Abstract
The fluorite structured actinide dioxides (AnO2), especially UO2, are the most common nuclear fuel materials. A comprehensive understanding of their surface chemistry is critical because of its relevance to the safe handling, usage, and storage of nuclear fuels. Because of the ubiquitous nature of water (H2O), its interaction with AnO2 has attracted significant attention for its significance in studies of nuclear fuels corrosion and the long-term storage of nuclear wastes. The last few years have seen extensive experimental and theoretical studies on the H2O–AnO2 interaction. Herein, we present a brief review of recent advances in this area. We focus on the atomic structures of AnO2 surfaces, the surface energies, surface oxygen vacancies, their influence on the oxidation states of actinide atoms, and the adsorption and reactions of H2O on stoichiometric and reduced AnO2 surfaces. Finally, a summary and outlook of future studies on surface chemistry of AnO2 are given. We intend for this review to encourage broader interests and further studies on AnO2 surfaces.
1. Introduction
The fluorite structured actinide dioxides (AnO2, particularly UO2) are the most common nuclear fuels currently used in nuclear reactors worldwide. Understanding the surface chemistry of these materials is of both fundamental and technological importance owing to its relevance to many aspects in the nuclear industry from the corrosion of nuclear fuels [1,2], hydrogen gas production, and release from nuclear fuels [3], to the handling and long-term storage of spent fuels. As the interaction of nuclear fuels with environmental species (e.g., water and oxygen) starts at their surfaces, there is a pressing need to study the chemical reactions on AnO2 surfaces to evaluate the environmental impact of spent nuclear wastes [4]. However, the understanding of the surface chemistry of AnO2 has been severely limited by the difficulties associated with the handling of radioactive actinides in experiments and the complexity of studying actinide systems using theoretical means.
Specialized laboratory equipment is required to handle actinides in experiments, because all the actinide elements are radioactive and very toxic [5,6]. Early actinides such as thorium and uranium are relatively abundant in nature and their most stable isotopes have long radioactive decay times, making them appropriate for laboratory work, for example, the most stable isotopes 232Th and 238U have half-lives of 1.4 × 1010 and 4.5 × 109 years, respectively. The transuranics, on the other hand, are not found in nature and have been artificially made; therefore, they are available in small quantities and are highly radioactive. Therefore, most experimental studies of AnO2 surface chemistry have focused on UO2, leaving the rest of the AnO2 essentially unexplored. Theoretical calculations can supplement experimental efforts to understand the surface chemistry of the AnO2 series and some studies have tried to fill this gap. However, the treatment of actinides using electronic structure calculations remains challenging, in comparison with the lighter elements of the periodic table, owing to a combination of factors: relativistic effects, strongly correlated 5f electrons, and noncollinear magnetism. For example, standard exchange-correlation functional of density functional theory (DFT), such as Local Density Approximation (LDA) and Generalized Gradient Approximation (GGA), yields over-delocalized solutions for 5f electrons, failing to correctly describe their localization behavior [7,8]. Different approaches have been proposed to circumvent this problem. One of the most popular methods is DFT + U, which involves the introduction of an empirical Hubbard U correction term to the Hamiltonian [9,10]. This method has found high appeal because it does not increase the computational cost and can greatly improve the description of the 5f electrons; therefore, it has been widely used to calculate the bulk and surface properties of AnO2 [7,8,11,12,13,14,15,16]. However, searching of ground state electronic structure in DFT + U calculations presents additional difficulties [17,18,19,20].
Significant experimental and computational efforts have been made to understand the surface chemistry of AnO2 in the last few years. Herein, we present a brief review of recent progress in the interaction of H2O with AnO2 surfaces. We start from the atomic structures of stoichiometric AnO2 surfaces and their surface energies. Then, the formation of oxygen vacancies and their influence on the oxidation states of actinide atoms are discussed. Finally, we discuss the adsorption and reactions of water on both the stoichiometric and reduced AnO2 surfaces with oxygen vacancies. This work is not a complete review of all the efforts in the field, but rather we focus on the recent relevant progress in hope that this review can encourage broader interests and further studies on the surface chemistry of AnO2.
