Structural, Vibrational and Electrochemical Analysis and Antibacterial Potential of Isomeric Chalcones Derived from Natural Acetophenone
Abstract
1. Introduction
2. Results and Discussion
2.1. NMR Data
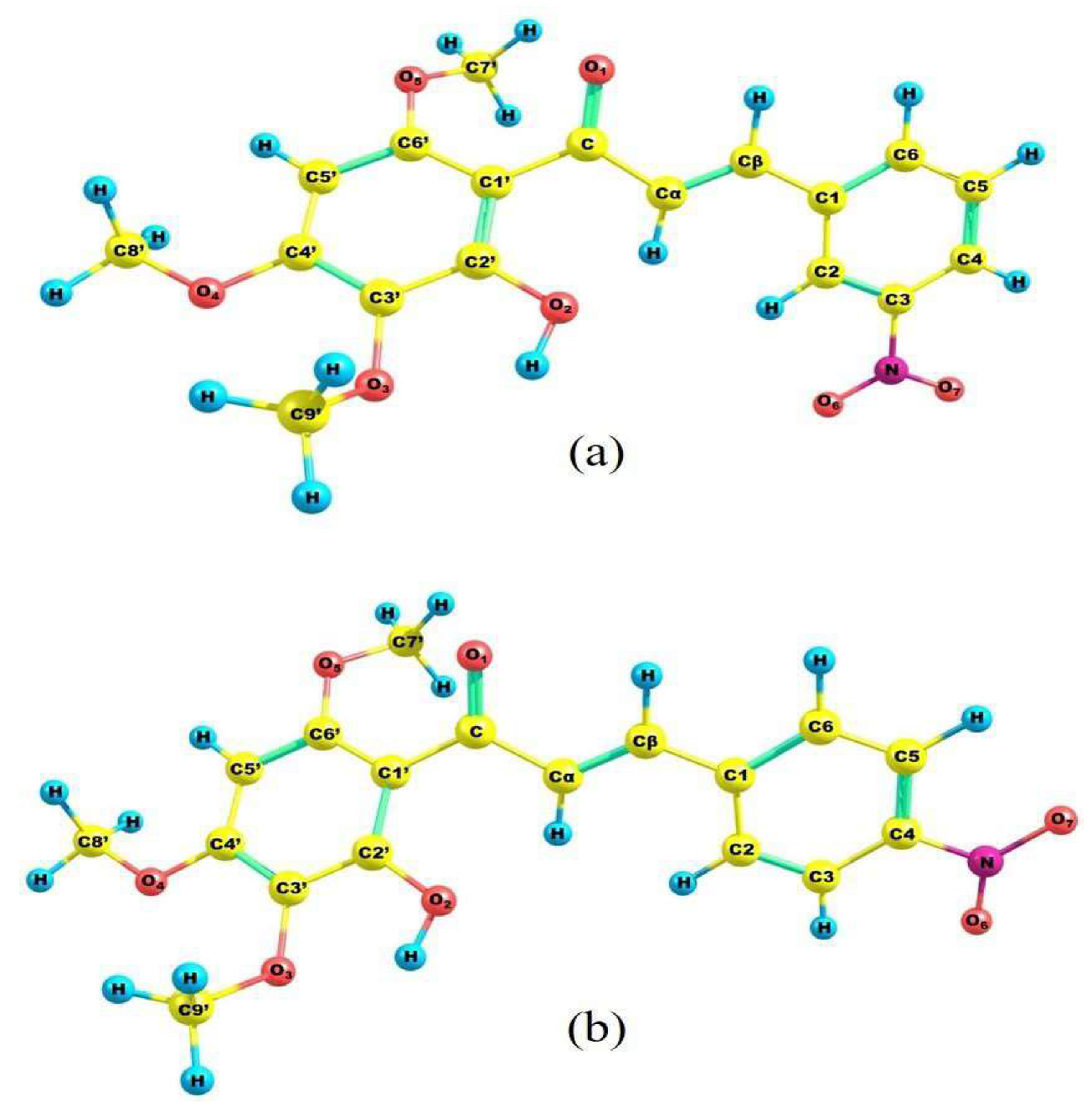
2.2. Molecular Structure
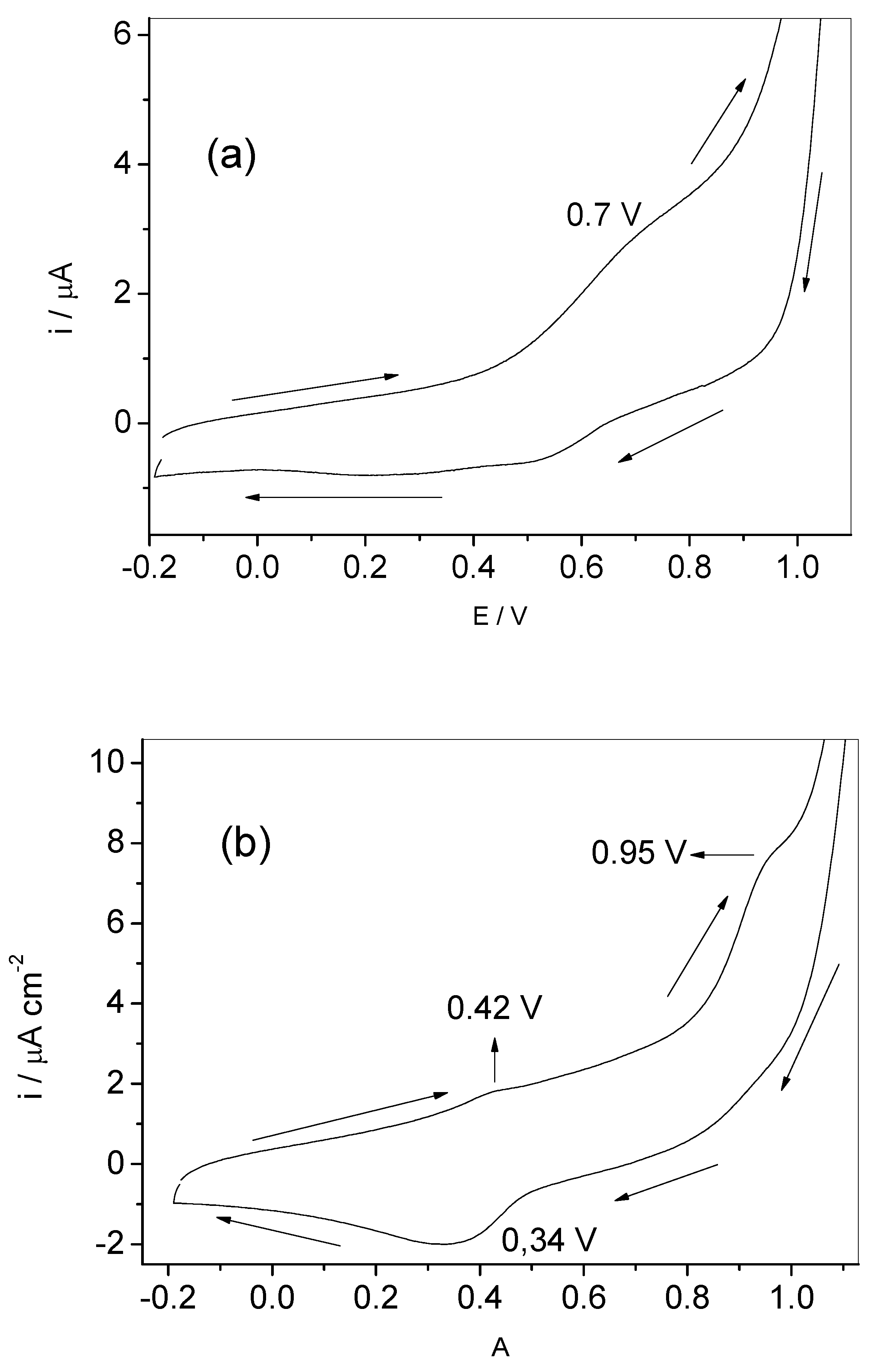
2.3. Electrochemical Analysis
2.4. Quantum Chemical Parameters
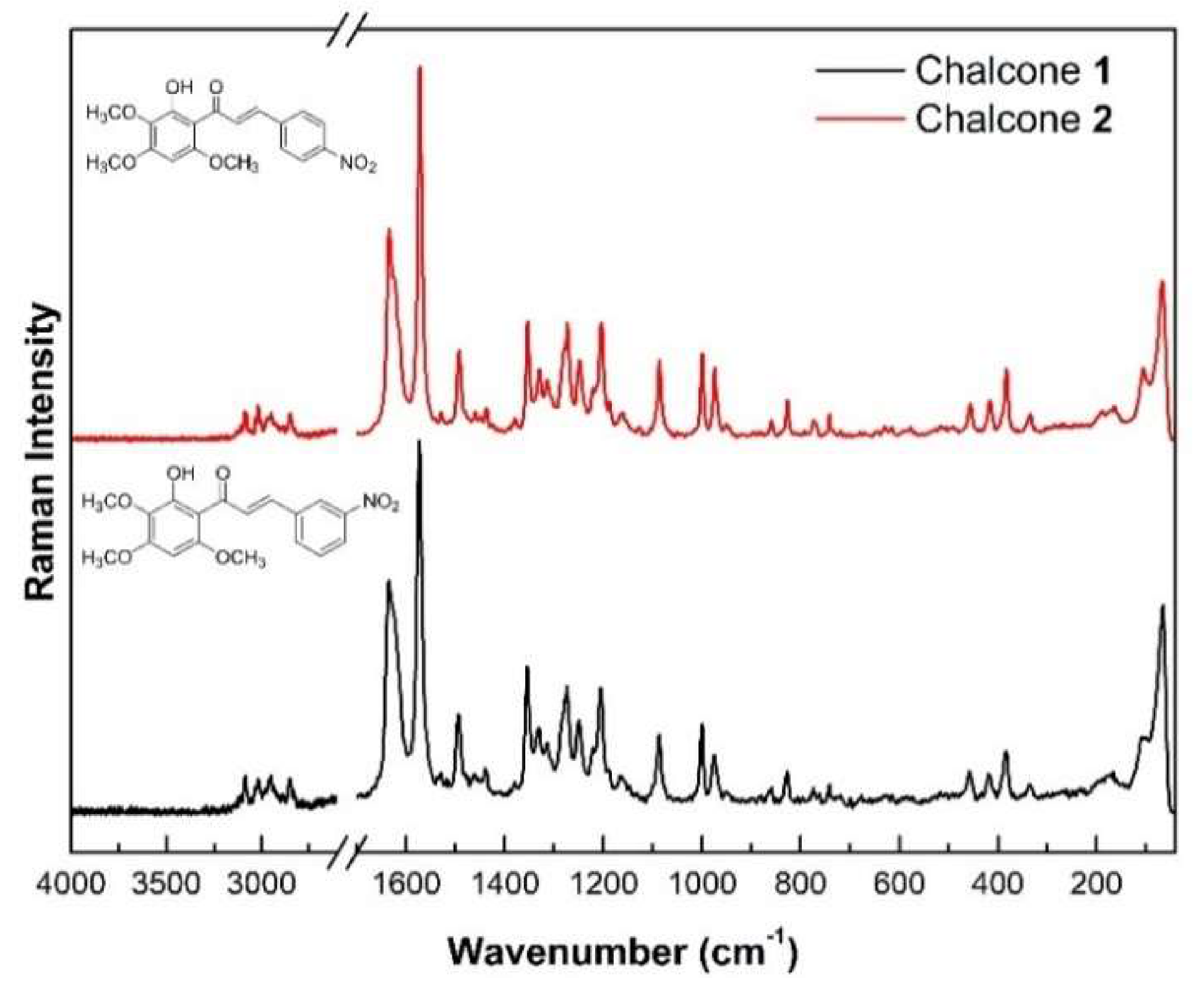
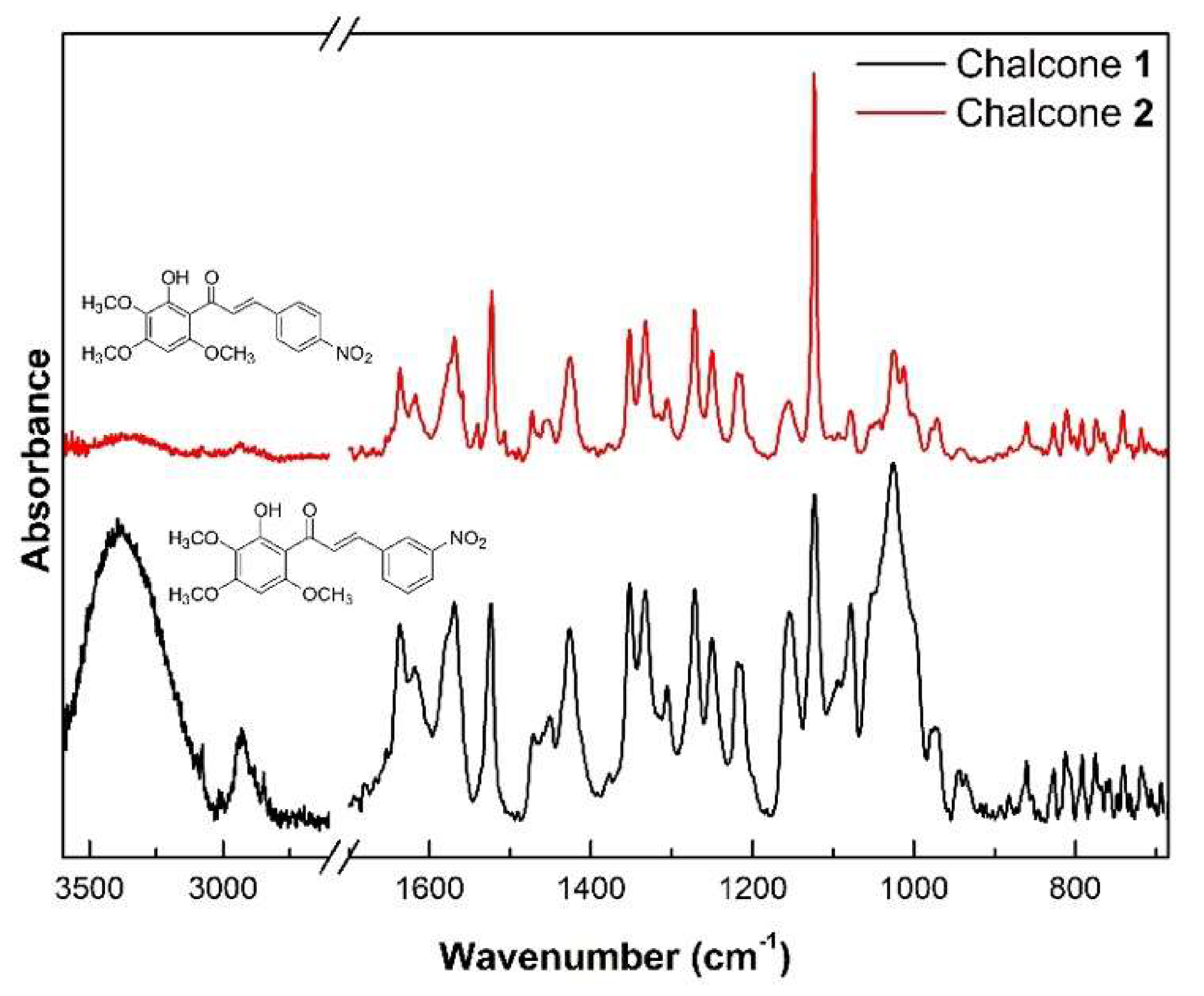
2.5. Vibrational Analysis
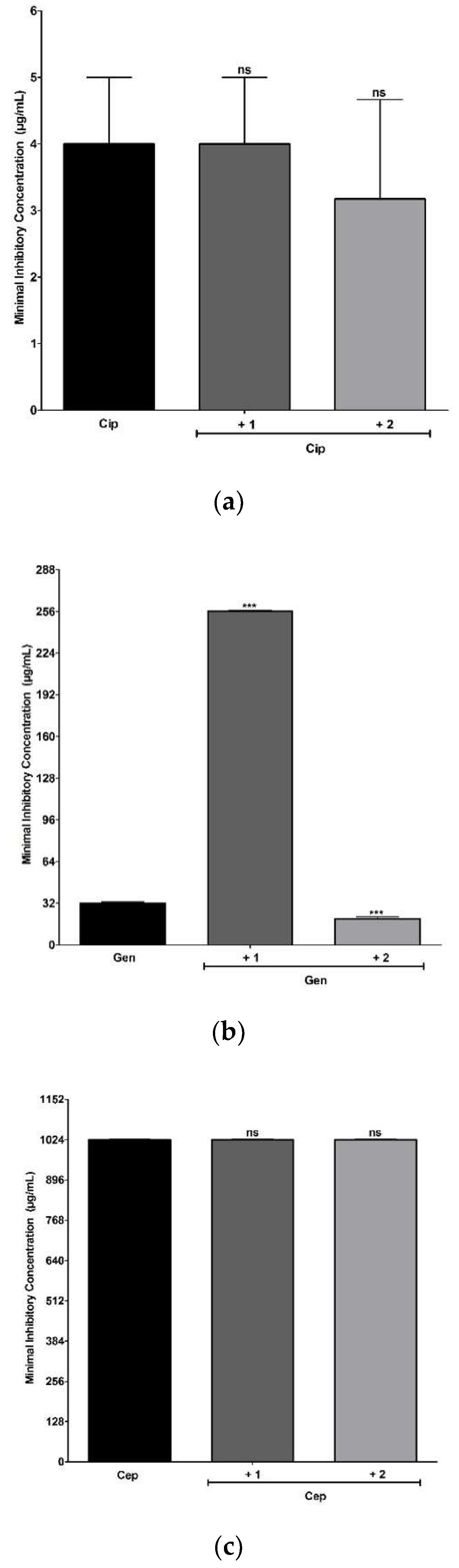
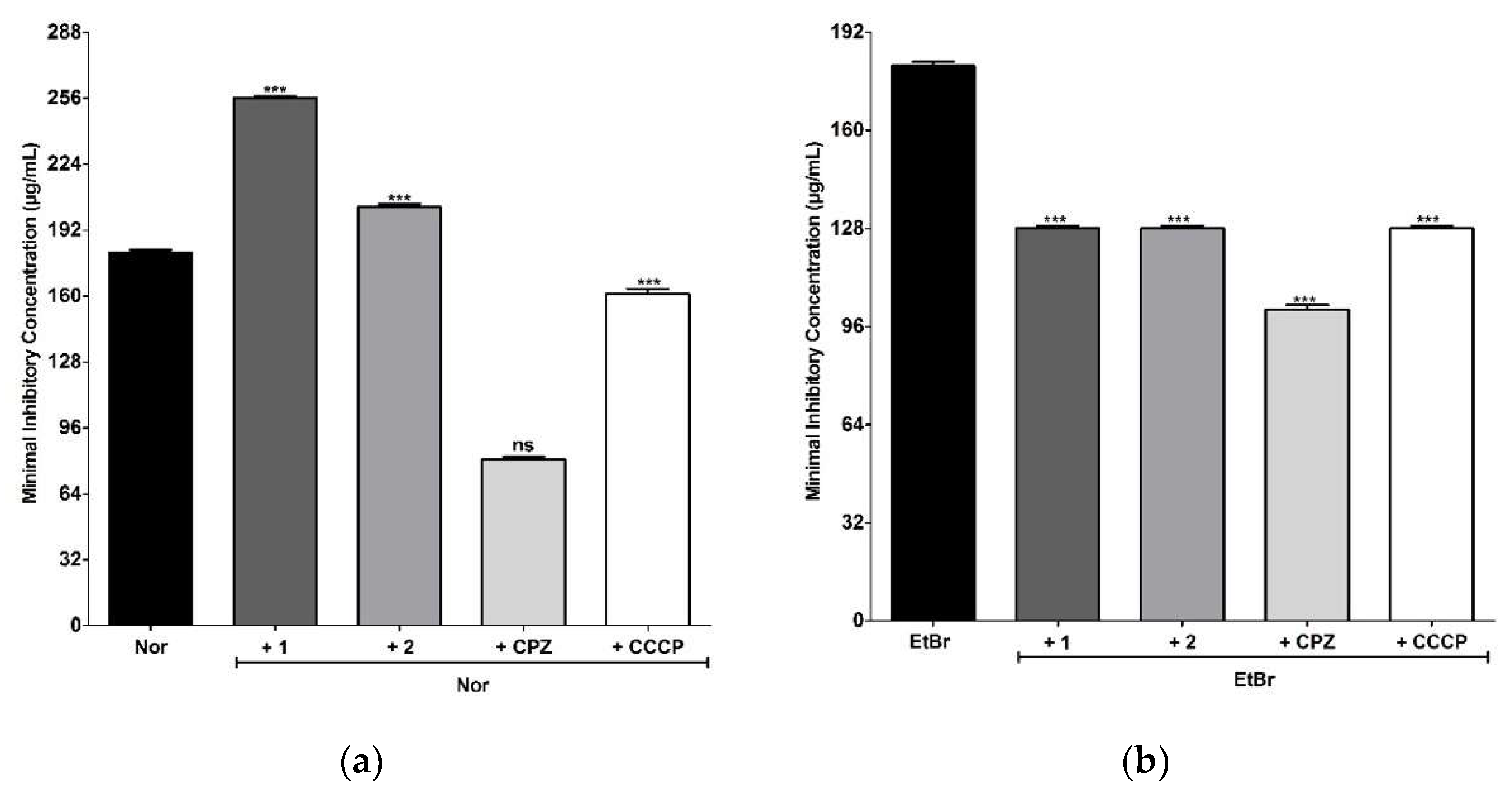
2.6. Antimicrobial and Modulating Activities of Antibiotics
3. Materials and Methods
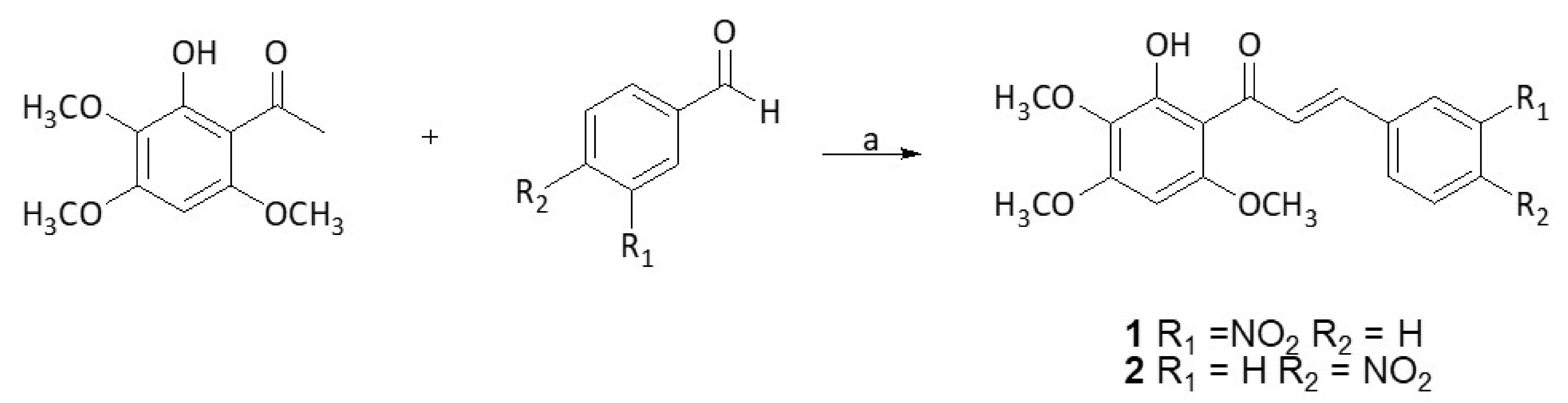
3.1. Synthesis of Chalcones
3.2. NMR
3.3. FT-Raman and ATR-FTIR
3.4. UV-Vis Spectroscopy
3.5. Cyclic Voltammetry Experiments
3.6. Computational Methods
3.7. Microbiological Characterization
3.7.1. Bacterial Material
3.7.2. Drugs and Substances
3.7.3. Intrinsic Antibacterial Activity
3.7.4. Modulation of Antibiotic
3.8. Ethidium Bromide MIC
3.9. Statistical Analysis of Microbiological Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ninomiya, M.; Koketsu, M. Minor Flavonoids (Chalcones, Flavanones, Dihydrochalcones, and Aurones). In Natural Products, 1st ed.; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 1, pp. 1867–1900. [Google Scholar]
