Simulation Study on the Influence of Multifrequency Ultrasound on Transient Cavitation Threshold in Different Media
Abstract
Featured Application
Abstract
1. Introduction
2. Principle and Method
3. Simulation Results
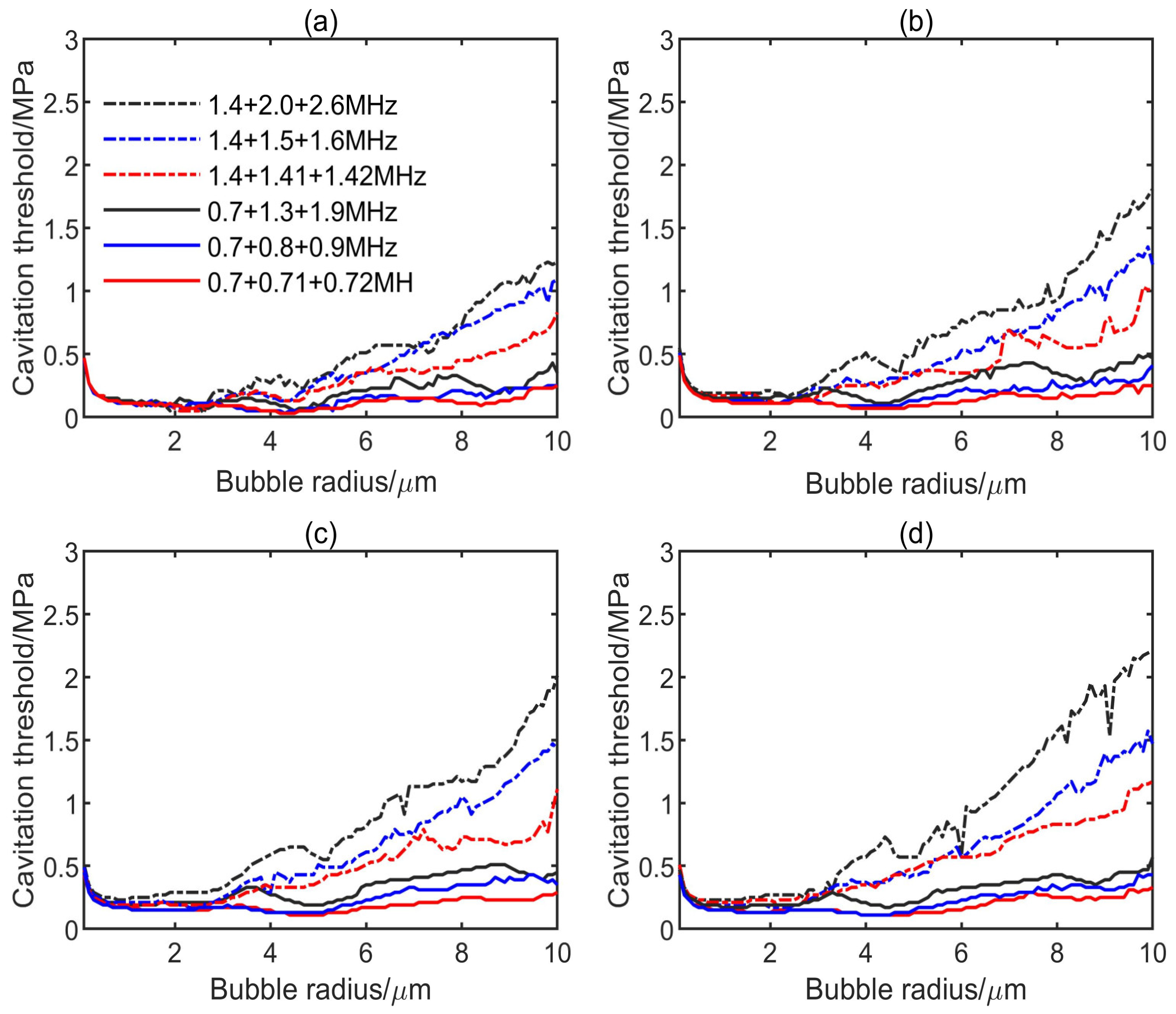