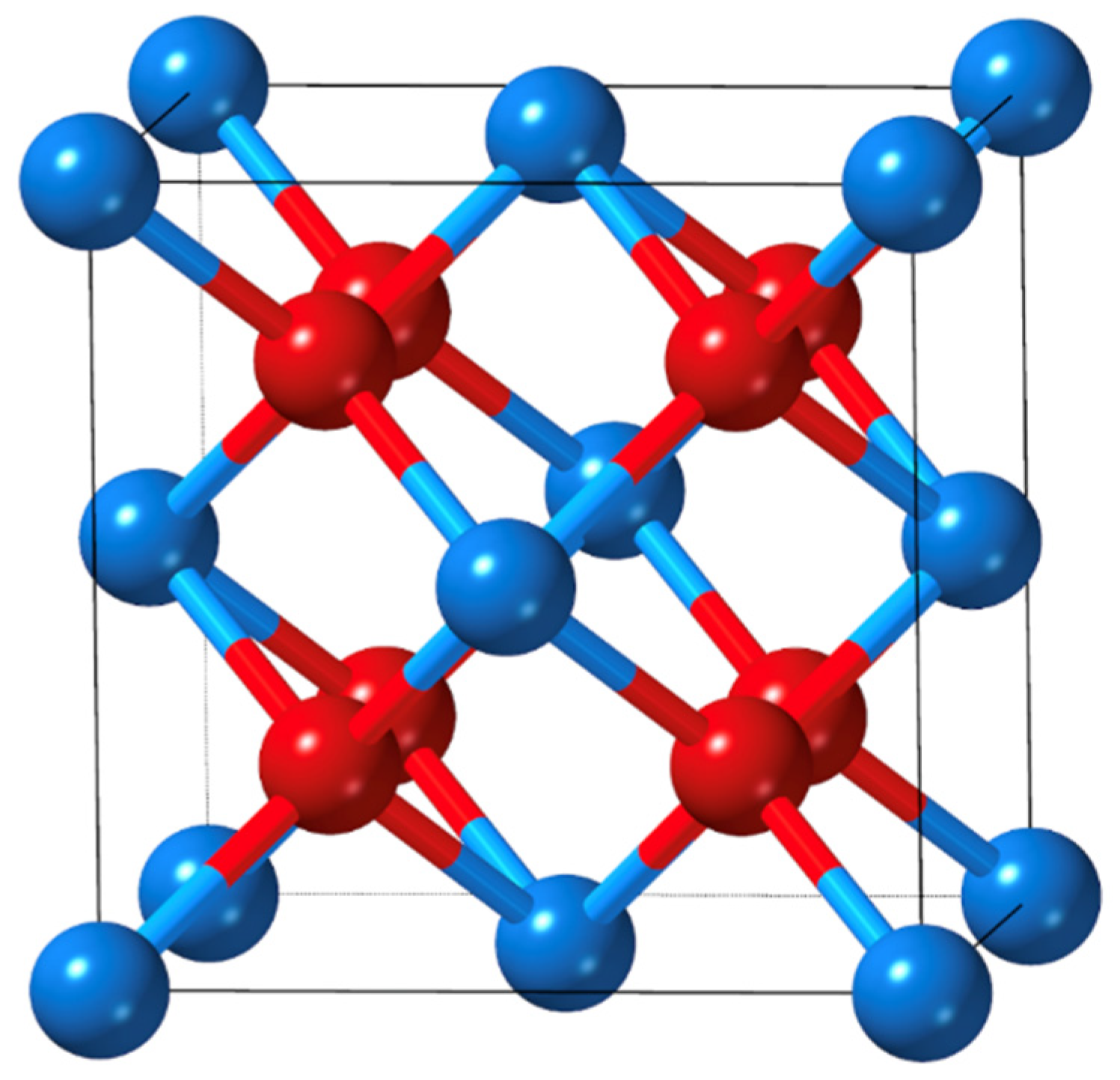
Bulk AnO2 from the elements Th through Cf has been observed to crystalize in a fluorite structure, as shown in Figure 1 [6]. In this structure, each actinide atom is eight-fold coordinated, and the oxygen atom is four-fold coordinated. Each actinide atom is formally in the +4 oxidation state, and the oxygen atoms are in the −2 state. The properties of actinide materials are closely dependent on the contraction and stabilization of 5f orbitals traversing the actinide series. For example, the lattice constant of bulk AnO2 shows a monotonic decrease from ThO2 to CfO2, however, it deviates from the trend at CmO2 as a result of orbital mixing and covalency between the metal 5f and the oxygen 2p orbitals. This increased orbital mixing can be tracked down to the proximity in energy between those two orbital manifolds and is now referred to as energy-driven covalency in the literature [21].
Figure 1.
Unit cell of fluorite AnO2. The blue and red atoms correspond to the actinide and oxygen atoms, respectively.
The surface properties (surface energy, reconstruction, reactivity, and so on) of AnO2 are highly dependent on the surface geometry. The top and side views of the low indexed (111), (110), and (100) surfaces of AnO2 are shown in Figure 2. Along the (111) direction, the surface consists of alternating oxygen and actinide layers with a tri-layer structure (O-An-O). Each tri-layer has a net dipole moment of zero in the direction perpendicular to the surface [22], and the most stable surface is terminated by an oxygen layer. Along the (110) direction, a series of neutral AnO2 planes are found; therefore, the surface is nonpolar. Along the (100) direction, AnO2 consists of alternating oxygen and actinide layers with the sequence of -O2-An-O2-An-O2-. Cleaving the crystal perpendicular to the (100) direction yields a polar surface with a net dipole moment, which is inherently unstable. A nonpolar stable surface is achieved by the removal of half of the oxygen atoms from the surface. This reconstruction leads to a slab-type unit cell of the form O-An-O2-(An-O2)n-An-O2-An-O, as shown in Figure 2. The surface that contains 50% oxygen vacancies on the topmost oxygen layer has been observed for UO2 (100) [23,24].
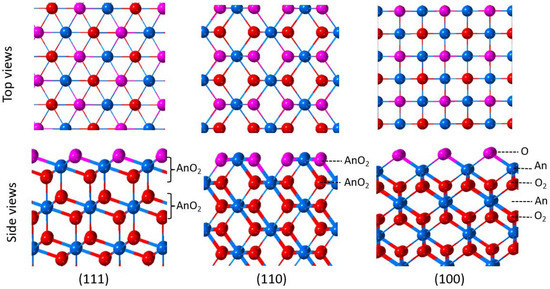
Figure 2.
Top and side views of the (111), (110), and (100) surfaces of AnO2.
