Bio-Kinetics of Simultaneous Nitrification and Aerobic Denitrification (SNaD) by a Cyanide- Degrading Bacterium Under Cyanide-Laden Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of the Bacterial Isolate of Interest
2.2. Batch Culture Experiments
2.3. Enzyme Extraction
2.4. Analytical Procedure(s)
2.5. Kinetics Model Developed
2.6. Regression of Experimental Data and Estimation of Model Kinetic Parameters
2.7. Data Handling and Kinetic Parameters
3. Results and Discussion
3.1. Identification of Bacterial Isolate
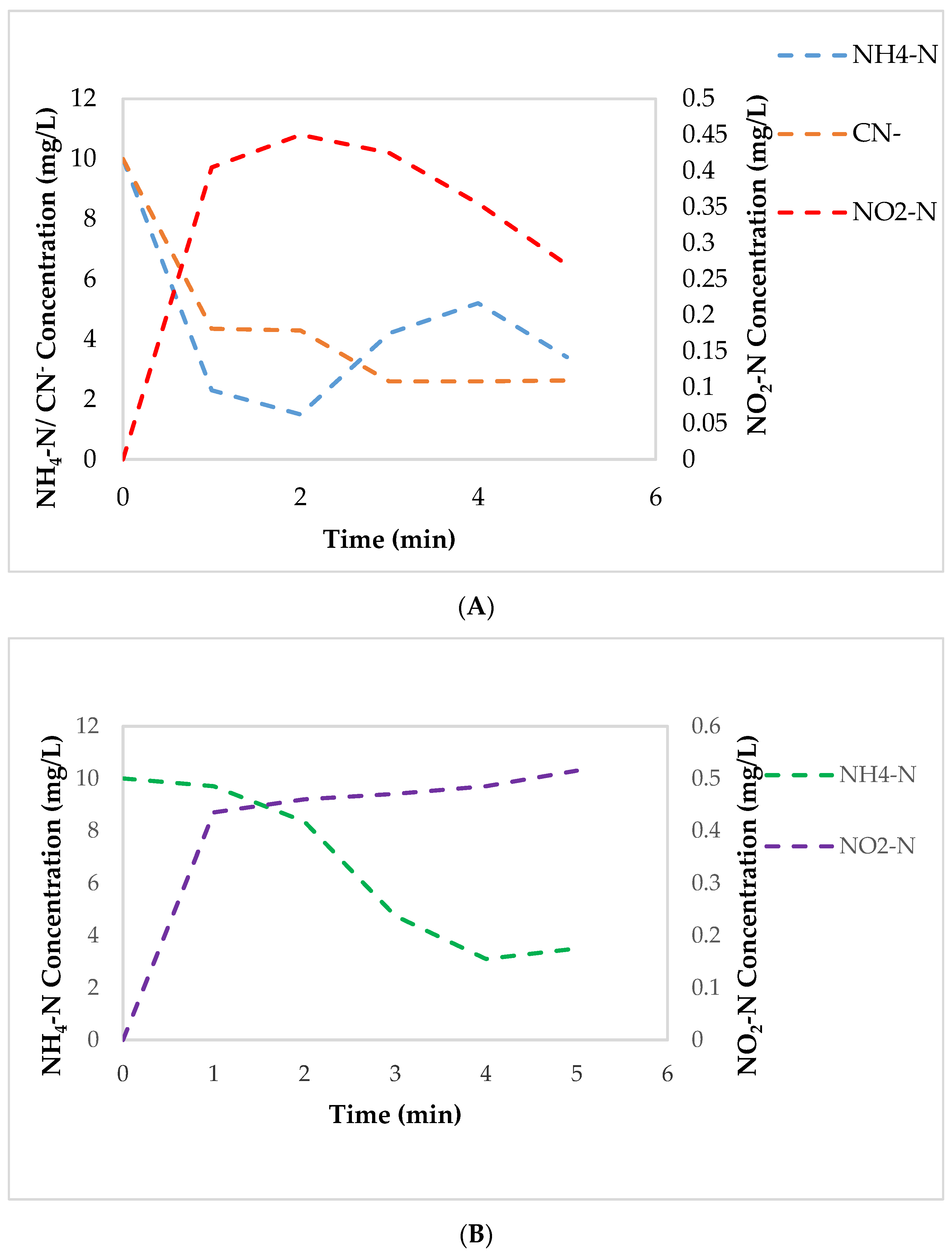
3.2. Degradation Kinetics of Cyanide and NH4-N
3.3. Model Parameter Determination and Statistical Analysis
3.4. Effect of Cyanide on AMO, NaR, and NiR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duan, J.; Fang, H.; Su, B.; Chen, J.; Lin, J. Characterization of a halophilic heterotrophic nitrification–aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, S.; Yang, X.; Qiu, S.; Li, B.; Peng, Y. Detection of nitrifiers and evaluation of partial nitrification for wastewater treatment: A review. Chemosphere 2015, 140, 85–98. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Puyol, D.; Carvajal-Arroyo, J.; Sierra-Alvarez, R.; Field, J. Inhibition of anaerobic ammonium oxidation by heavy metals. J. Chem. Technol. Biotechnol. 2014, 90, 830–837. [Google Scholar] [CrossRef]
- Kim, Y.; Park, D.; Lee, D.; Park, J. Inhibitory effects of toxic compounds on nitrification process for cokes wastewater treatment. J. Hazard. Mater. 2008, 152, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.; Santo Domingo, J. Impact of Heavy Metals on Transcriptional and Physiological Activity of Nitrifying Bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef]
- Inglezakis, V.; Malamis, S.; Omirkhan, A.; Nauruzbayeva, J.; Makhtayeva, Z.; Seidakhmetov, T.; Kudarova, A. Investigating the inhibitory effect of cyanide, phenol and 4-nitrophenol on the activated sludge process employed for the treatment of petroleum wastewater. J. Environ. Manag. 2017, 203, 825–830. [Google Scholar] [CrossRef]