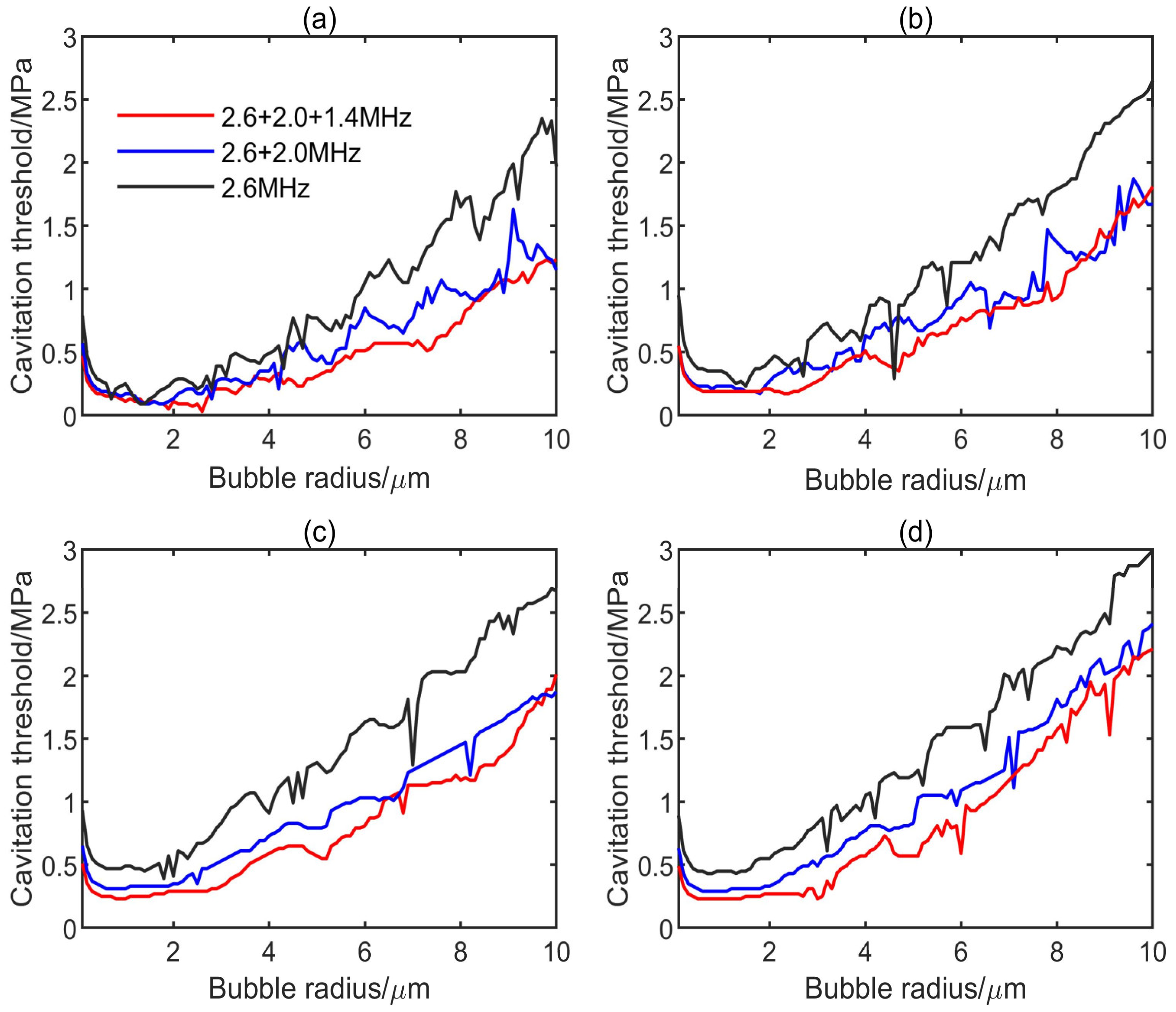
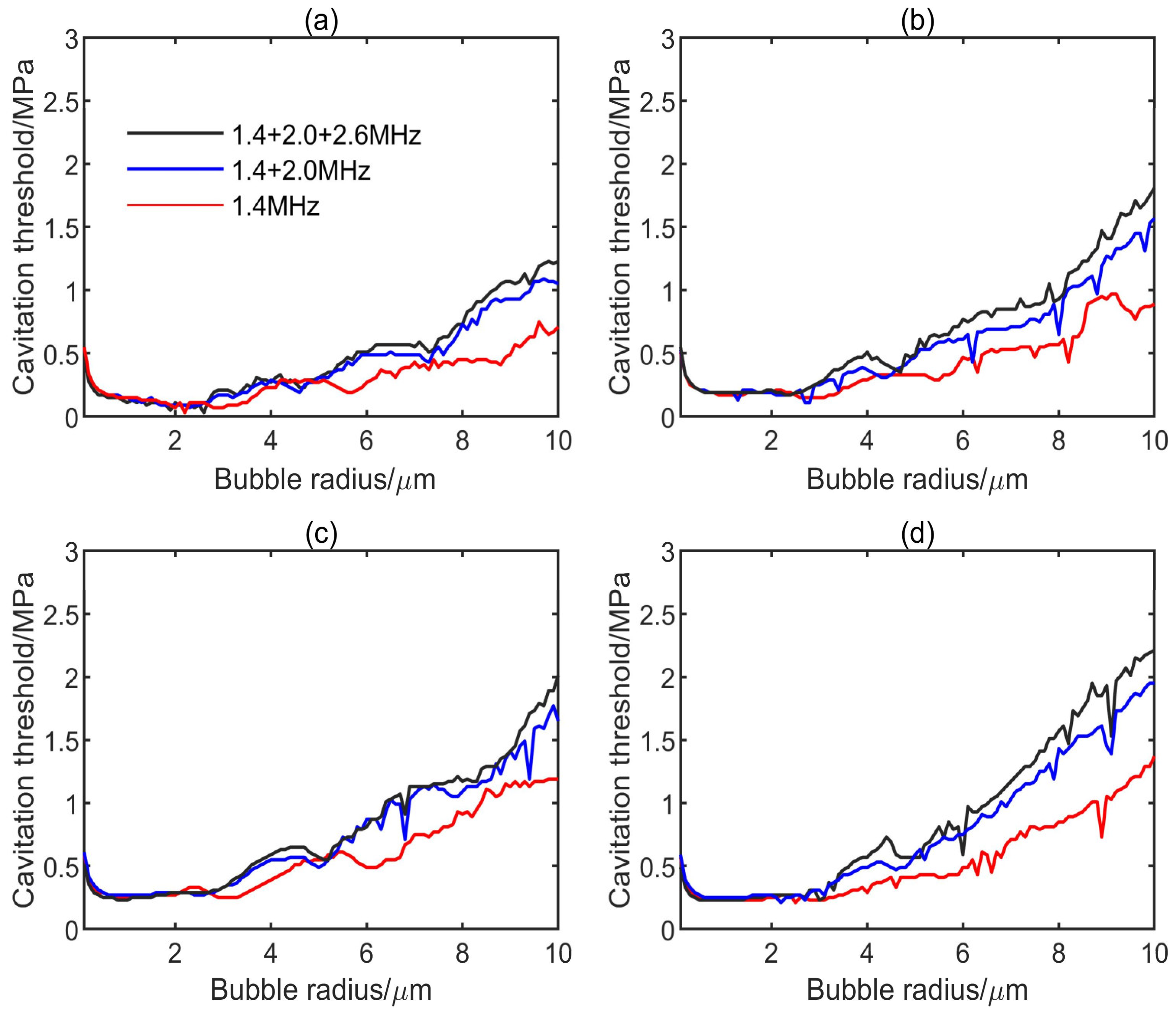
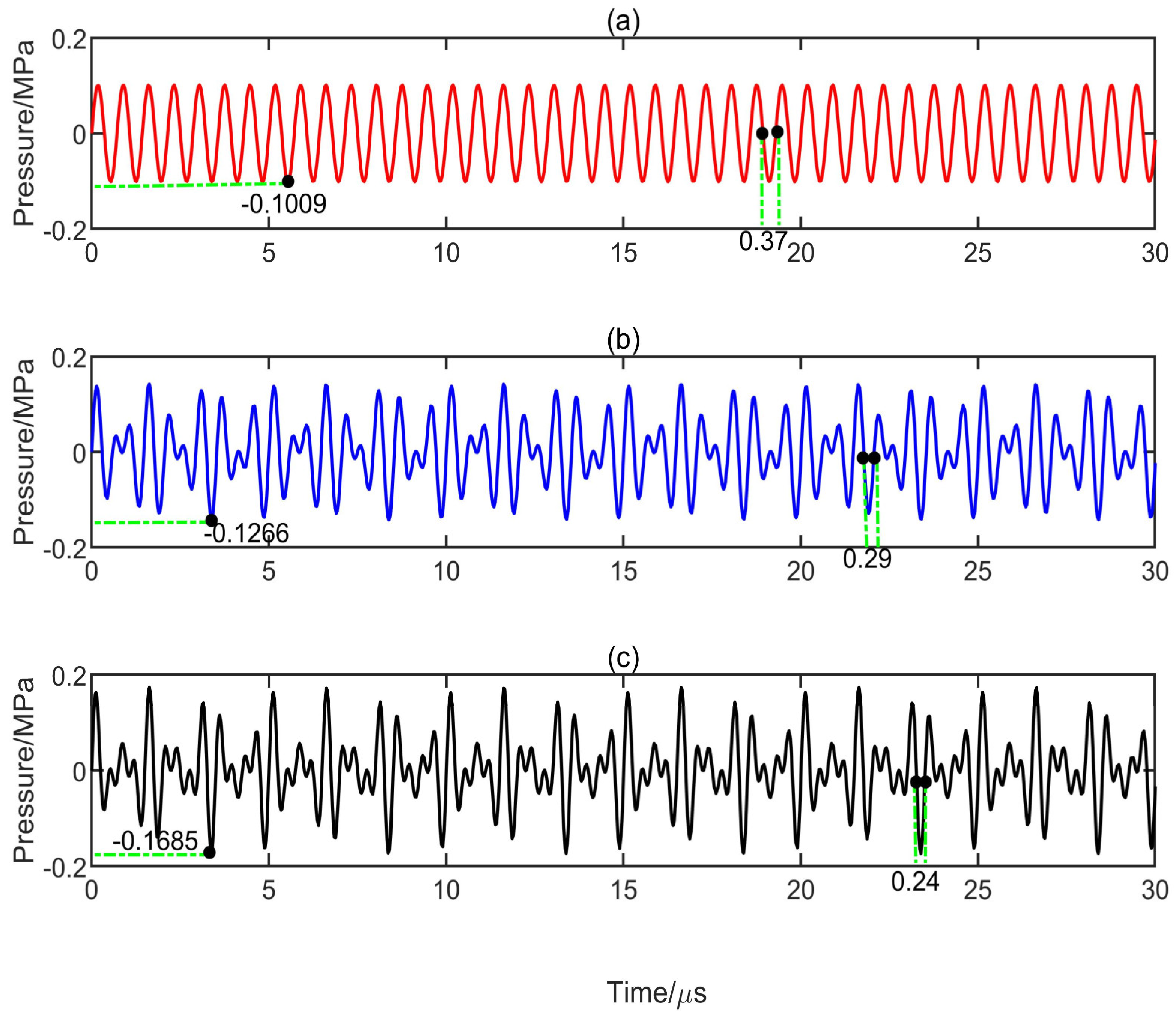
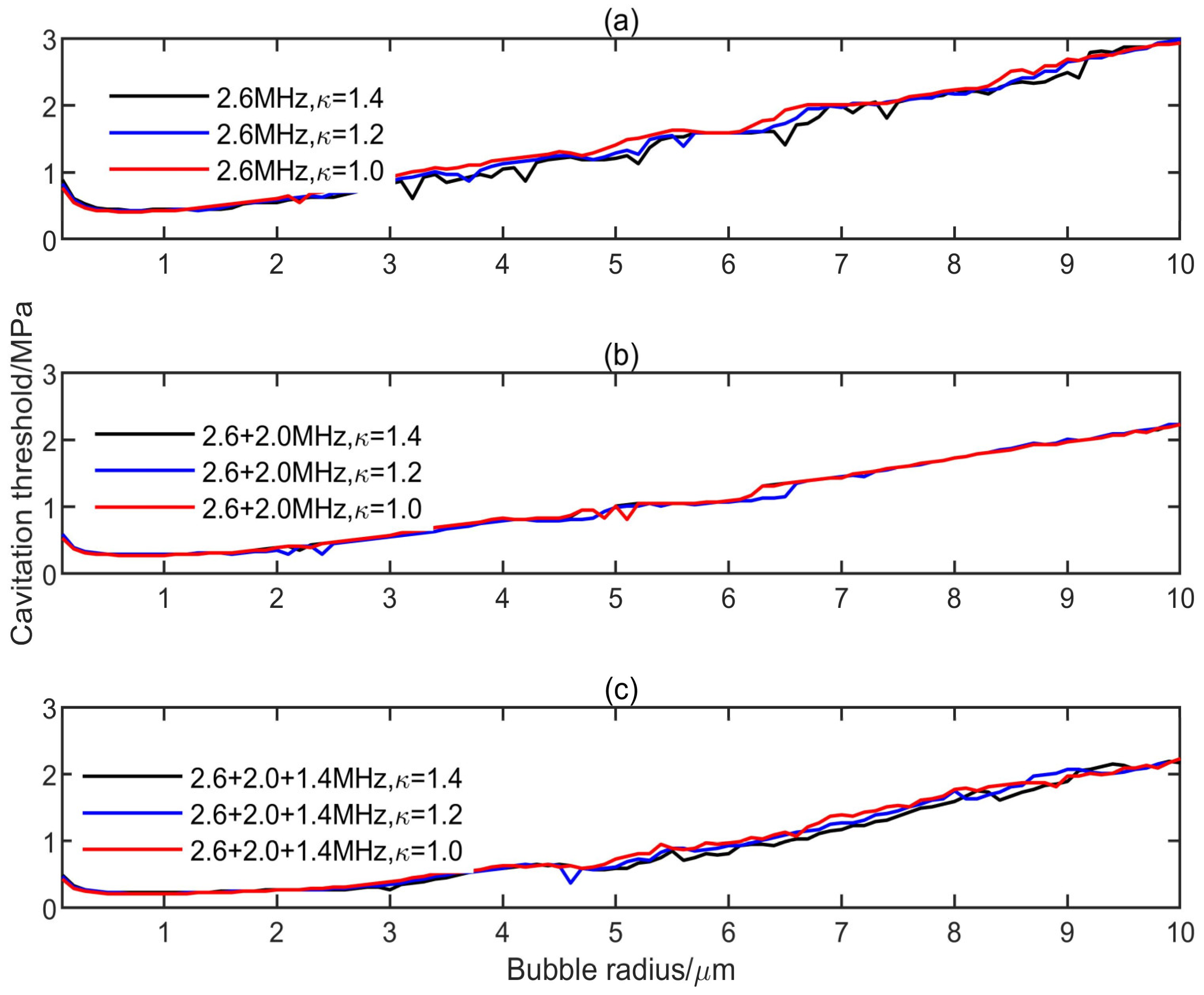
3.1. Influence of Multifrequency Ultrasonic Combination on Transient Cavitation Threshold
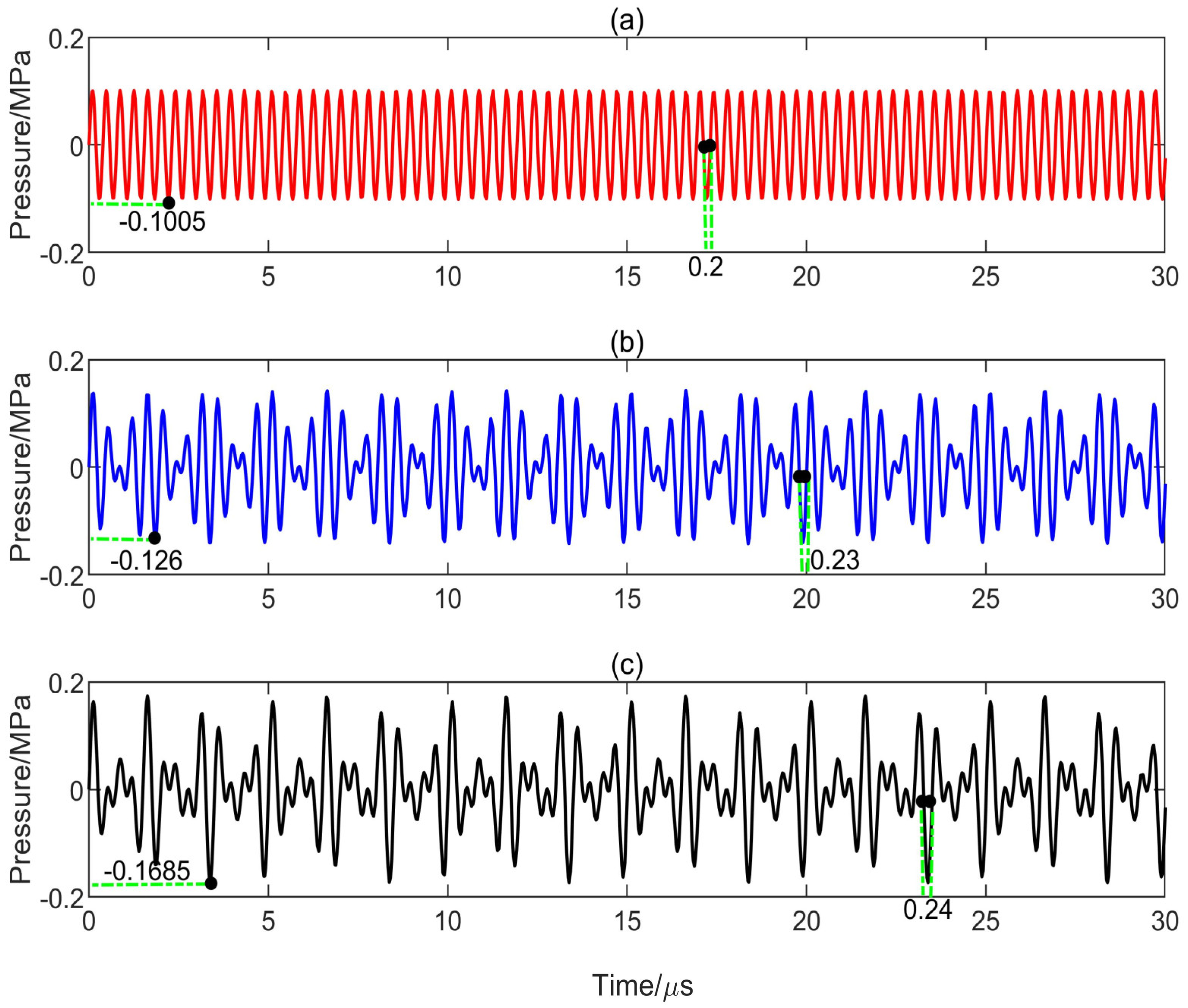
3.2. Effect of Peak Negative Pressure and Its Duration on Transient Cavitation Threshold
3.3. Effect of Phase Angle Difference on Transient Cavitation Threshold
3.4. Effect of Polytropic Index on Transient Cavitation Threshold
4. Conclusions
- (1)
- On the premise of the same frequency difference and initial bubble radius, the transient cavitation threshold of the triple-frequency combination with higher frequency is higher than that of the triple-frequency combination with lower frequency. With the increase of the initial bubble radius, the threshold difference between the single-frequency ultrasound and the multifrequency combination increases, and the influence of the frequency difference on the transient cavitation threshold of the triple-frequency combination with higher frequency is more obvious.