Calculated surface energies of AnO2 have been reported in several papers, particularly for ThO2, UO2, and PuO2. Table 1, Table 2 and Table 3 summarize the surface energies for the low indexed (111), (110), and (100) surfaces, comparing the different reports that employed DFT and those that relied on other classical models [25,26,27,28,29,30,31,32,33]. It is seen that DFT calculations give rise to smaller surface energies than the classical models for ThO2, UO2, and PuO2 surfaces. A variation in the surface energies is also observed for DFT calculations owing to the use of different level of theory and parameters in the calculations. Despite the variation in the calculated surface energies, all these calculations predict the (111) surface to have the lowest surface energy, hence being the most stable surface for AnO2. Therefore, nanocrystals of AnO2 are expected to be mostly terminated by the (111) surface and grow in octahedral shape, according to the Wulff constructions [34]. The relative stability of surfaces can be modified through the selective adsorption of organic ligands on different surfaces, which is key for the controlled synthesis of nanocrystals with different morphologies [35,36].

Table 1.
ThO2 surface energies in units of J/m2. DFT, density functional theory.

Table 2.
UO2 surface energies in units of J/m2.

Table 3.
PuO2 surface energies in units of J/m2.
2. Oxygen Vacancies on AnO2 Surfaces
The effect of oxygen vacancies is crucial for understanding the reactivity of AnO2 surfaces as radiation damage can lead to the formation of vacancies on these surfaces. Indeed, studies of the reactivity of H2O on UO2 surfaces indicate weak reactivity on stoichiometric UO2 (111) surface, while dissociative adsorption and hydrogen (H2) formation upon reduction of the surfaces with oxygen vacancies [45,46]. Similarly, oxygen vacancies have been demonstrated to have a strong impact on the surface chemistry of transition metal and lanthanide oxides, as evidenced by the catalytic properties of CeO2, a close sibling of AnO2 sharing the crystal structure and oxidation states [38]. The formation of an oxygen vacancy in CeO2 leaves two excess electrons in the host material, which localize at two 4f orbitals of Ce sites, reducing them to Ce3+ [47]. The formation of Ce3+ has been demonstrated to alter the reactivity of CeO2 (111) surface, such as the activation of the acetaldehyde for coupling reactions [48]. The integrated experimental and theoretical efforts have made great progress in understanding its surface reactivity and reaction mechanism. In this section, we will illustrate the progress for the AnO2 systems.
