Molecular Assembling in Mixtures of Hydrophilic 1-Butyl-1-Methylpyrrolidinium Dicyanamide Ionic Liquid and Water
Abstract
1. Introduction
2. Materials and Methods
3. Results
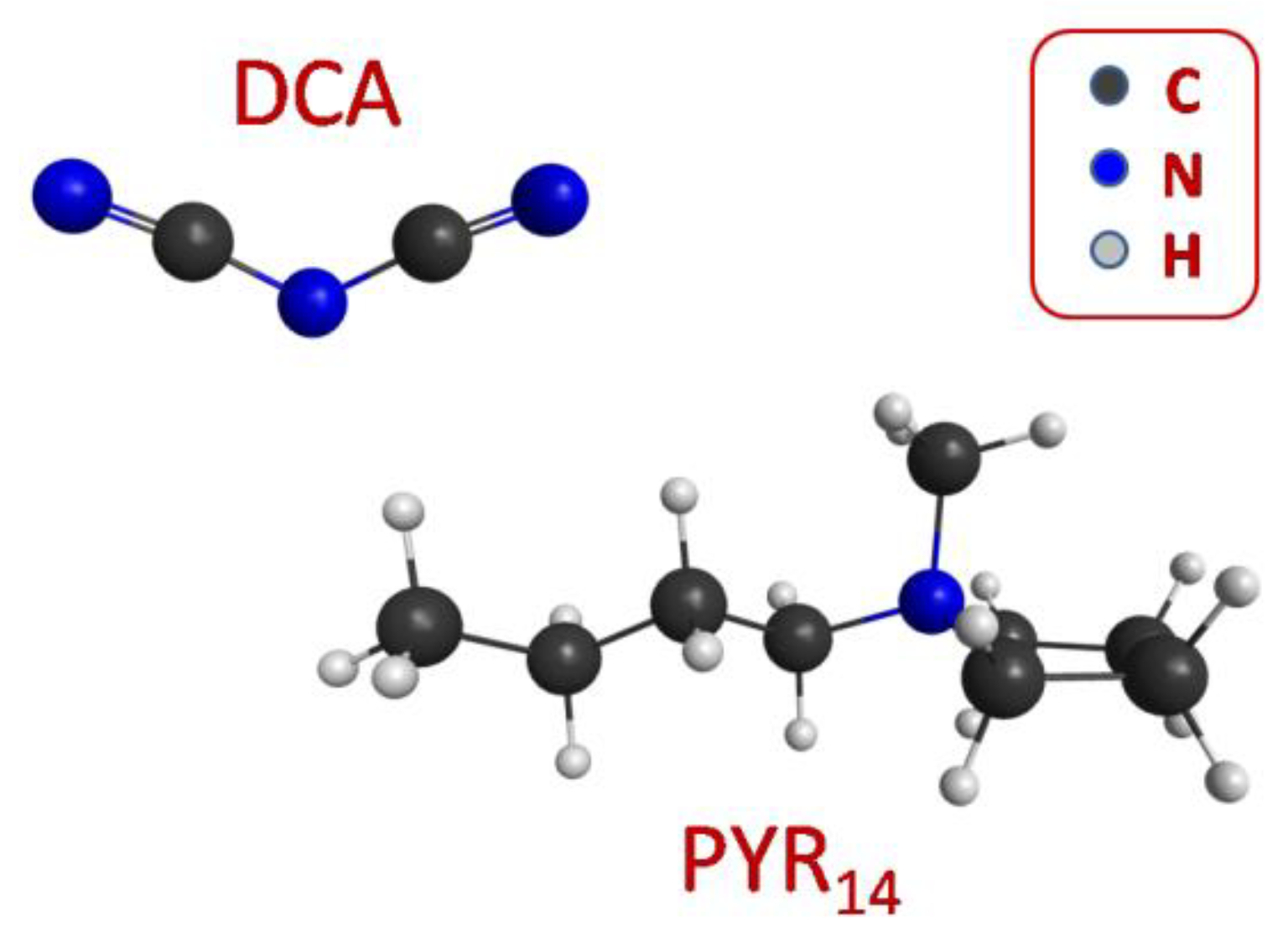
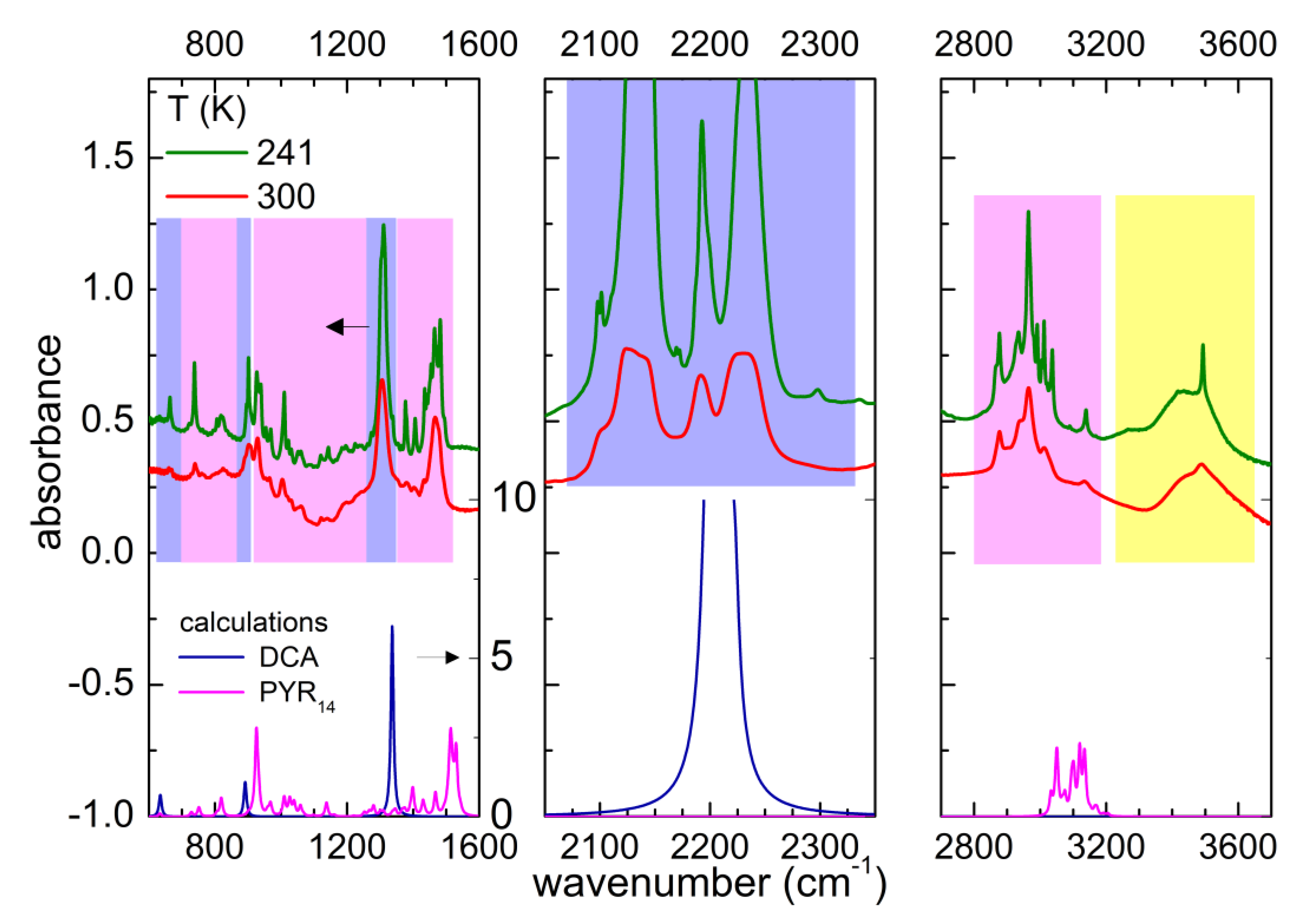
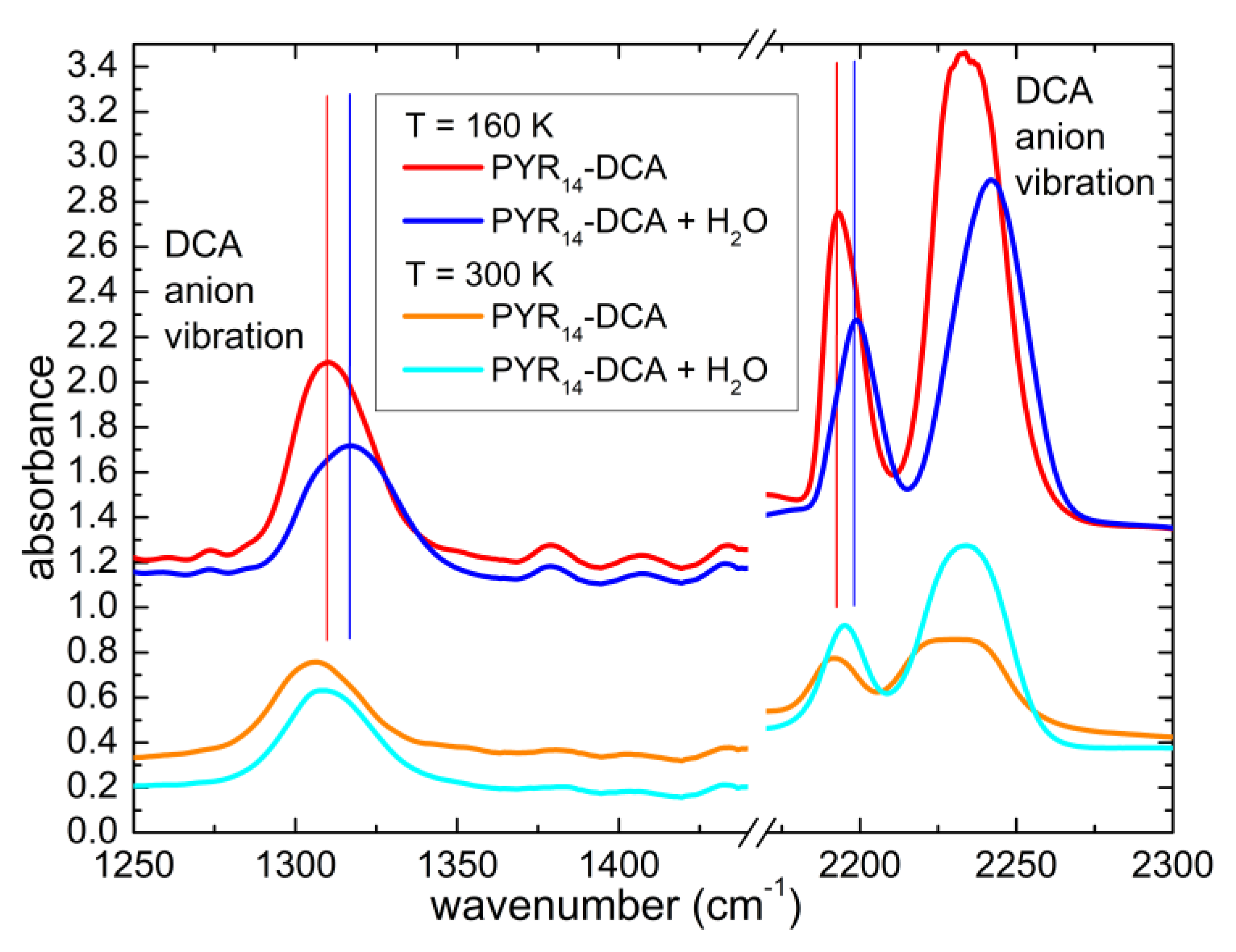
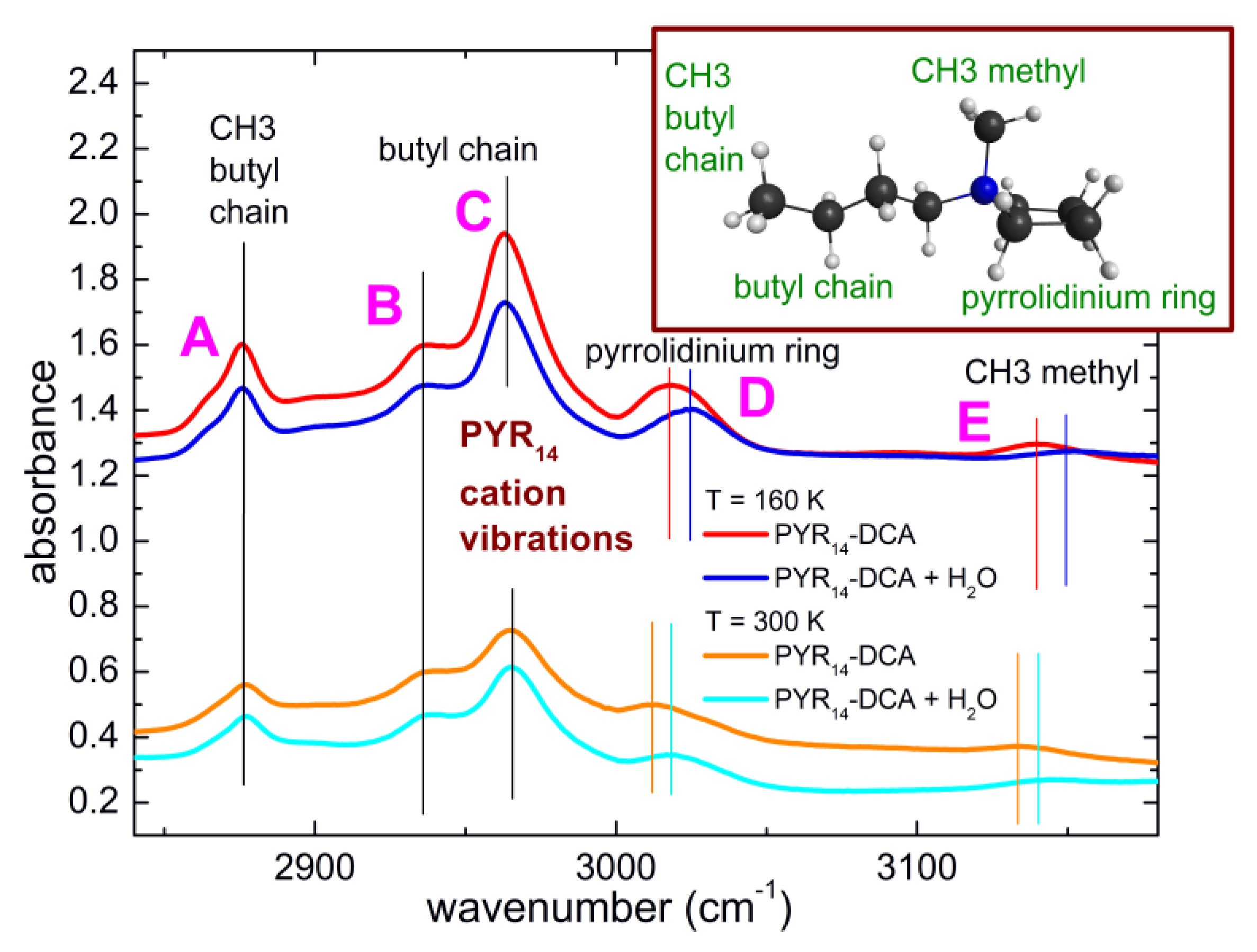
3.1. Line Attribution at Room Temperature and Estimate of the Initial Water Content of PYR14-DCA
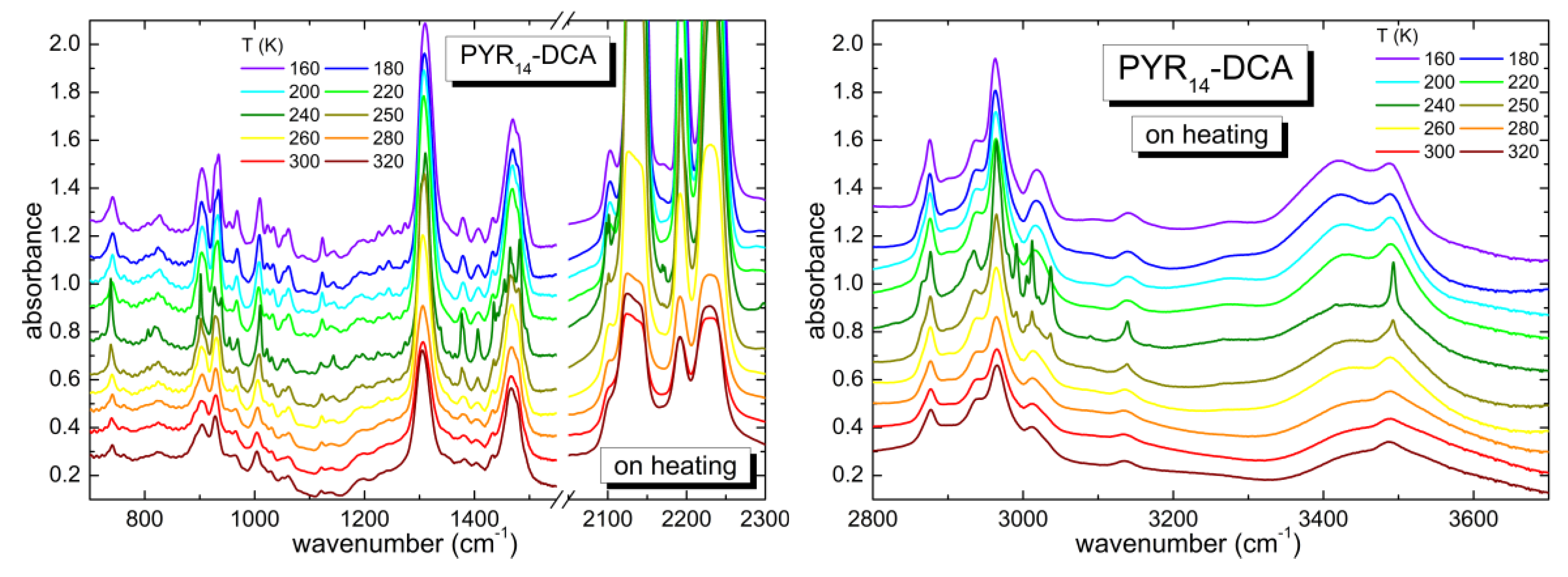
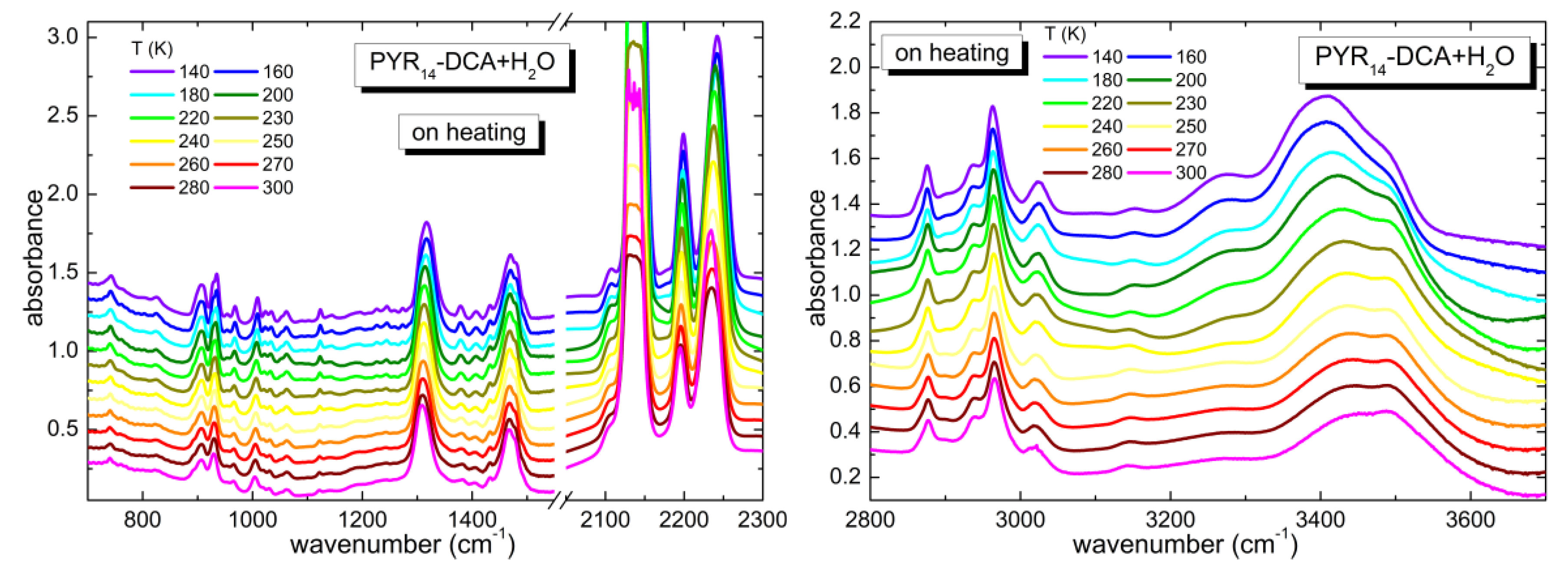
3.2. Temperature Dependence of the As-Received Sample
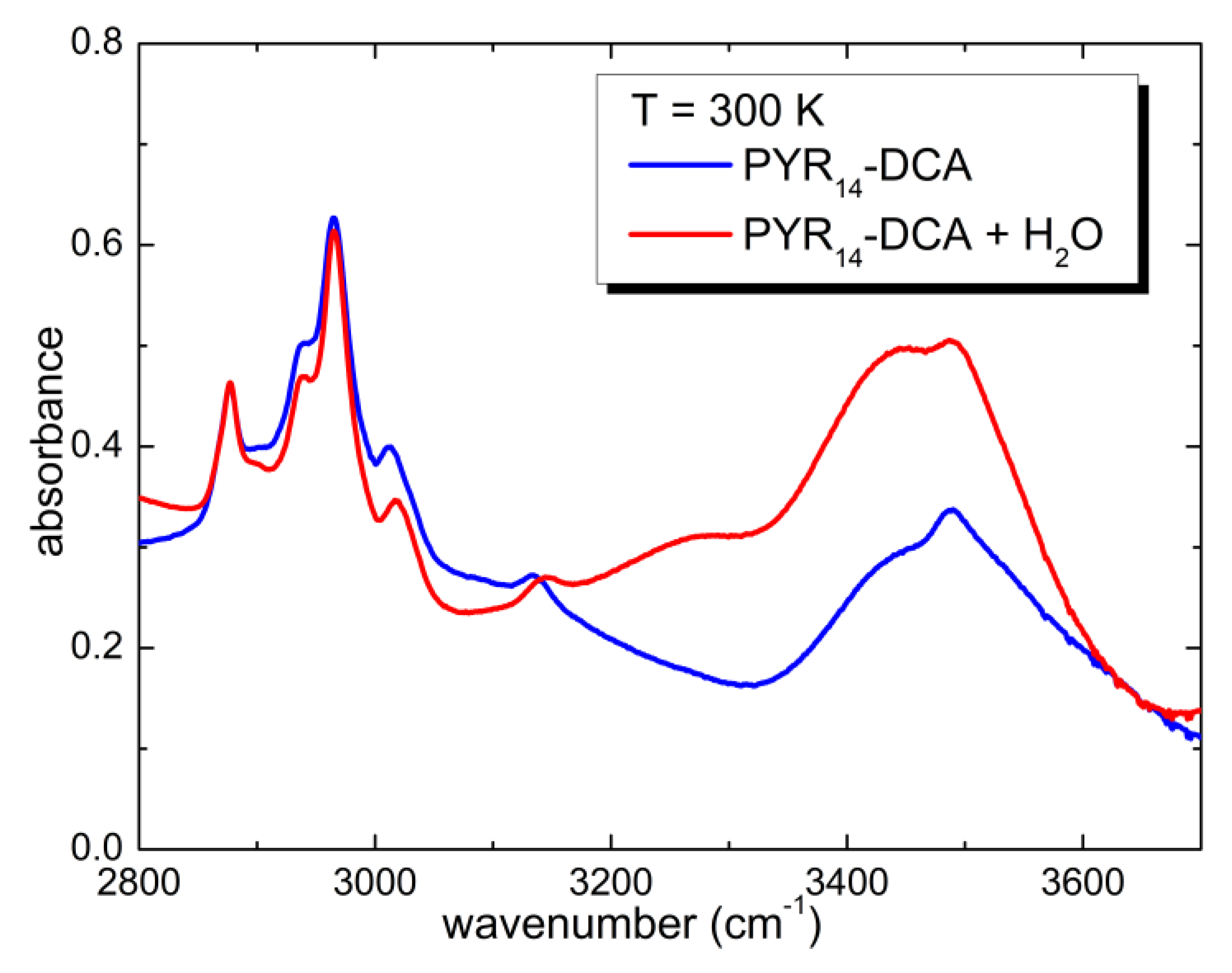
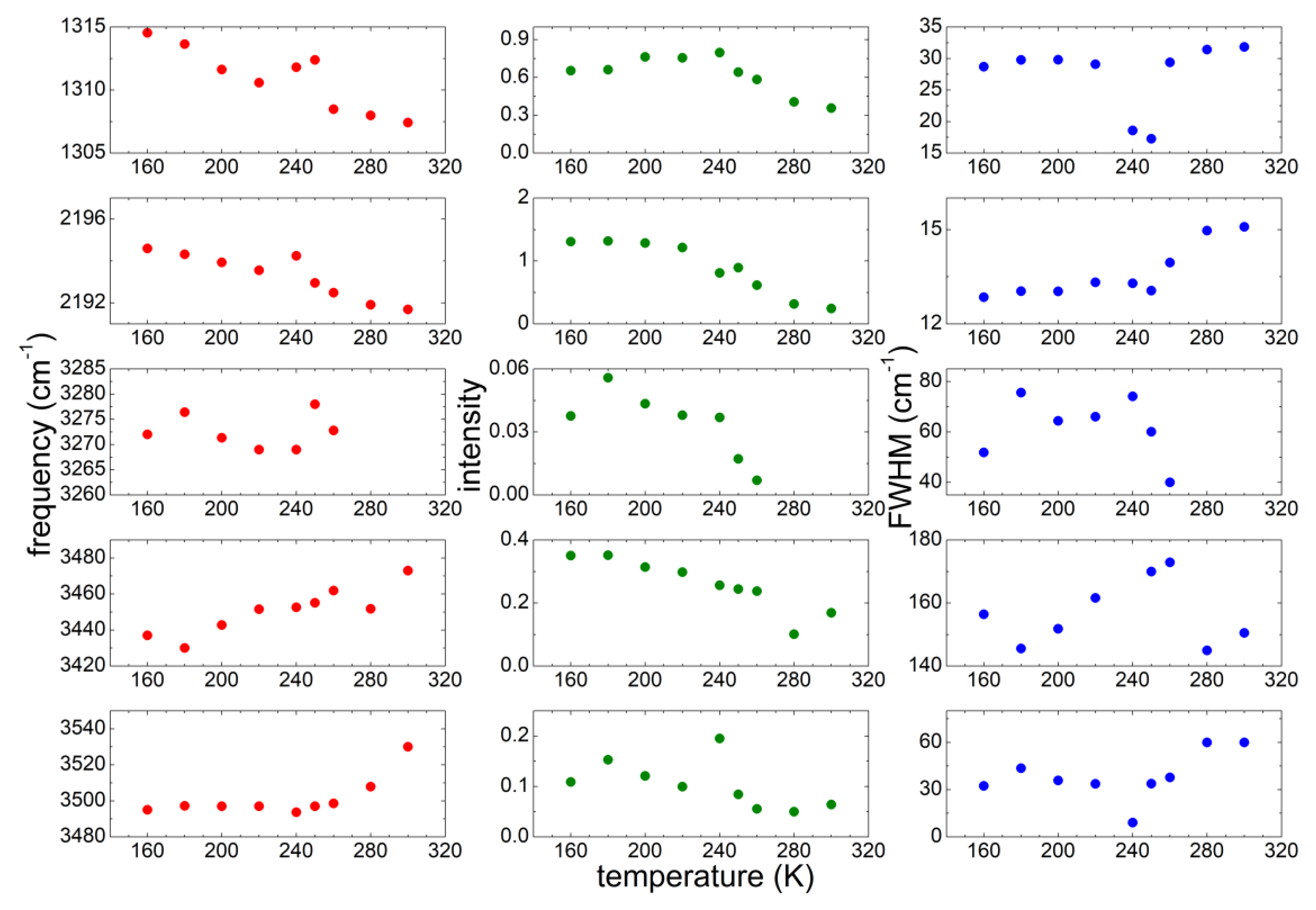
3.3. Temperature Dependence of the Intentionally Hydrated Sample
3.4. Interaction of Water with the Ionic Liquid
4. Discussion
4.1. Temperature Evolution of Water
4.2. Interaction of Water with the Anion and Cation of the Ionic Liquid
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Isosaari, P.; Srivastava, V.; Sillanpää, M. Ionic liquid-based water treatment technologies for organic pollutants: Current status and future prospects of ionic liquid mediated technologies. Sci. Total Environ. 2019, 690, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Mukherjee, A. Effect of water and ionic liquids on biomolecules. Biophys. Rev. 2018, 10, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Borodin, O.; Self, J.; Persson, K.A.; Wang, C.; Xu, K. Uncharted waters: Super-concentrated electrolytes. Joule 2020, 4, 69–100. [Google Scholar] [CrossRef]