- (2)
- When the lowest frequency of triple frequencies is the same, the larger the frequency difference is, the higher the corresponding transient cavitation threshold is. When the bubble radius is small, the frequency difference has little effect on the transient cavitation threshold of the triple-frequency combination.
- (3)
- The level of transient cavitation threshold is related to the peak negative pressure and duration of ultrasound. Compared with the single-frequency ultrasound, the introduction of a low-frequency component in the multifrequency combination can significantly reduce the transient cavitation threshold in the medium. However, the introduction of a high-frequency component in the multifrequency combination can significantly increase the transient cavitation threshold in the medium.
- (4)
- When the phase angle difference of the multifrequency excitation is zero, the corresponding transient cavitation threshold in the medium is the lowest. The change of the polytropic index has little effect on the transient cavitation threshold of different frequency combinations.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holland, C.K.; Apfel, R.E. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J. Acoust. Soc. Am. 1990, 88, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Black, J.J.; Qin, B.; Pacella, J.; Chen, X.; Villanueva, F.S. Fluid viscosity affects the fragmentation and inertial cavitation threshold of lipid-encapsulated microbubbles. Ultrasound Med. Biol. 2016, 42, 782–794. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, P.; He, G.; Ge, S.; Liu, L.; Zhou, X. Hemocoagulase combined with microbubble-enhanced ultrasound cavitation for augmented ablation of microvasculature in rabbit VX2 liver tumors. Ultrasound Med. Biol. 2017, 43, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Thudium, M.; Bette, B.; Tonguc, T.; Ghaei, S.; Conrad, R.; Becher, M.U.; Mücke, M.; Luechters, G.; Strunk, S.; Marinova, M. Multidisciplinary management and outcome in pancreatic cancer patients treated with high-intensity focused ultrasound. Int. J. Hyperther. 2020, 37, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, X.; Lin, W. Tissue ablation using multi-frequency focused ultrasound. In Proceedings of the IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 2177–2180. [Google Scholar]
- Law, S.K.B.; Zhou, Y. High-Intensity Focused Ultrasound Ablation by the Dual-Frequency Excitation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 66, 18–25. [Google Scholar]
- Wang, Y.; Zhang, Z.; He, R.; Liu, D.; Mintah, B.K.; Dabbour, M.; Ma, H. Improvement in enzymolysis efficiency and changes in conformational attributes of corn gluten meal by dual-frequency slit ultrasonication action. Ultrason. Sonochem. 2020, 64, 105038. [Google Scholar] [CrossRef]
- Ma, J.; Guo, S.; Wu, D.; Geng, X.; Jiang, X. Design, fabrication, and characterization of a single-aperture 1.5-MHz/3-MHz dual-frequency HIFU transducer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1519–1529. [Google Scholar] [CrossRef]
- He, P.Z.; Shou, W.D.; Duan, S.M.; Xia, R.M. Dual-frequency high intensity focused ultrasound (HIFU) accelerating therapy. In Proceedings of the IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 1–4 September 2005; pp. 213–216. [Google Scholar]
- Gilles, B.; Béra, J.C.; Mestas, J.L.; Cathignol, D. Reduction of ultrasound inertial cavitation threshold using bifrequency excitation. Appl. Phys. Lett. 2006, 89, 94106. [Google Scholar] [CrossRef]
- Saletes, I.; Gilles, B.; Auboiroux, V.; Bendridi, N.; Salomir, R.; Béra, J.C. In vitro demonstration of focused ultrasound thrombolysis using bifrequency excitation. Biomed. Res. Int. 2014, 1, 10. [Google Scholar]