- Ávila, H.P.; Smania, E.D.F.A.; Monache, F.D.; Smânia, A. Structure-activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Hazarkhani, H.; Kumar, P.; Kondiram, K.S.; Gadwal, I.M.S. Highly Selective Claisen–Schmidt Condensation Catalyzed by Silica Chloride Under Solvent-Free Reaction Conditions. Synth. Commun. 2010, 40, 2887–2896. [Google Scholar] [CrossRef]
- Orlíková, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2014, 15, 87–120. [Google Scholar] [CrossRef]
- Gaonkar, S.; Vignesh, U.N. Synthesis and pharmacological properties of chalcones: A review. Res. Chem. Intermed. 2017, 43, 6043–6077. [Google Scholar] [CrossRef]
- Verma, S.; Srivastava, A.K.; Pandey, O.P. A Review on Chalcones Synthesis and their Biological Activity. PharmaTutor 2018, 6, 22–39. [Google Scholar] [CrossRef]
- Rani, A.; Anand, A.; Kumar, K.; Kumar, V. Recent developments in biological aspects of chalcones: The odyssey continues. Expert Opin. Drug Discov. 2019, 14, 249–288. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, P.N.; Lemos, T.L.G.; Santos, H.S.; De Carvalho, M.C.S.; Pinheiro, D.P.; Filho, M.O.D.M.; Pessoa, C.; Barros-Nepomuceno, F.W.A.; Rodrigues, T.H.S.; Ribeiro, P.R.V.; et al. Synthesis, structural characterization, and cytotoxic evaluation of chalcone derivatives. Med. Chem. Res. 2019, 28, 2037–2049. [Google Scholar] [CrossRef]
- Zhang, B.; Duan, D.; Ge, C.; Yao, J.; Liu, Y.; Li, X.; Fang, J. Synthesis of Xanthohumol Analogues and Discovery of Potent Thioredoxin Reductase Inhibitor as Potential Anticancer Agent. J. Med. Chem. 2015, 58, 1795–1805. [Google Scholar] [CrossRef]
- Rampa, A.; Bartolini, M.; Pruccoli, L.; Naldi, M.; Iriepa, I.; Moraleda, I.; Belluti, F.; Gobbi, S.; Tarozzi, A.; Bisi, A. Exploiting the Chalcone Scaffold to Develop Multifunctional Agents for Alzheimer’s Disease. Molecules 2018, 23, 1902. [Google Scholar] [CrossRef]
- Hameed, A.; Masood, S.; Hameed, A.; Ahmed, E.; Sharif, A.; Abdullah, M.I. Anti-malarial, cytotoxicity and molecular docking studies of quinolinyl chalcones as potential anti-malarial agent. J. Comput. Mol. Des. 2019, 33, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.L.; Hossain, S.; Cole, A.M.; Phanstiel, O.; Iv, O.P. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg. Med. Chem. 2016, 24, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Prabhakar, P.K.; Doble, M. Synthesis, antioxidant evaluation, and quantitative structure-activity relationship studies of chalcones. Med. Chem. Res. 2010, 20, 482–492. [Google Scholar] [CrossRef]
- Božić, D.D.; Milenković, M.; Ivković, B.; Ćirković, I. Antibacterial activity of three newly-synthesized chalcones & synergism with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus. Indian J. Med. Res. 2014, 140, 130–137. [Google Scholar]
- Tran, T.-D.; Do, T.-H.; Tran, N.C.; Ngo, T.-D.; Huynh, T.-N.-P.; Tran, C.-D.; Thai, K.-M. Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg. Med. Chem. Lett. 2012, 22, 4555–4560. [Google Scholar] [CrossRef]
- De Mello, T.F.; Cardoso, B.M.; Bitencourt, H.R.; Donatti, L.; Aristides, S.M.; Lonardoni, M.V.C.; Silveira, T.G. Ultrastructural and morphological changes in Leishmania (Viannia) braziliensis treated with synthetic chalcones. Exp. Parasitol. 2016, 160, 23–30. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Dos Santos, H.S.; Bandeira, P.; Julião, M.; Freire, P.; Lima, V.; Cruz, B.; Da Silva, P.; Coutinho, H.D.M.; Sena, D. Structural, spectroscopic and microbiological characterization of the chalcone 2E-1-(2ʹ-hydroxy-3ʹ,4ʹ,6ʹ-trimethoxyphenyl)-3-(phenyl)-prop-2-en-1-one derived from the natural product 2-hydroxy-3,4,6-trimethoxyacetophenone. J. Mol. Struct. 2019, 1179, 739–748. [Google Scholar] [CrossRef]
- Santiago, R.; Freire, P.; Ayala, A.P.; Teixeira, A.M.; Dos Santos, H.S.; Bandeira, P.; Gonçalves, F.; Oliveira, M.; Cruz, B.; Sena, D. Crystal structure, vibrational spectra and quantum chemical parameters of 2-hydroxy-3,4,6-trimethoxyacetophenone isolated from the Croton anisodontus Müll. Arg. (Euphorbiaceae). J. Mol. Struct. 2018, 1171, 815–826. [Google Scholar] [CrossRef]
- Besharati-Seidani, T.; Keivanloo, A.; Kaboudin, B.; Yoshida, A.; Yokomatsu, T. Regioselective synthesis of 2,3-disubstituted 1-alkyl pyrrolo[2,3-b] quinoxalines through palladium-catalyzed Heck reaction of chalcones and evaluation of their anti-bacterial activities. Tetrahedron 2018, 74, 2350–2358. [Google Scholar] [CrossRef]
- Al-Amood, H.; Al-Hadithi, H.T.; Dadhil, F.G. Structure—Antimicrobial Activity Relationship Investigation of Some Butadiene and Chalcone Derivatives. J. Life Sci. 2013, 7, 705–711. [Google Scholar]