- Han, Y.; Jin, X.; Wang, Y.; Liu, Y.; Chen, X. Inhibitory effect of cyanide on nitrification process and its eliminating method in a suspended activated sludge process. Environ. Sci. Pollut. Res. Int. 2014, 21, 2706–2713. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.O.; Jackson, V.A. Biodegradation of free cyanide and subsequent utilisation of biodegradation by-products by Bacillus consortia: Optimisation using response surface methodology. Environ. Sci. Pollut. Res. Int. 2015, 22, 10434–10443. [Google Scholar] [CrossRef]
- Mpongwana, N.; Ntwampe, S.; Mekuto, L.; Akinpelu, E.; Dyantyi, S.; Mpentshu, Y. Isolation of high-salinity-tolerant bacterial strains, Enterobacter sp., Serratia sp., Yersinia sp., for nitrification and aerobic denitrification under cyanogenic conditions. Water Sci. Technol. 2016, 73, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1942, 3, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Annuar, M.S.M.; Tan, I.K.P.; Ibrahim, S.; Ramachandran, K.B. A kinetic model for growth and biosynthesis of medium-chain-length poly-(3-hydroxyalkanoates) in Pseudomonas putida. Braz. J. Chem. Eng. 2008, 25, 217–228. [Google Scholar] [CrossRef]
- Arai, H.; Kodama, T.; Igarashi, Y. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol. Microbiol. 1997, 25, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Yunjie, R.; Mohammad, J.T.; Dedong, K.; Huifeng, L.; Heping, Z.; Xiangyang, X.; Yu, L.; Lei, C. Nitrogen Removal Performance and Metabolic Pathways Analysis of a Novel Aerobic Denitrifying Halotolerant Pseudomonas balearica strain RAD-17. Microorganism 2020, 8, 72. [Google Scholar]
- Kim, Y.; Park, D.; Lee, D.; Park, J. Instability of biological nitrogen removal in a cokes wastewater treatment facility during summer. J. Hazard. Mater. 2007, 141, 27–32. [Google Scholar] [CrossRef]
- Mpongwana, N.; Ntwampe, S.K.O.; Omodanisi, E.I.; Chidi, B.S.; Razanamahandry, L.C. Sustainable Approach to Eradicate the Inhibitory Effect of Free-Cyanide on Simultaneous Nitrification and Aerobic Denitrification during Wastewater Treatment. Sustainability 2019, 11, 6180. [Google Scholar] [CrossRef] [Green Version]
