Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
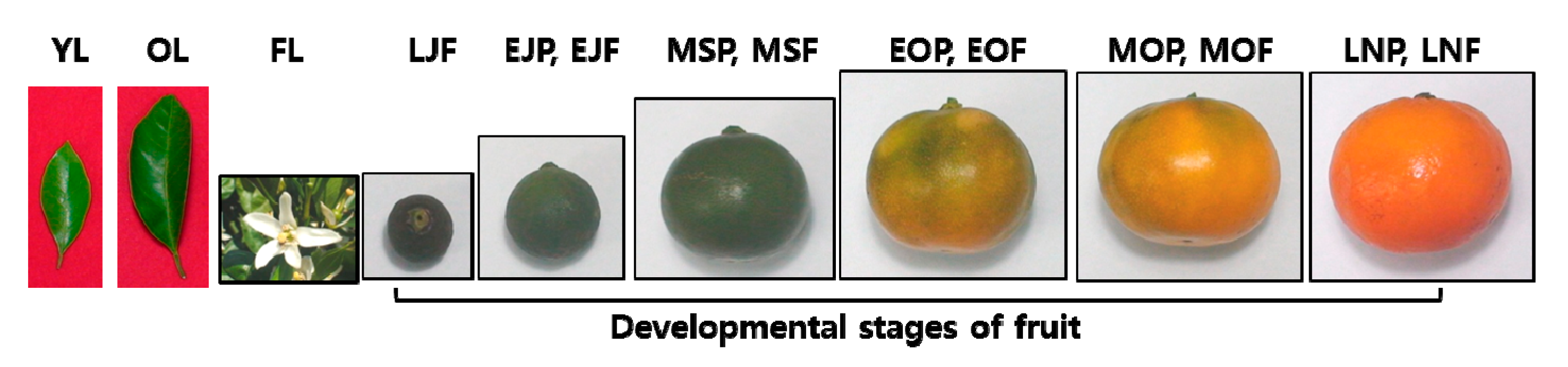
2.2. Plant Materials
2.3. Sample Preparation
2.4. In Vitro Elastase and Collagenase Inhibition Assay
2.5. GC-MS Analysis of Citrus Extracts
2.6. Analysis of Total Phenolic Content (TPC)
2.7. Analysis of Total Flavonoid Content (TFC)
2.8. DPPH Radical Scavenging and Ferric Reducing Anti-Oxidant Power (FRAP) Activity Assay
2.9. Cytotoxicity Assay
2.10. Statistical Analysis
3. Results
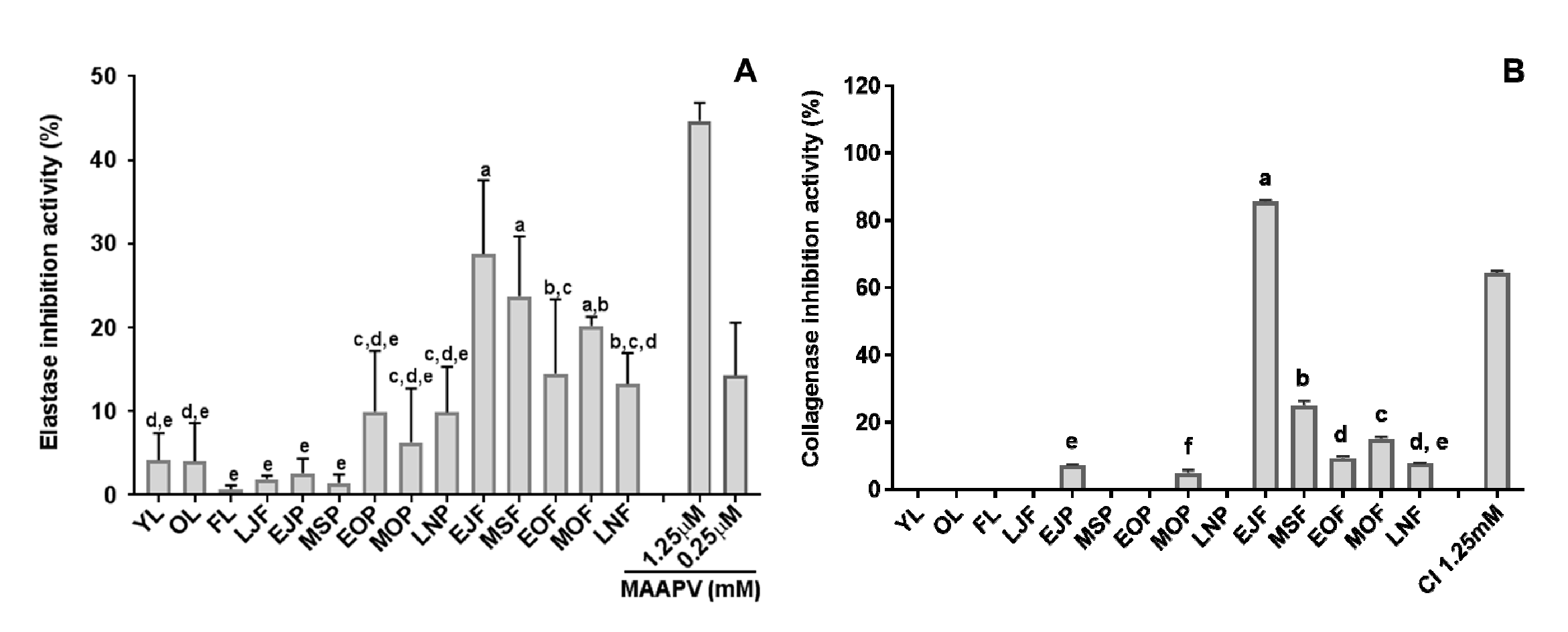
3.1. Elastase and Collagenase Inhibitory Activity
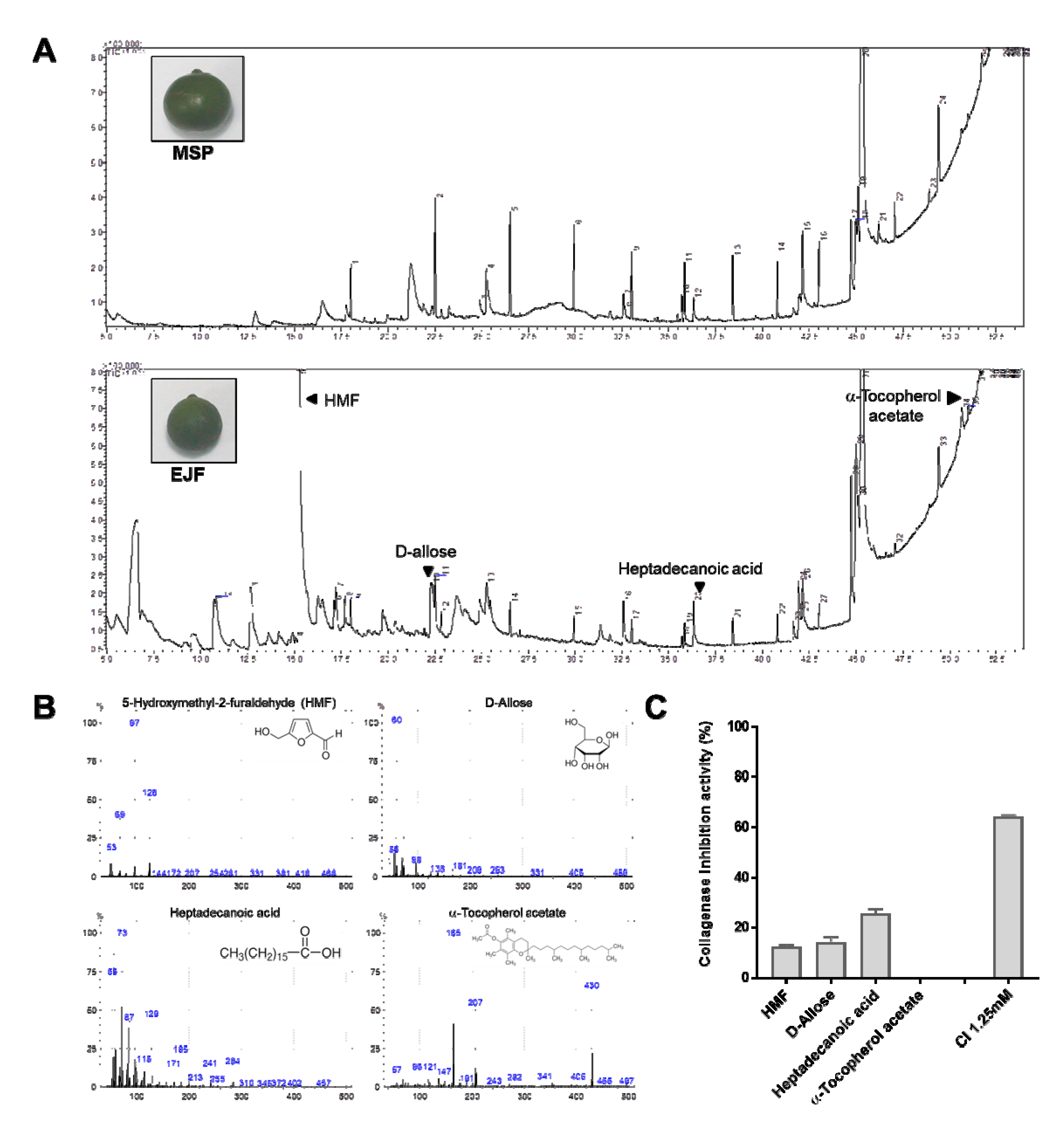
3.2. Chemical Composition by GC-MS Analysis and Anti-Aging Effect
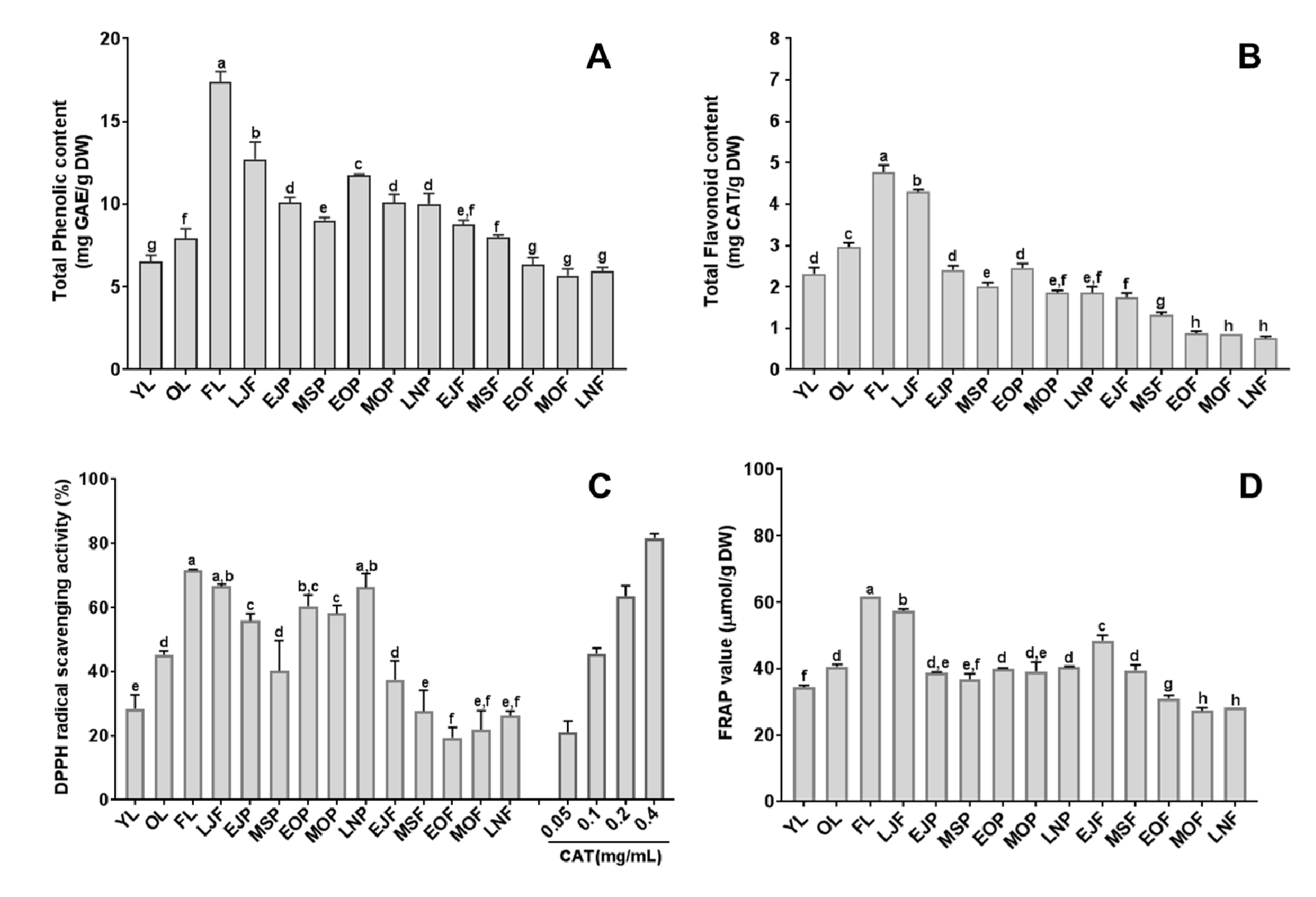
3.3. Total Phenolic and Flavonoid Contents of Citrus Fruits and Tissues
3.4. Anti-Oxidant Activity of Citrus Fruits and Tissues
3.5. Cytotoxicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayaprakasha, G.K.; Patil, B.S. In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chem. 2007, 101, 410–418. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, W.; Huang, H.; Ye, Y.; Sun, P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chem. 2019, 279, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Elkhatim, K.; Elagib, R.; Hassan, A. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Cardenosa, V.; Barros, L.; Barreira, J.C.M.; Arenas, F.; Moreno-Rojas, J.M.; Ferreira, I.C.F.R. Different Citrus rootstocks present high dissimilarities in their antioxidant activity and vitamins content according to the ripening stage. J. Plant Physiol. 2015, 174, 124–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Xi, W.; Shen, Y.; Qiao, L.; Zhong, L.; Ye, X.; Zhou, Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. [Google Scholar] [CrossRef]
- Legua, P.; Forner, J.B.; Hernández, F.; Forner-Giner, M.A. Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Sci. Hortic. 2014, 174, 60–64. [Google Scholar] [CrossRef]
- AL-Jabri, N.N.; Hossain, M.A. Chemical composition and antimicrobial potency of locally grown lemon essential oil against selected bacterial strains. J. King Saud Univ.-Sci. 2018, 30, 14–20. [Google Scholar] [CrossRef]
- Rawson, N.; Ho, C.T.; Li, S. Efficacious anti-cancer property of flavonoids from citrus peels. Food Sci. Hum. Well. 2014, 3, 104–109. [Google Scholar] [CrossRef]
- Park, H.Y.; Choi, H.D.; Eom, H.; Choi, I. Enzymatic modification enhances the protective activity of citrus flavonoids against alcohol-induced liver disease. Food Chem. 2013, 139, 231–240. [Google Scholar] [CrossRef]
- Kumud, M.; Sanju, N. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar]