2.1. Experimental Work
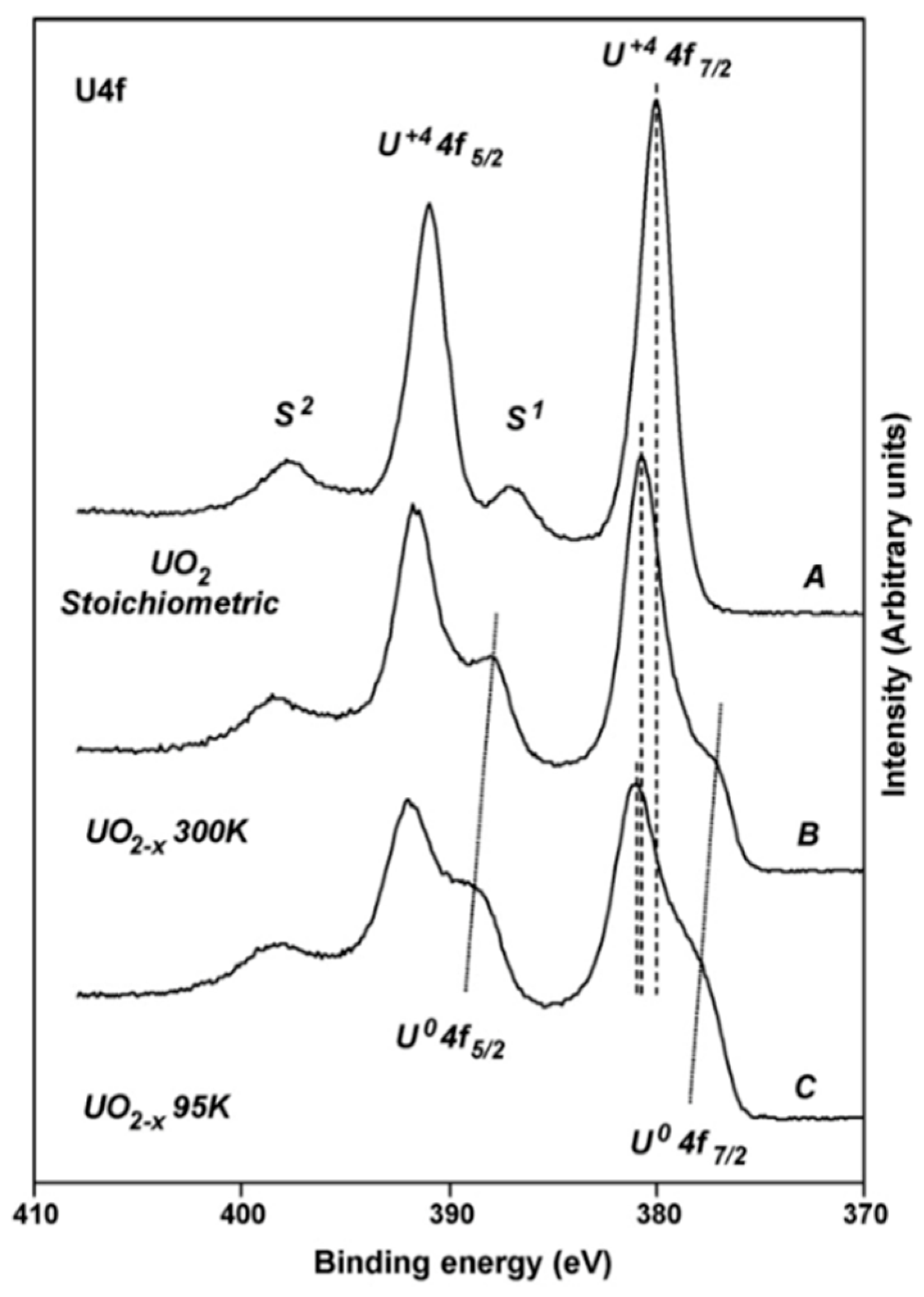
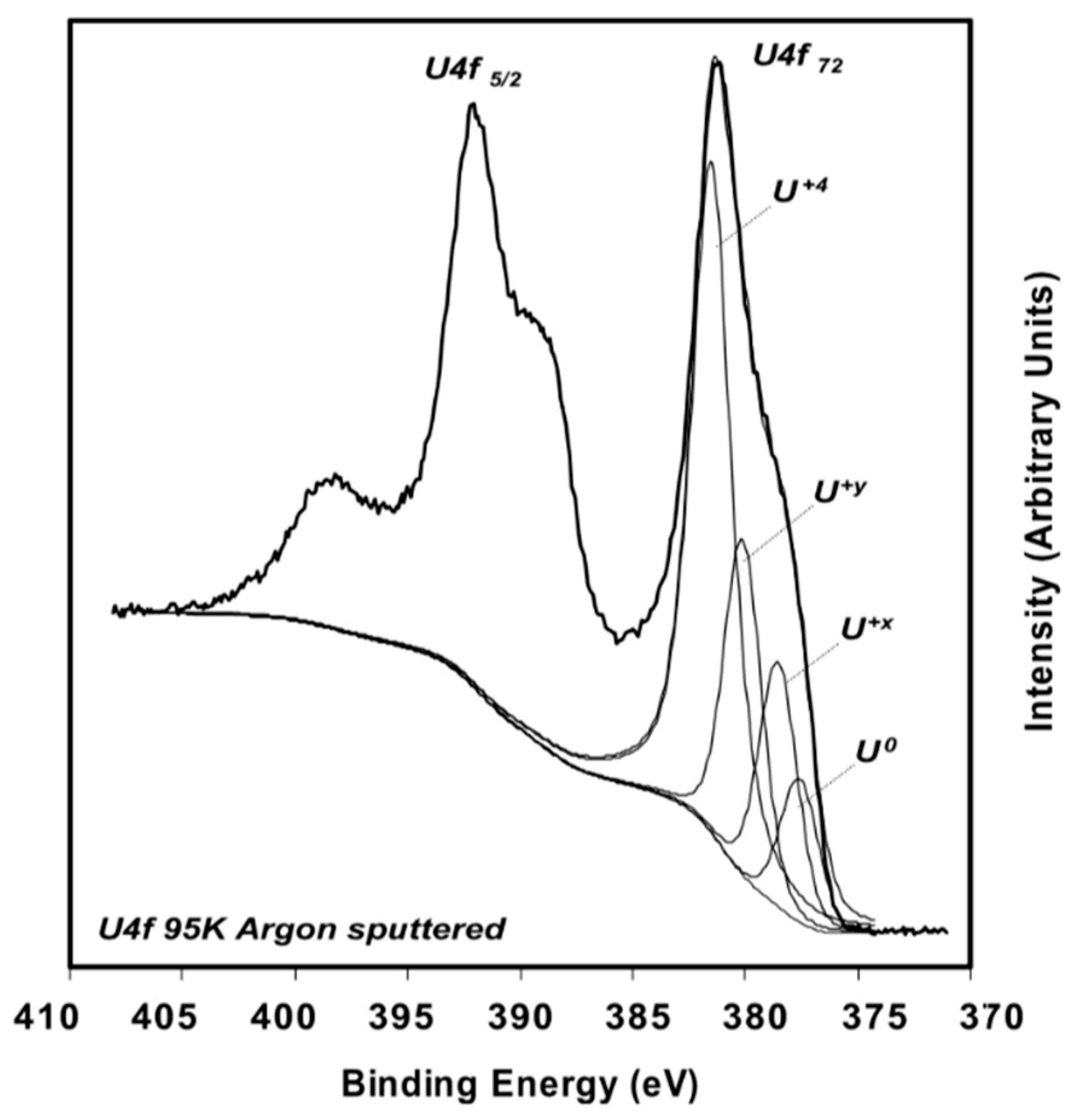
A thorough experimental study of oxygen vacancies on AnO2 surfaces has only been performed on UO2 (111) surface. Artificial oxygen vacancies on UO2 (111) surfaces were created using Ar+ sputtering, and the oxidation states of surface actinide atoms were studied using X-ray photoelectron spectroscopy (XPS) [45]. A comprehensive review of XPS spectra of uranium oxide powder, thin films, and single crystals can be found in the work of [49]. Figure 3 shows the XPS spectra of stoichiometric UO2 (111) surface (A), the surface after Ar+ sputtering at 300 K (B), and after Ar+ sputtering at 90 K (C) [45]. The stoichiometric surface has the U4f 7/2 and U4f 5/2 peaks at 380.0 and 390.8 eV, and two characteristic satellite peaks S1 and S2 at 386.7 and 397.3 eV [45]. Similar peak positions have been reported in various studies for the U4+, suggesting that uranium atoms on the stoichiometric surface remain in U4+ as in the bulk UO2 [50,51]. After sputtering with Ar+ at 300 K, a shoulder on the lower binding energy side of the 4f peaks appears. The broad shoulder has been attributed to uranium atoms in different oxidation states from +4 to 0 (uranium metal) and the slight shift of the U4f 7/2 and U4f 5/2 is owing to the formation of an n-type semiconductor upon the creation of oxygen vacancies [45]. The formation of different oxidation states of uranium atoms on the surfaces with oxygen vacancies is evidenced in the fit of XPS spectra, as shown in Figure 4 [49]. An improved fit of the shoulder of U4f 7/2 peak can be obtained via the inclusion of peaks corresponding to the different oxidation states between +4 and 0, indicating the presence of uranium atoms in the reduced states [49]. However, deriving the structure of an oxygen vacancy at the atomic layer of UO2 surface and its electronic properties from these XPS data is still not possible without the complementation of DFT simulations. This is because most of the surface analysis techniques such as XPS and Auger electron spectroscopy (AES) have a penetration depth of a few nanometers [52], thus the signal from XPS and AES typically represents an average over a few nanometers of the materials.
