Emission Performance of Zinc Silicate Glass-Ceramics Phosphor Derived from the ZnO–B2O3–SLS Glass System. Appl. Sci. 2020, 10, 4940. https://doi.org/10.3390/app10144940
Mohd Shofri MFS, Mohd Zaid MH, Matori KA, Fen YW, Yaakob Y, Jaafar SH, Wahab SAA, Iwamoto Y. Phase Transformation, Optical and Emission Performance of Zinc Silicate Glass-Ceramics Phosphor Derived from the ZnO–B2O3–SLS Glass System. Applied Sciences. 2020; 10(14):4940. https://doi.org/10.3390/app10144940
Chicago/Turabian StyleMohd Shofri, Muhammad Faris Syazwan, Mohd Hafiz Mohd Zaid, Khamirul Amin Matori, Yap Wing Fen, Yazid Yaakob, Suhail Huzaifa Jaafar, Siti Aisyah Abdul Wahab, and Yuji Iwamoto. 2020. "Phase Transformation, Optical and Emission Performance of Zinc Silicate Glass-Ceramics Phosphor Derived from the ZnO–B2O3–SLS Glass System" Applied Sciences 10, no. 14: 4940. https://doi.org/10.3390/app10144940
APA StyleMohd Shofri, M. F. S., Mohd Zaid, M. H., Matori, K. A., Fen, Y. W., Yaakob, Y., Jaafar, S. H., Wahab, S. A. A., & Iwamoto, Y. (2020). Phase Transformation, Optical and Emission Performance of Zinc Silicate Glass-Ceramics Phosphor Derived from the ZnO–B2O3–SLS Glass System. Applied Sciences, 10(14), 4940. https://doi.org/10.3390/app10144940