- BagherKarimi, M.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrog. Energy 2019, 44, 28919–28938. [Google Scholar]
- Hejazifar, M.; Lanaridi, O.; Bica-Schroder, K. Ionic liquid based microemulsions: A review. J. Mol. Liq. 2020, 303, 112264. [Google Scholar] [CrossRef]
- Domańska, U. Experimental data of fluid phase equilibria-correlation and prediction models: A review. Processes 2019, 7, 277. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef]
- Cammarata, L.; Kazarian, S.G.; Salter, P.A.; Welton, T. Molecular states of water in room temperature ionic liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192–5200. [Google Scholar] [CrossRef]
- Aliaga, C.; Baker, G.A.; Baldelli, S. Sum frequency generation studies of ammonium and pyrrolidinium ionic liquids based on the bis-trifluoromethanesulfonimide anion. J. Phys. Chem. B 2008, 112, 1676–1684. [Google Scholar] [CrossRef]
- Fazio, B.; Triolo, A.; Di Marco, G. Local organization of water and its effect on the structural heterogeneities in room-temperature ionicliquid/H2O mixtures. J. Raman Spectrosc. 2008, 39, 233–237. [Google Scholar] [CrossRef]
- Bešter-Rogač, M.; Stoppa, A.; Hunger, J.; Hefter, G.; Buchner, R. Association of ionic liquids in solution: A combined dielectric and conductivity study of [bmim][Cl] in water and in acetonitrile. Phys. Chem. Chem. Phys. 2011, 13, 17588–17598. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, T.A.; Husson, P.; DeVine, J.A.; Costa Gomes, M.F.; Greenbaum, S.G.; Castner, E.W. Interactions between water and 1-butyl-1-methylpyrrolidinium ionic liquids. J. Chem. Phys. 2015, 143, 064503. [Google Scholar] [CrossRef] [PubMed]
- Yaghini, N.; Pitawala, J.; Matic, A.; Martinelli, A. Effect of water on the local structure and phase behavior of imidazolium-based protic ionic liquids. J. Phys. Chem. B 2015, 119, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Śmiechowski, M. Anion–Water interactions of weakly hydrated anions: Molecular dynamics simulations of aqueous NaBF4 and NaPF6. Mol. Phys. 2016, 114, 1831–1846. [Google Scholar] [CrossRef]
- Abe, H.; Takekiyo, T.; Yoshimura, Y.; Saihara, K.; Shimizu, A. Anomalous freezing of nano-confined water in room-temperature ionic liquid 1-butyl-3-methylimidazolium nitrate. ChemPhysChem 2016, 17, 1136–1142. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Zhao, Y.; Xuan, X.; Zhao, Y.; Wang, J. Hydrogen bonding mediated ion pairs of some aprotic ionic liquids and their structural transition in aqueous solution. Sci. China Chem. 2017, 60, 970–978. [Google Scholar] [CrossRef]
- Kundu, K.; Chandra, G.K.; Umapathy, S.; Kiefer, J. Spectroscopic and computational insights into the ion–solvent interactions in hydrated aprotic and protic ionic liquids. Phys. Chem. Chem. Phys. 2019, 21, 20791–20804. [Google Scholar] [CrossRef]