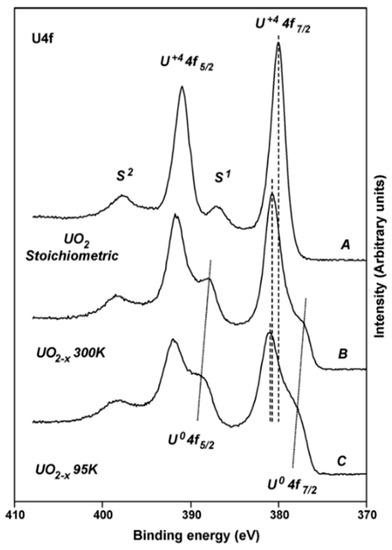
Figure 3.
X-ray photoelectron spectroscopy (XPS) spectra of stoichiometric UO2 (111) surface and the surface after sputtering with Ar+ [45].
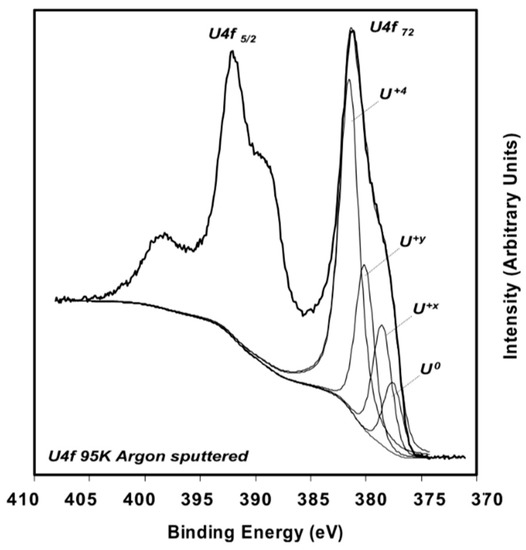
Figure 4.
XPS spectra of reduced UO2 (111) surface [49]. The broad shoulder of U4f 7/2 peak can be better fitted by considering different oxidation states of uranium from +4 to 0.
2.2. Theoretical Calculations
Several DFT simulations have been reported on the study of oxygen vacancies on the top-surface and the subsurface of AnO2, providing insights in terms of the electronic structure of the reduced surfaces, the oxidation states of actinide atoms, the formation energies of vacancies, and the electronic structure of excess electrons owing to oxygen vacancies.
Table 4 shows the calculated formation energies of oxygen vacancies on (111) surface of ThO2, UO2 and PuO2. The formation energy is defined as , where and are the total energy of the slab with and without oxygen vacancy, respectively. is the total energy of one isolated O2 molecule in its ground state. From these calculations, it is seen that the oxygen vacancy formation energies decrease from ThO2 to PuO2 [44,53,54,55]. Recent studies found that the oxygen vacancy formation energies are correlated with the reduction potential of An4+, in accordance with the f-band energy drops as going down the actinide series [53,56]. Interestingly, the fundamental question regarding the relative stability of oxygen vacancy in the top-surface and subsurface is still debatable, although similar DFT + U calculations were used [53,54]. The conclusions for PuO2 (111) surface are consistent that the oxygen vacancy formation energy is slightly lower in the top-surface than in the subsurface in previous studies [44,53], and the energy difference is within 0.05 eV. However, it has been demonstrated that oxygen vacancy in the subsurface is more stable than in the top-surface on CeO2 (111) surface, which has an identical structure to AnO2 [47]. Besides the debate of the relative stability of oxygen vacancy in the top-surface and subsurface of AnO2, inconsistent results about the distribution of the excess electrons were also reported. On the UO2 (110) surface, some theoretical calculations based on Perdew-Burke-Ernzerhof (PBE) functional and an effective Hubbard U value of 4.0 eV found that the excess electrons are localized on two uranium atoms, resulting in two U3+ next to the vacancy site [54]. Other theoretical studies reported the two electrons to be delocalized over three uranium atoms by natural population analysis for the embedded cluster calculations [53].

Table 4.