- Duque, L.; Bravo, K.; Osorio, E. A holistic anti-aging approach applied in selected cultivated medicinal plants: A view of photoprotection of the skin by different mechanisms. Ind. Crops Products 2017, 97, 431–439. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Chattuwatthana, T.; Okello, E. Anti-collagenase, anti-elastase and antioxidant activities of Pueraria candollei var. mirifica root extract and Coccinia grandis fruit juice extract: An In vitro study. Eur. J. Med. Plants 2015, 5, 318–327. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin aging. Mech. Aging Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Comp. Alter. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkarm, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agri. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rennert, B.; Melzig, M.F. Free fatty acids inhibit the activity of Clostridium histolyticum collagenase and human neutrophil elastase. Planta Med. 2002, 68, 767–769. [Google Scholar] [CrossRef]
- Noguchi, C.; Kamitori, K.; Hossain, A.; Hoshikawa, H.; Katagi, A.; Dong, Y.; Sui, L.; Tokuda, M.; Yamaguchi, F. D-Allose inhibits cancer cell growth by reducing GLUT1 expression. Tohoku J. Exp. Med. 2016, 238, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tanaka, S.; Hirooka, K.; Toyoshima, T.; Kawai, N.; Tamiya, T.; Shiraga, F.; Tokuda, M.; Keep, R.F.; Itano, T. Anti-oxidative effects of D-allose, a rare sugar, on