- Sivakumar, P.M.; Priya, S.; Doble, M. Synthesis, Biological Evaluation, Mechanism of Action and Quantitative Structure-Activity Relationship Studies of Chalcones as Antibacterial Agents. Chem. Biol. Drug Des. 2009, 73, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.L.; Castaño, J.; Jaramillo, M.C. Actividad inhibitoria de dihidroxifenilpropenona sobre β-lactamasa de Enterobacter cloacae: Estudio preliminar en el desarrollo de fármacos para enfrentar la resistencia bacteriana. Biomédica 2013, 34, 114. [Google Scholar] [CrossRef][Green Version]
- Chu, W.-C.; Bai, P.-Y.; Yang, Z.-Q.; Cui, D.-Y.; Hua, Y.-G.; Yang, Y.; Yang, Q.; Zhang, E.; Qin, S. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. Eur. J. Med. Chem. 2018, 143, 905–921. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R. Antibiotic effectiveness: Balancing conservation against innovation. Science 2014, 345, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Wachino, J.-I.; Jin, W.; Kimura, K.; Kurosaki, H.; Sato, A.; Arakawa, Y. Sulfamoyl Heteroarylcarboxylic Acids as Promising Metallo-β-Lactamase Inhibitors for Controlling Bacterial Carbapenem Resistance. mBio 2020, 11, 03144-19. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. The Role of Frontier Orbitals in Chemical Reactions (Nobel Lecture). Angew. Chem. Int. Ed. 1982, 21, 801–809. [Google Scholar] [CrossRef]
- Parr, R.G. Density Functional Theory of Atoms and Molecules; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 1980; pp. 5–15. [Google Scholar]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Paulai, F.R.; Serrano, S.H.P.; Tavares, L.C. Aspectos mecanísticos da bioatividade e toxicidade de nitrocompostos. Química Nova 2009, 32, 1013–1020. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2018, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Prabhu, S.R.; Jayarama, A.; Chandrasekharan, K.; Upadhyaya, V.; Ng, S.W. Synthesis, growth, structural characterization, Hirshfeld analysis and nonlinear optical studies of a methyl substituted chalcone. J. Mol. Struct. 2017, 1136, 244–252. [Google Scholar] [CrossRef]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- ACD/Structure Viewer—Freeware, version 2016.1.1; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2016.
- Andrienko, G.A. Chemcraft. Available online: www.chemcraftprog.com (accessed on 24 June 2020).