- Abe, H.; Takekiyo, T.; Yoshimura, Y.; Shimizu, A. Static and dynamic properties of nano-confined water in room-temperature ionic liquids. J. Mol. Liq. 2019, 290, 111216. [Google Scholar] [CrossRef]
- van der Vegt, N.F.A.; Haldrup, K.; Roke, S.; Zheng, J.; Lund, M.; Bakker, H.J. Water-mediated ion pairing: Occurrence and relevance. Chem. Rev. 2016, 116, 7626–7641. [Google Scholar] [CrossRef]
- Fumino, K.; Stange, P.; Fossog, V.; Hempelmann, R.; Ludwig, R. Equilibrium of contact and solvent-separated ion pairs in mixtures of protic ionic liquids and molecular solvents controlled by polarity. Angew. Chem. Int. Ed. 2013, 52, 12439–12442. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Rudas, T.; Neumayr, G.; Benkner, S.; Steinhauser, O. On the collective network of ionic liquid/water mixtures. I. Orientational structure. J. Chem. Phys. 2007, 127, 234503. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.M.; Méndez-Morales, T.; Carrete, J.; Gómez-González, V.; Docampo-Álvarez, B.; Gallego, L.J.; Cabeza, O.; Russina, O. Solvation of molecular cosolvents and inorganic salts in ionic liquids: A review of molecular dynamics simulations. J. Mol. Liq. 2015, 210, 178–188. [Google Scholar] [CrossRef]