ischemia–reperfusion damage following focal cerebral ischemia in rat. Neurosci. Lett. 2011, 48, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Gao, D.; Hei, Y.; Zhang, X.; Chen, X.; Fei, Z. D-Allose protects the blood brain barrier through PPAR gamma-mediated anti-inflammatory pathway in the mice model of ischemia reperfusion injury. Brain Res. 2016, 1642, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Hou, R.; Zhang, P. D-allose alleviates ischemia/reperfusion (I/R) injury in skin flap via MKP-1. Mol. Med. 2020, 26, 21. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Ki, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. Agri. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother. Res. 2011, 25, 965–974. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Z.; Zhang, L.; Quan, Y.; Wei, K. Experimental study of the protective effect of mesosilica-supported 5-hydroxymethylfurfural on UV-induced aging of human dermal fibroblasts. RSC Adv. 2018, 8, 25021–25030. [Google Scholar] [CrossRef]
- German-Báez, L.J.; Valdez-Flores, M.; Figureueroa-Pérez, M.G.; Garduño-Félix, K.G.; Valdez-Ortiz, R.; Meza-Ayala, K.A.; Valdez-Ortiz, A. Anti-aging and nutraceutical characterization of plant infusions used in traditional medicine. Pakistan J. Nutr. 2017, 16, 285–292. [Google Scholar]