- Sin, G.; Kaelin, D.; Kampschreur, M.J.; Takacs, I.; Wett, B.; Gernaey, K.V.; Rieger, L.; Siegrist, H.; van Loosdrecht, M. Modelling nitrite in wastewater treatment systems: A discussion of different modelling concepts. Water Sci. Technol. 2008, 58, 1155–1171. [Google Scholar] [CrossRef]
- Ge, Q.; Yue, X.; Wang, G. Simultaneous heterotrophic nitrification and aerobic denitrification at high initial phenol concentration by isolated bacterium Diaphorobacter sp. PD-7. Chin. J. Chem. Eng. 2015, 23, 835–841. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. A kinetic study of hydrogenotrophic denitrification. Process Biochem. 2006, 41, 1401–1408. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H.; Yin, Y.; Qin, Y.; Tang, A.; Liu, H.; Liu, Y. Synergic effect of adsorption and biodegradation enhance cyanide removal by immobilized Alcaligenes sp. strain DN25. J. Hazard. Mater. 2019, 364, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Thi, S.S.; Wuertz, S. Inhibition factors and kinetic model for anaerobic ammonia oxidation in a granular sludge bioreactor with Candidatus Brocadia. Chem. Eng. J. 2019, 123618. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Zhang, Y.; Zhang, J.; Li, J.; Wang, S.; Chen, G. Isolation and characterization of Acinetobacter sp. JQ1004 and evaluation of its inhibitory kinetics by free ammonia. Desalin. Water Treat. 2019, 147, 316–325. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]


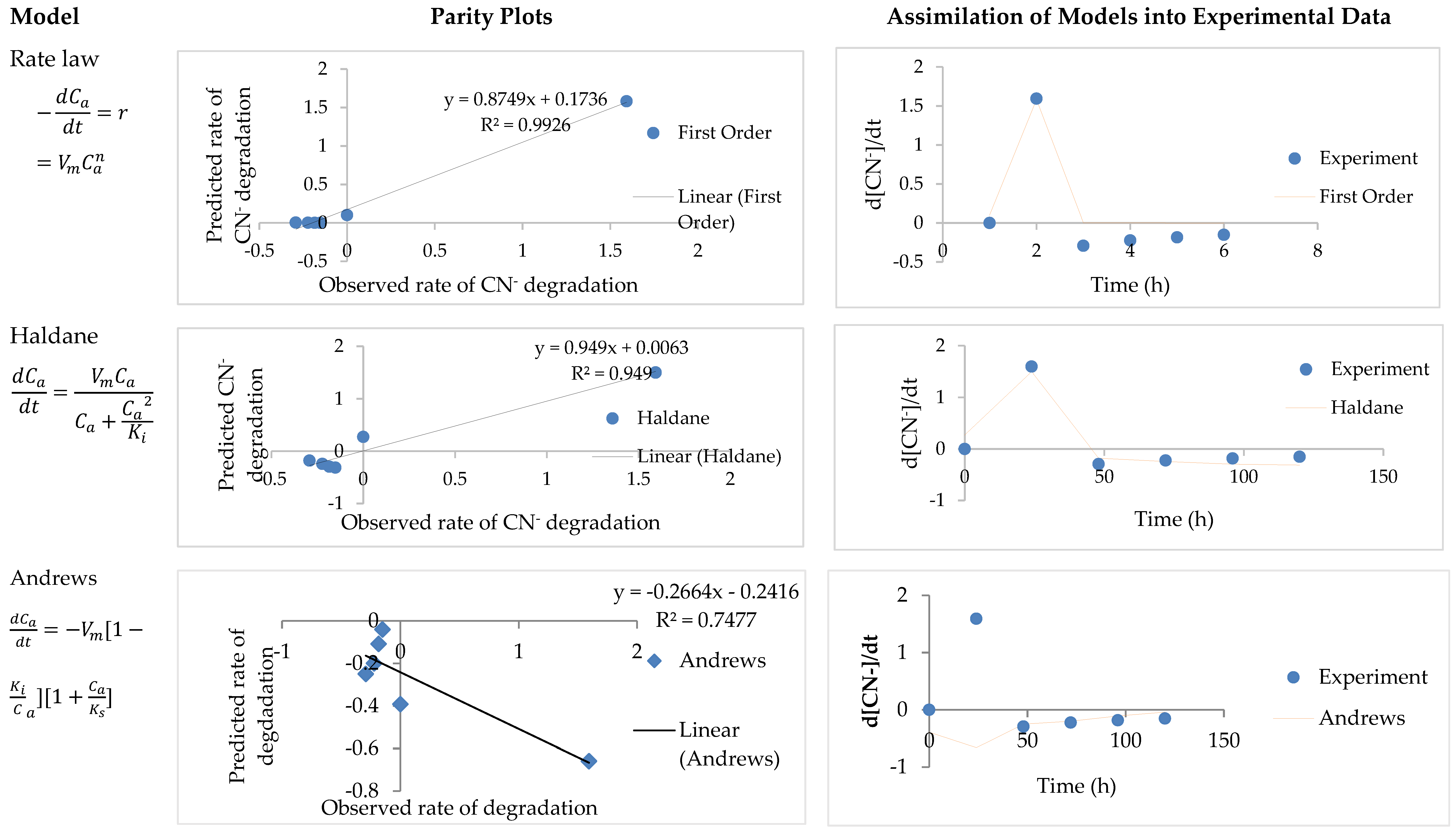
| Values of Kinetic Parameters (±95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| Model | Vm (h−1) | Ki (mgL−1) | Ks (mgL−1) | n (-) | R2 | Variance |
| Rate law | - | 0.0000629 | - | 2.27 | 0.91 | 0.02 |
| Haldane | 0.45 | 23.94 | - | - | 0.92 | 0.02 |
| Model with Substrate Inhibition | ||||||
| Andrews | 0.36 | 27.54 | 13.22 | - | 0.94 | 0.02 |
| Values of Kinetic Parameter (±95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| Model | Vm (h−1) | Ki (mgL−1) | Ks (mgL−1) | n (-) | R2 | Variance |
| Rate law | - | 3.33 × 10−5 | - | 2.65 | 0.92 | 0.05 |
| Haldane | 0.36 | 11.36 | - | - | 0.95 | 365.43 |
| Model | Kinetic Parameters | |||||