- Heid, E.; Docampo-Álvarez, B.; Varela, L.M.; Prosenz, K.; Steinhauser, O.; Schröder, C. Langevin behavior of the dielectric decrement in ionic liquid water mixtures. Phys. Chem. Chem. Phys. 2018, 20, 15106–15117. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Hunger, J.; Stoppa, A.; Buchner, R.; Steinhauser, O. On the collective network of ionic liquid/water mixtures. II. Decomposition and interpretation of dielectric spectra. J. Chem. Phys. 2008, 129, 184501. [Google Scholar]
- Macchieraldo, R.; Esser, L.; Elfgen, R.; Voepel, P.; Zahn, S.; Smarsly, B.M.; Kirchner, B. Hydrophilic ionic liquid mixtures of weakly and strongly coordinating anions with and without water. ACS Omega 2018, 3, 8567–8582. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef]
- Mariani, A.; Russina, O.; Caminiti, R.; Triolo, A. Structural organization in a methanol:ethylammonium nitrate (1:4) mixture: A joint X-ray/Neutron diffraction and computational study. J. Mol. Liq. 2015, 212, 947–956. [Google Scholar] [CrossRef]
- Russina, O.; Sferrazza, A.; Caminiti, R.; Triolo, A. Amphiphile meets amphiphile: Beyond the polar−apolar dualism in ionic liquid/alcohol mixtures. J. Phys. Chem. Lett. 2014, 5, 1738–1742. [Google Scholar] [CrossRef]
- Abe, H.; Kohki, E.; Nakada, A.; Kishimura, H. Phase behavior in quaternary ammonium ionic liquid-propanol solutions: Hydrophobicity, molecular conformations, and isomer effects. Chem. Phys. 2017, 491, 136–142. [Google Scholar] [CrossRef]