- Suo, D.; Guo, S.; Lin, W.; Jiang, X.; Jing, Y. Thrombolysis using multi-frequency high intensity focused ultrasound at MHz range: An in vitro study. Phys. Med. Biol. 2015, 60, 7403–7418. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yasuda, J.; Takagi, R.; Miyashita, T.; Goto, K.; Yoshizawa, S.; Umemura, S.I. Highly efficient cavitation-enhanced heating with dual-frequency ultrasound exposure in high-intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2014, 53, 07KF11. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, Y.; Zhu, C.; Mason, T.J. Enhancement of ultrasonic cavitation yield by multi-frequency sonication. Ultrason. Sonochem. 2002, 9, 231–236. [Google Scholar] [CrossRef]
- Guo, S.; Jing, Y.; Jiang, X. Temperature rise in tissue ablation using multi-frequency ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 1699–1707. [Google Scholar]
- Wallace, N.; Dicker, S.; Lewin, P.; Wrenn, S.P. Inertial cavitation threshold of nested microbubbles. Ultrasonics 2015, 58, 67–74. [Google Scholar] [CrossRef]
- Liu, R.; Xu, S.; Hu, H.; Huo, R.; Wang, S.; Wan, M. Wavelet-transform-based active imaging of cavitation bubbles in tissues induced by high intensity focused ultrasound. J. Acoust. Soc. Am. 2016, 140, 798–805. [Google Scholar] [CrossRef]
- Yang, X.; Church, C.C. A model for the dynamics of gas bubbles in soft tissue. J. Acoust. Soc. Am. 2005, 118, 3595–3606. [Google Scholar] [CrossRef]
- Fourest, T.; Deletombe, E.; Faucher, V.; Arrigoni, M.; Dupas, J.; Laurens, J.M. Comparison of Keller-Miksis model and finite element bubble dynamics simulations in a confined medium. Application to the Hydrodynamic Ram. Eur. J. Mech. B Fluid 2018, 68, 66–75. [Google Scholar] [CrossRef]
- Man, V.H.; Li, M.S.; Derreumaux, P.; Nguyen, P.H. Rayleigh-Plesset equation of the bubble stable cavitation in water: A nonequilibrium all-atom molecular dynamics simulation study. J. Chem. Phys. 2018, 148, 094505. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Lin, K.W.; Warnez, M.T.; Singh, R.; Mancia, L.; Putnam, A.J.; Johnsen, E.; Cain, C.; Xu, Z. Effects of tissue stiffness, ultrasound frequency, and pressure on histotripsy-induced cavitation bubble behavior. Phys. Med. Biol. 2015, 60, 2271–2292. [Google Scholar] [CrossRef]
- Suo, D.; Govind, B.; Zhang, S.; Jing, Y. Numerical investigation of the inertial cavitation threshold under multi-frequency ultrasound. Ultrason. Sonochem. 2018, 41, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Shampine, L.F.; Reichelt, M.W. The MATLAB ODE Suite. SIAM J. Sci. Comput. 1997, 18, 1–22. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y. Numerical investigation of the inertial cavitation threshold by dual-frequency excitation in the fluid and tissue. Ultrason. Sonochem. 2018, 42, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Maiga, M.A.; Coutier-Delgosha, O.; Buisine, D. Analysis of the critical pressure of cavitation bubbles. Meccanica 2018, 53, 787–801. [Google Scholar] [CrossRef]
- Church, C.C.; Labuda, C.; Nightingale, K. A Theoretical Study of Inertial Cavitation from Acoustic Radiation Force Impulse Imaging and Implications for the Mechanical Index1. Ultrasound Med. Biol. 2015, 41, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, W.; Kurz, T. Physics of bubble oscillations. Rep. Prog. Phys. 2010, 73, 106501. [Google Scholar] [CrossRef]
- Gaudron, R.; Warnez, M.T.; Johnsen, E. Bubble dynamics in a viscoelastic medium with nonlinear elasticity. J. Fluid Mech. 2015, 766, 54–75. [Google Scholar] [CrossRef]
- Khismatullin, D.B. Resonance frequency of microbubbles: Effect of viscosity. J. Acoust. Soc. Am. 2004, 116, 1463–1473. [Google Scholar] [CrossRef]
- Yount, D.E. A microscopic investigation of bubble formation nuclei. J. Acoust. Soc. Am. 1984, 76, 1511–1521. [Google Scholar] [CrossRef]
- Blatteau, J.E. Gas nuclei, their origin, and their role in bubble formation. Aviation Space Environ. Med. 2006, 77, 1068–1076. [Google Scholar]
- Gateau, J.; Taccoen, N.; Tanter, M.; Aubry, J.F. Statistics of acoustically induced bubble-nucleation events in in vitro blood: A feasibility study. Ultrasound Med. Biol. 2013, 39, 1812–1825. [Google Scholar] [CrossRef]