Formation energies of top-surface and subsurface oxygen vacancy in AnO2 (111) surfaces.
The inclusion of a Hubbard-like on-site Coulomb interaction in DFT + U yields a more accurate treatment of the correlated electrons, which overcomes deficiencies of the pure LDA/GGA functionals and has achieved a wide range of successes in treating AnO2 [7,8]. However, the inclusion of Hubbard term introduces multiple energy local minima, which complicates the search for the ground state [17,18,19,20]. Several methods, such as U-ramping [19] and occupation matrix control (OMC) [17,18], have been proposed to overcome this difficulty presented by DFT + U. These methods have been shown to locate low-energy solutions for a variety of systems containing strongly correlated d and f electrons [17,18,19]. Recently, these approaches have been applied to the study of reduced AnO2 (111) surfaces [55]. It was found for ThO2, UO2, and PuO2 (111) surfaces that the oxygen formation energy is 0.23–0.38 eV lower for the subsurface vacancy. This is consistent with the calculations for CeO2 (111) surface, where explicit searching of the low-energy solutions has been carried out by assigning excess electrons to different sites near the vacancy [47,57]. The unexpected lower formation energy for oxygen vacancy energy in the subsurface has been explained in terms of smaller Madelung potential owing to defect-induced lattice relaxation [47]. The excess electrons on the ThO2 surface stay at the vacancy site, forming a lone electron pair, as shown in Figure 5a,b. On the UO2 surface, one of the excess electrons stays at the vacancy site, and the other moves to uranium 5f orbitals, reducing it to U3+ (Figure 5c,d). On the PuO2 surface, both excess electrons localize at the Pu 5f orbitals reducing two next-nearest Pu sites (Figure 5e,f). More advanced experimental surface-sensitive characterization will be needed in order to settle the debate and validate these predicted theoretical results.
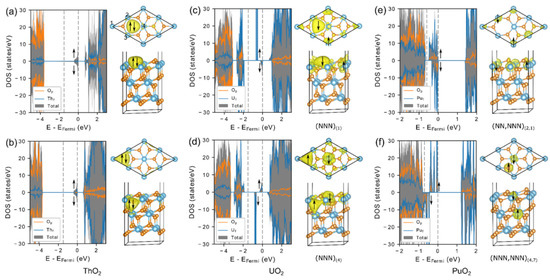
Figure 5.
Density of states (DOS) and partial charge density plots of reduced ThO2, UO2, and PuO2 (111) surfaces with oxygen vacancy in the top-surface (a,c,e) and in the subsurface (b,d,f) [55].
3. Water on AnO2 Surfaces
The interaction of water with AnO2 has attracted numerous works owing to its importance for the storage of nuclear fuels [1,2,4]. In particular, the adsorption, dissociation, and reaction pathways of water on UO2 and PuO2 surfaces have been of great interest. For UO2, the interaction of water with both perfect and reduced single crystalline (111) films and polycrystalline films has been carried out [58,59]. On PuO2 surfaces, several experimental studies have been performed on polycrystalline films [1,60,61,62,63].
3.1. Experiments
The interaction of H2O with AnO2 surfaces initiates with its adsorption and dissociation on the surfaces. It has been shown that the adsorption of H2O molecules on UO2 (111) surface is reversible (80%) at 300 K, indicating that H2O is weakly adsorbed on perfect UO2 (111) surface [58]. On the contrary, H2O is strongly adsorbed on surfaces with oxygen vacancies. Figure 6 shows the XPS spectra of reduced surface after exposure to heavy water (D2O), then followed by annealing at different temperatures [45]. It is clearly seen that the shoulders for the U4f 7/2 peaks disappear after exposure to D2O and subsequent annealing. This implies the recovery of uranium atoms to the formal oxidation state of +4. More interestingly, temperature programmed desorption (TPD) experiments for H2 release upon exposing water to an Ar+ sputtered UO2 surface show that H2 production can only occur on reduced (111) surface with oxygen vacancies (Figure 7). The amount of H2 desorption increases with the Ar+ sputtering time, namely the concentration of oxygen vacancies on the surfaces [58]. These experiments demonstrate that the interaction of H2O with AnO2 surfaces is dependent on the surface oxygen vacancies, and oxygen vacancies prompt the dissociation of H2O and the production of H2 on AnO2 surfaces. The interaction of H2O with PuO2 films has been studied using XPS and ultra-violet photoelectron spectroscopies (UPS) [60,61]. It was found that H2O dissociates and forms a thin hydroxyl (OH-) layer with small amounts of molecularly adsorbed water at 298 K. While under 80–120 K, H2O is adsorbed as thick ice multilayers and no significant OH- is detected. The top surface layer can be reduced to Pu2O3 when the ice covered PuO2 is warmed up under ultraviolet light, while the mechanism of the reduction is still unclear [61]. Another study has reported that H2O can catalyze the oxidation of PuO2 surfaces and the formation of stable compounds PuO2+x (x <= 0.27) containing Pu6+ [1].
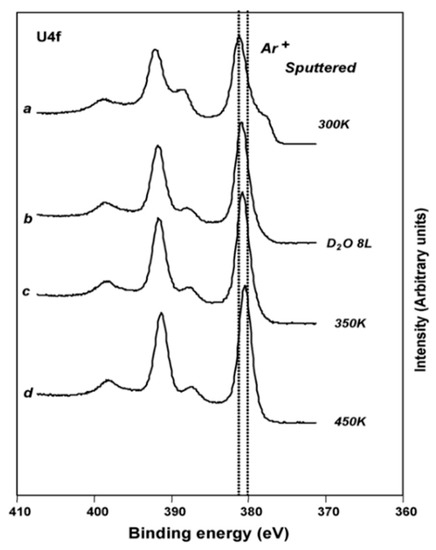
Figure 6.