- Abe, H.; Murata, K.; Kiyokawa, S.; Yoshimura, Y. Surface tension anomalies in room temperature ionic liquids-acetone solutions. Chem. Phys. Lett. 2018, 699, 275–278. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Brubach, J.-B.; Roy, P.; Paolone, A. Crystallization of mixtures of hydrophilic ionic liquids and water: Evidence of microscopic inhomogeneities. J. Colloid Interf. Sci. 2019, 552, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Faye, M.; Bordessoule, M.; Kanouté, B.; Brubach, J.B.; Roy, P.; Manceron, L. Improved mid infrared detector for high spectral or spatial resolution and synchrotron radiation use. Rev. Sci. Instr. 2016, 87, 063119. [Google Scholar] [CrossRef]
- Vitucci, F.M.; Palumbo, O.; Trequattrini, F.; Brubach, J.-B.; Roy, P.; Meschini, I.; Croce, F.; Paolone, A. Interaction of 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide with an electrospun PVdF membrane: Temperature dependence of the anion conformers. J. Chem. Phys. 2015, 143, 094707. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, B.; Irran, E.; Schnick, W. Syntheses, vibrational spectroscopy, and crystal structure determination from X-Ray powder diffraction data of alkaline earth dicyanamides M[N(CN)2]2 with M = Mg, Ca, Sr, and Ba. J. Solid State Chem. 2001, 157, 241–249. [Google Scholar] [CrossRef]
- Dahl, K.; Sando, G.M.; Fox, D.M.; Sutto, T.E.; Owrutsky, J.C. Vibrational spectroscopy and dynamics of small anions in ionic liquid solutions. J. Chem. Phys. 2005, 123, 084504. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.W.; Grasselli, J.G.; Ritchey, W.M. The synthesis and infrared and nuclear magnetic resonance spectra of ammonium dicyanamide. J. Phys. Chem. 1964, 68, 431–433. [Google Scholar] [CrossRef]