- Kanashiro, A.; Souza, J.G.; Kabeya, L.M.; Azzolini, A.E.C.S.; Lucisano-Valim, Y.M. Elastase release by stimulated neutrophils inhibited by flavonoids: Importance of the catechol group. Verlag der Zeitschrift für Naturforschung 2007, 62, 357–361. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Lawand, C.; Ryan, D.J.; McDonald, J.E.; Donner, H.; Forney, C.F. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage. J. Amer. Soc. Horticul. Sci. 2003, 126, 917–923. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar]
- Park, J.H.; Lee, M.; Park, E. Antioxidant activity of orange flesh and peel extracted with various solvents. Prev. Nut. Food Sci. 2014, 19, 291–298. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eun, C.-H.; Kang, M.-S.; Kim, I.-J. Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Appl. Sci. 2020, 10, 4838. https://doi.org/10.3390/app10144838
Eun C-H, Kang M-S, Kim I-J. Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Applied Sciences. 2020; 10(14):4838. https://doi.org/10.3390/app10144838
Chicago/Turabian StyleEun, Chang-Ho, Mi-Sook Kang, and In-Jung Kim. 2020. "Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity" Applied Sciences 10, no. 14: 4838. https://doi.org/10.3390/app10144838
APA StyleEun, C.-H., Kang, M.-S., & Kim, I.-J. (2020). Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Applied Sciences, 10(14), 4838. https://doi.org/10.3390/app10144838