XPS spectra of reduced UO2 (111) surface after exposure to D2O [45].
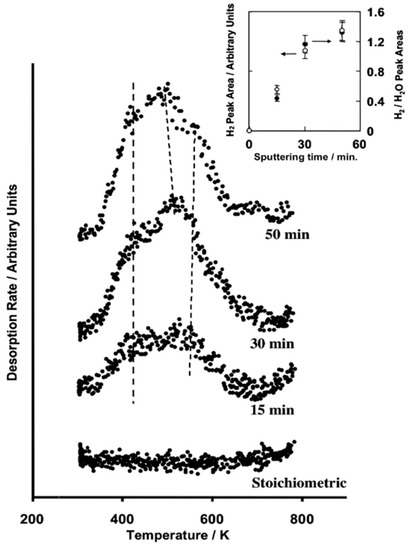
Figure 7.
Temperature programed desorption of H2 after H2O adsorption at 300 K over Ar+ sputtered UO2 (111) single crystal [58]. The inset shows the computed H2 peak area as well as the H2/H2O peak area ratios.
3.2. Theoretical Calculations
Contrary to the lack of experimental data, several theoretical groups have pioneered modeling of H2O on stoichiometric and reduced AnO2 surfaces [22,44,53,54,64]. These simulations shed light on the nature of the fundamental interaction between H2O and AnO2 surfaces. We will discuss these advances in two main aspects: (i) adsorption and dissociation of water and (ii) mechanism of H2 production.
3.2.1. Adsorption and dissociation of H2O on AnO2 surfaces
The adsorption and dissociation of H2O on AnO2 surfaces have been extensively studied in several papers [44,53,54,64,65]. H2O molecules can adsorb on surfaces in the molecular form or the dissociated form. For the molecular adsorption (Figure 8a,b), the oxygen atom from the H2O molecule binds via its lone pair with the positive actinide atom on the surface, while, at the same time, one of the hydrogen atoms forms a hydrogen bond with a nearby oxygen atom on the surface. If the H2O dissociates at the surface into OH- and H+, the hydroxyl group bonds with one surface actinide atom, and the proton bonds with one surface oxygen atom (Figure 8c,d). On the basis of these studies [44,53,54,64], the following general conclusions have been reached on the adsorption and dissociation of H2O on AnO2 surfaces:
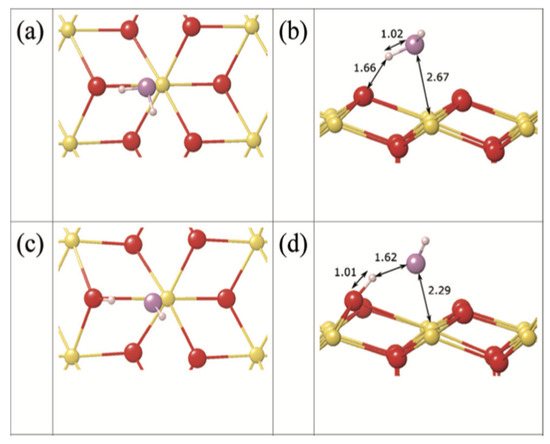
Figure 8.
H2O on ThO2 (111) surface [38]. Top and side views of molecular adsorption (a,b) and dissociative adsorption (c,d).
- (i)
- A mixture of molecular and dissociated adsorption of H2O occurs on the (111) surface of AnO2.
- (ii)
- Dissociative adsorption of H2O preferentially occurs on the (110) and (100) surfaces of AnO2.
- (iii)
- The adsorption energies of H2O are correlated with the surface energies; a stronger adsorption energy is expected on the surface with a higher surface energy.
- (iv)
- The presence of oxygen vacancies on the surface of AnO2 favors the dissociative adsorption mechanism. The distribution of excess electrons on reduced AnO2 surfaces is different upon the adsorption of H2O.
Alexandrov et al. first studied the adsorption configurations of H2O on ThO2 surfaces using both accurate calorimetric measurements and first-principle calculations [38]. They observed coverage-dependent adsorption energies and demonstrated a mixture of molecular and dissociative adsorption of H2O on ThO2 (111) surface. They also showed that the adsorption energy of water molecules is correlated with the surface energies; a higher surface energy results in a stronger water adsorption on ThO2 surfaces [38]. Bo et al. [22] found that, for a single water molecule on UO2 (111) surface, the two adsorption configurations, molecular adsorption and dissociative adsorption, exhibit comparable adsorption energies (0.61 vs. 0.68 eV, as shown in Table 5). However, the adsorption in the molecular form, on (110) and (100) surfaces, is far less stable than the dissociative adsorption (0.62 vs. 1.27 eV and 1.02 vs 1.71 eV, respectively) [22]. Bo’s results are consistent with those of Wellington et al. [66] and Tegner et al. [67], who also found that the molecular and dissociative adsorptions have similar adsorption energies on UO2 and PuO2 (111) surfaces, while there is a clear preference for dissociative adsorption on (110) surfaces. Both groups reported the mixture of molecular and dissociative adsorption to be the most stable adsorption configuration at a higher coverage of H2O on (111) surfaces [22,66,67]. The calculated adsorption energies of H2O on UO2 surfaces increase from (111), (110), to (100) surface, as summarized in Table 5, which is consistent with the observation by Alexandrov et al. that stronger H2O adsorption energies are expected on ThO2 surfaces with higher surface energies [38]. Tegner et al. further studied the adsorption of up to five layers of H2O on PuO2 and UO2 surfaces, and found significant variation in the adsorption energies as a result of intra- and interlayer hydrogen bonding network [68].