- Gateau, J.; Aubry, J.F.; Chauvet, D.; Boch, A.L.; Fink, M.; Tanter, M. In vivo bubble nucleation probability in sheep brain tissue. Phys. Med. Biol. 2011, 56, 7001–7015. [Google Scholar] [CrossRef]
- Webb, I.R.; Payne, S.J.; Coussios, C.C. The effect of temperature and viscoelasticity on cavitation dynamics during ultrasonic ablation. J. Acoust. Soc. Am. 2011, 130, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.S.; Choi, J.J.; Baseri, B.; Konofagou, E.E. Identifying the inertial cavitation threshold and skull effects in a vessel phantom using focused ultrasound and microbubbles. Ultrasound Med. Biol. 2010, 36, 840–852. [Google Scholar] [CrossRef]
- Guédra, M.; Inserra, C.; Gilles, B. Accompanying the frequency shift of the nonlinear resonance of a gas bubble using a dual-frequency excitation. Ultrason. Sonochem. 2017, 38, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.; Pandit, A.B. Experimental investigation of cavitational bubble dynamics under multi-frequency system. Ultrason. Sonochem. 2008, 15, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Cain, C.A.; Hall, T.L.; Fowlkes, J.B.; Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 2013, 39, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Aydin, O.; Lin, K.W.; Durmaz, Y.Y.; Fowlkes, B.; ElSayed, M.; Xu, Z. The role of positive and negative pressure on cavitation nucleation in nanodroplet-mediated histotripsy. Phys. Med. Biol. 2015, 61, 663–682. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Xu, Z.; Maxwell, A.D.; Mancia, L.; Zhang, X.; Lin, K.W.; Duryea, A.; Sukovich, J.; Hall, T.; Johnsen, E.; et al. Effects of temperature on the histotripsy intrinsic threshold for cavitation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 1064–1077. [Google Scholar] [CrossRef]
- Yasuda, J.; Asai, A.; Yoshizawa, S.; Umemura, S.I. Efficient generation of cavitation bubbles in gel phantom by ultrasound exposure with negative-followed by positive-peak-pressure-emphasized waves. Jpn. J. Appl. Phys. 2013, 52, 07HF11. [Google Scholar] [CrossRef]
- Behnia, S.; Sojahrood, A.J.; Soltanpoor, W.; Jahanbakhsh, O. Suppressing chaotic oscillations of a spherical cavitation bubble through applying a periodic perturbation. Ultrason. Sonochem. 2009, 16, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Sadighi-Bonabi, R.; Lahiji, F.A.F.; Razeghi, F. The effect of viscosity, applied frequency and driven pressure on the laser induced bubble luminescence in water–sulfuric acid mixtures. Phys. Lett. A 2016, 380, 2219–2226. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H. Hydrodynamic formation of a microlayer underneath a boiling bubble. Int. J Heat Mass Transf. 2018, 120, 1229–1240. [Google Scholar] [CrossRef]

| Medium | Speed/c (m/s) | Density/ρ (g/m3) | Surface Tension/σ (mN/m) | Viscosity/μ (mPa.s) | Shear Modulus/G (MPa) |
|---|---|---|---|---|---|
| Water | 1500 | 1000 | 68 | 1 | 0 |
| Blood | 1570 | 1050 | 56 | 5 | 0 |
| Brain | 1540 | 1050 | 56 | 9 | 0.012 |
| Liver | 1549 | 1100 | 56 | 9 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Zou, X.; Qian, S. Simulation Study on the Influence of Multifrequency Ultrasound on Transient Cavitation Threshold in Different Media. Appl. Sci. 2020, 10, 4778. https://doi.org/10.3390/app10144778
Dong H, Zou X, Qian S. Simulation Study on the Influence of Multifrequency Ultrasound on Transient Cavitation Threshold in Different Media. Applied Sciences. 2020; 10(14):4778. https://doi.org/10.3390/app10144778
Chicago/Turabian StyleDong, Hu, Xiao Zou, and Shengyou Qian. 2020. "Simulation Study on the Influence of Multifrequency Ultrasound on Transient Cavitation Threshold in Different Media" Applied Sciences 10, no. 14: 4778. https://doi.org/10.3390/app10144778
APA StyleDong, H., Zou, X., & Qian, S. (2020). Simulation Study on the Influence of Multifrequency Ultrasound on Transient Cavitation Threshold in Different Media. Applied Sciences, 10(14), 4778. https://doi.org/10.3390/app10144778