- Rauhut, G.; Pulay, P. Transferable Scaling Factors for Density Functional Derived Vibrational Force Fields. J. Phys. Chem. 1995, 99, 3093–3100. [Google Scholar] [CrossRef]
- Jamróz, M.; Dobrowolski, J. Potential energy distribution (PED) analysis of DFT calculated IR spectra of the most stable Li, Na, and Cu(I) diformate molecules. J. Mol. Struct. 2001, 565, 475–480. [Google Scholar] [CrossRef]
- Leal, A.L.A.B.; Bezerra, C.F.; Rocha, J.E.; Dos Santos, A.T.L.; Da Cruz, R.P.; Carneiro, J.N.P.; Sales, D.L.; De Freitas, T.S.; Tintino, S.R.; Almeida, W.D.O.; et al. Piper cernuum Vell.: Chemical profile and antimicrobial potential evaluation. Ind. Crop. Prod. 2019, 140, 111577. [Google Scholar] [CrossRef]
- Salazar, G.J.T.; De Sousa, J.P.; Lima, C.N.F.; Lemos, I.C.S.; Da Silva, A.R.P.; De Freitas, T.S.; Coutinho, H.D.M.; Da Silva, L.E.; Amaral, W.D.; Deschamps, C. Phytochemical characterization of the Baccharis dracunculifolia DC (Asteraceae) essential oil and antibacterial activity evaluation. Ind. Crop. Prod. 2018, 122, 591–595. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.; Campina, F.F.; Silva, R.L.; Costa, M.D.S.; Menezes, I.R.; Calixto-Júnior, J.T.; Siqueira-Júnior, J.P.; Coutinho, H.D.M.; Leal-Balbino, T.C.; et al. Evaluation of the tannic acid inhibitory effect against the NorA efflux pump of Staphylococcus aureus. Microb. Pathog. 2016, 97, 9–13. [Google Scholar] [CrossRef]



| 1 | 2 | |||
|---|---|---|---|---|
| C | δC | δH | δC | δH |
| 1′ | 106.9 | 106.9 | ||
| 2′ | 158.9 | 158.8 | ||
| 3′ | 131.1 | 131.2 | ||
| 4′ | 159.6 | 159.6 | ||
| 5′ | 87.2 | 6.02 (s) | 87.4 | 6.04 (s) |
| 6′ | 159.2 | 159.1 | ||
| MeO-3′ | 60.9 | 3.84 (s) | 60.9 | 3.90 (s) |
| MeO-4′ | 56.3 | 3.97 (s) | 56.4 | 4.02 (s) |
| MeO-6′ | 56.2 | 3.98 (s) | 56.3 | 4.10 (s) |
| C=O | 192.7 | 192.7 | ||
| 1 | 137.5 | 137.6 | ||
| 2 | 122.3 | 8.45 (s) | 122.3 | 8.09 (d, J = 8.09 Hz) |
| 3 | 148.9 | 124.4 | 7.69 (d, J = 8.1 Hz) | |
| 4 | 124.4 | 8.22 (d, J = 8.13 Hz) | 149.0 | |
| 5 | 130.1 | 7.59 (t) | 130.1 | 7.69 (d, J = 8.1 Hz) |
| 6 | 134.5 | 7.86 (d, J = 7.60 Hz) | 134.4 | 8.09 (d, J = 8.09 Hz) |
| Cα | 130.6 | 7.75 (d, J = 15.60 Hz) | 130.7 | 8.33 (d, J = 16.1 Hz) |
| Cβ | 139.6 | 7.94 (d, J = 15.60 Hz) | 139.3 | 8.36 (d, J = 15.4 Hz) |
| Chalcone | EHOMO | ELUMO | ∆E | I | A | µ | χ | η | ω |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −5.74 | −2.44 | 3.30 | 5.74 | 2.44 | −4.09 | 4.09 | 1.65 | 5.07 |
| 2 | −5.83 | −2.76 | 3.07 | 5.83 | 2.73 | −4.30 | 4.30 | 1.54 | 6.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.T.d.; Freitas, T.S.d.; Sena, D.M., Jr.; Bandeira, P.N.; Julião, M.S.d.S.; Marinho, E.S.; Alcanfor, A.A.C.; Marinho, E.M.; Lima-Neto, P.d.; Nogueira, C.E.S.; et al. Structural, Vibrational and Electrochemical Analysis and Antibacterial Potential of Isomeric Chalcones Derived from Natural Acetophenone. Appl. Sci. 2020, 10, 4713. https://doi.org/10.3390/app10144713
Silva PTd, Freitas TSd, Sena DM Jr., Bandeira PN, Julião MSdS, Marinho ES, Alcanfor AAC, Marinho EM, Lima-Neto Pd, Nogueira CES, et al. Structural, Vibrational and Electrochemical Analysis and Antibacterial Potential of Isomeric Chalcones Derived from Natural Acetophenone. Applied Sciences. 2020; 10(14):4713. https://doi.org/10.3390/app10144713
Chicago/Turabian StyleSilva, Priscila Teixeira da, Thiago Sampaio de Freitas, Diniz Maciel Sena, Jr., Paulo Nogueira Bandeira, Murilo Ségio da Silva Julião, Emmanuel Silva Marinho, Ana Aline Coêlho Alcanfor, Emanuelle Machado Marinho, Pedro de Lima-Neto, Carlos Emídio Sampaio Nogueira, and et al. 2020. "Structural, Vibrational and Electrochemical Analysis and Antibacterial Potential of Isomeric Chalcones Derived from Natural Acetophenone" Applied Sciences 10, no. 14: 4713. https://doi.org/10.3390/app10144713
APA StyleSilva, P. T. d., Freitas, T. S. d., Sena, D. M., Jr., Bandeira, P. N., Julião, M. S. d. S., Marinho, E. S., Alcanfor, A. A. C., Marinho, E. M., Lima-Neto, P. d., Nogueira, C. E. S., Coutinho, H. D. M., Leal, A. L. A. B., Barreto, H. M., Martins, N., Rodrigues Teixeira, A. M., & Santos, H. S. d. (2020). Structural, Vibrational and Electrochemical Analysis and Antibacterial Potential of Isomeric Chalcones Derived from Natural Acetophenone. Applied Sciences, 10(14), 4713. https://doi.org/10.3390/app10144713