|---|---|---|---|---|---|---|
| n | Reference | |||||
| Haldane | 0.323 | 9.65 | 152.40 | - | [19] | SNaD under high phenol concentrations using A strain capable of phenol degradation (Haldane model with substrate inhibition) |
| 0.45 | - | 23.94 | - | This study | SNaD in CN− (Haldane model without substrate inhibition) | |
| Rate law | - | - | 0.047 | 1 | [20] | Degradation of ammonia nitrogen under high phenol concentrations. |
| - | - | 6.29 × 10−5 | 2.27 | This study | SNaD in CN− | |
| Andrews | 0.0485 | 28.63 | 24.284 | [20] | Denitrification with nitrate inhibition | |
| 0.36 | 13.22 | 27.54 | - | This study | SNaD in CN− | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpongwana, N.; Ntwampe, S.K.O.; Omodanisi, E.I.; Chidi, B.S.; Razanamahandry, L.C.; Dlangamandla, C.; Mukandi, M.R. Bio-Kinetics of Simultaneous Nitrification and Aerobic Denitrification (SNaD) by a Cyanide- Degrading Bacterium Under Cyanide-Laden Conditions. Appl. Sci. 2020, 10, 4823. https://doi.org/10.3390/app10144823
Mpongwana N, Ntwampe SKO, Omodanisi EI, Chidi BS, Razanamahandry LC, Dlangamandla C, Mukandi MR. Bio-Kinetics of Simultaneous Nitrification and Aerobic Denitrification (SNaD) by a Cyanide- Degrading Bacterium Under Cyanide-Laden Conditions. Applied Sciences. 2020; 10(14):4823. https://doi.org/10.3390/app10144823
Chicago/Turabian StyleMpongwana, Ncumisa, Seteno Karabo Obed Ntwampe, Elizabeth Ife Omodanisi, Boredi Silas Chidi, Lovasoa Christine Razanamahandry, Cynthia Dlangamandla, and Melody Ruvimbo Mukandi. 2020. "Bio-Kinetics of Simultaneous Nitrification and Aerobic Denitrification (SNaD) by a Cyanide- Degrading Bacterium Under Cyanide-Laden Conditions" Applied Sciences 10, no. 14: 4823. https://doi.org/10.3390/app10144823
APA StyleMpongwana, N., Ntwampe, S. K. O., Omodanisi, E. I., Chidi, B. S., Razanamahandry, L. C., Dlangamandla, C., & Mukandi, M. R. (2020). Bio-Kinetics of Simultaneous Nitrification and Aerobic Denitrification (SNaD) by a Cyanide- Degrading Bacterium Under Cyanide-Laden Conditions. Applied Sciences, 10(14), 4823. https://doi.org/10.3390/app10144823