- Le Caer, S.; Klein, G.; Ortiz, D.; Lima, M.; Devineau, S.; Pin, S.; Brubach, J.-B.; Roy, P.; Pommeret, S.; Leibl, W.; et al. The effect of myoglobin crowding on the dynamics of water: An infrared study. Phys. Chem. Chem. Phys. 2014, 16, 22841–22852. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Zhang, H.; Wang, J. Molecular origin for the difficulty in separation of 5-hydroxymethylfurfural from imidazolium based ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 6712–6721. [Google Scholar] [CrossRef]
- Haddad, B.; Brandán, S.A.; Assenine, M.A.; Paolone, A.; Villemin, D.; Bresson, S. Bidentate cation-anion coordination in the ionic liquid 1-ethyl-3-methylimidazolium hexafluorophosphate supported by vibrational spectra and NBO, AIM and SQMFF calculations. J. Mol. Struct. 2020, 1212, 128104. [Google Scholar] [CrossRef]
- Brubach, J.-B.; Mermet, A.; Filabozzi, A.; Colavita, P.; Gerschel, A.; Roy, P. Infrared investigation of water encapsulated in non ionic reverse micelles. J. Phys. IV 2000, 10, 215–218. [Google Scholar] [CrossRef]
- DallaBernardina, S.; Paineau, E.; Brubach, J.-B.; Judeinstein, P.; Rouzière, S.; Launois, P.; Roy, P. Water in carbon nanotubes: The peculiar hydrogen bond network revealed by infrared spectroscopy. J. Am. Chem. Soc. 2016, 138, 10437–10443. [Google Scholar] [CrossRef] [PubMed]
- Russina, O.; Lo Celso, F.; Plechkova, N.V.; Triolo, A. Emerging evidences of mesoscopic-scale complexity in neat ionic liquids and their mixtures. J. Phys. Chem. Lett. 2017, 8, 1197–1204. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, O.; Trequattrini, F.; Brubach, J.-B.; Roy, P.; Paolone, A. Molecular Assembling in Mixtures of Hydrophilic 1-Butyl-1-Methylpyrrolidinium Dicyanamide Ionic Liquid and Water. Appl. Sci. 2020, 10, 4837. https://doi.org/10.3390/app10144837
Palumbo O, Trequattrini F, Brubach J-B, Roy P, Paolone A. Molecular Assembling in Mixtures of Hydrophilic 1-Butyl-1-Methylpyrrolidinium Dicyanamide Ionic Liquid and Water. Applied Sciences. 2020; 10(14):4837. https://doi.org/10.3390/app10144837
Chicago/Turabian StylePalumbo, Oriele, Francesco Trequattrini, Jean-Blaise Brubach, Pascale Roy, and Annalisa Paolone. 2020. "Molecular Assembling in Mixtures of Hydrophilic 1-Butyl-1-Methylpyrrolidinium Dicyanamide Ionic Liquid and Water" Applied Sciences 10, no. 14: 4837. https://doi.org/10.3390/app10144837
APA StylePalumbo, O., Trequattrini, F., Brubach, J.-B., Roy, P., & Paolone, A. (2020). Molecular Assembling in Mixtures of Hydrophilic 1-Butyl-1-Methylpyrrolidinium Dicyanamide Ionic Liquid and Water. Applied Sciences, 10(14), 4837. https://doi.org/10.3390/app10144837