Table 5.
Adsorption energies of one H2O molecule on perfect UO2 surfaces. The bold italic numbers correspond to adsorption energies in the dissociative form. The rest are adsorption energies in the molecular form.
Ab-initio molecular dynamics (AIMD) simulations have also been carried out to explore the mechanism of adsorption and dissociation of H2O on AnO2 surfaces. It was found that the dissociative adsorption of H2O is feasible for a large range of temperature and water partial pressures on UO2 (111) surface [69]. The dissociation of H2O on PuO2 (110) surface is a two-step hydroxylation process both for single H2O molecule and H2O clusters [70]. The dissociation is initiated by the dehydrogenation of water molecules to form a hydroxyl group with surface oxygen atom, followed by successive surface hydroxylation on plutonium with the remaining hydroxide ion of the dissociating molecule. The H2O molecule dissociates as the consequence of hybridizations between the molecular orbitals of water and the electronic state of the surface with a reaction energy barrier of 0.18 eV. In contrast, the dissociation of H2O clusters is exothermic by −0.42 eV with no energy barrier [70].
On reduced AnO2 surfaces with oxygen vacancies, H2O has much stronger adsorption energies, as shown in Table 6. On perfect ThO2, UO2, and PuO2 (111) surfaces, the adsorption energy of water is smaller than 1.1 eV from DFT calculations [38,54,55,64,67], while the adsorption energies are larger than 1.8 eV on surfaces with oxygen vacancies [53,54,55,64]. These DFT calculations are in agreement with experimental results by Senanayake et al. that the adsorption of H2O on UO2 (111) surface is weak and reversible (80%) at 300 K [58]. DFT simulations also found that the dissociation of H2O on reduced AnO2 surfaces can occur spontaneously at room temperature, as there is a very small energy barrier for the dissociation [54,55]. After H2O dissociation, the hydroxylate group moves to the oxygen vacancy site, and the proton binds with one surface oxygen atom as shown in Figure 9. The excess electrons are distributed differently on reduced AnO2 surfaces upon the adsorption of H2O. On reduced ThO2 (111) surface, the excess electrons still stay at the vacancy site after H2O dissociation. One proton from the H2O molecule is attracted to the vacancy site and forming H-. However, on both reduced surfaces, UO2 and PuO2 (111), the OH- group from H2O tends to fill the vacancy site. When OH- moves to the vacancy site for UO2 surface, it will push the excess electron to a neighboring uranium site and reduce it to U3+.

Table 6.
Adsorption energies of one H2O molecule on AnO2 (111) surface. The bold italic numbers correspond to the dissociative adsorption energies. The rest are the molecular adsorption energies.
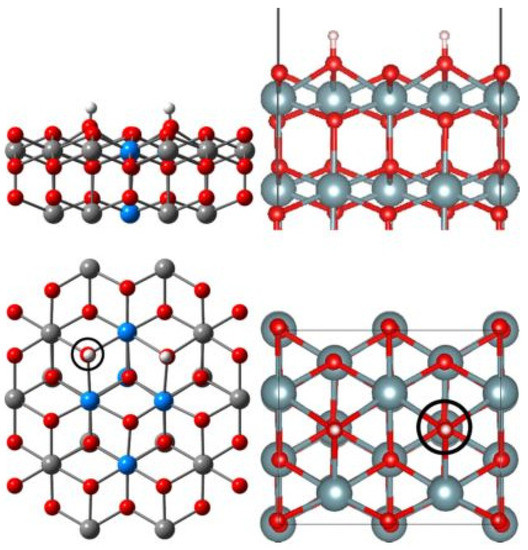
Figure 9.
Dissociative adsorption of H2O molecule on UO2 (111) surface with oxygen vacancy [53]. The left is the side and top views using the embedded cluster model, the right is using the periodic model. The position of the oxygen vacancy is indicated with a black circle.
3.2.2. H2 production from H2O splitting
The formation of H2 owing to the interaction of H2O with AnO2 through radiolytic process or chemical reactions can lead to potential pressurization of the containers of nuclear fuels. The formation of H2 on AnO2 surfaces owing to chemical reactions with H2O has rarely been studied in theoretical calculations [54,55]. Bo et al. first systematically investigated the formation and desorption mechanism of H2 on reduced UO2 (111) surface [54]. They found that H2 formation can occur on UO2 (111) surface with the oxygen vacancies [54]. Figure 10 shows the three possible reaction pathways [54]. The first formation pathway in Figure 10a involves the direct dissociation of H2O at a vacancy site, forming O-H-H structure on the surface, followed by the desorption of H2 from the surface and oxygen atom healing the vacancy. This pathway has an energy barrier of 0.56 eV, and the overall reaction is exothermic by 2.62 eV [54]. The second pathway in Figure 10b involves the dissociation of H2O on the surface, forming hydroxylated surface, and then the combination of the hydrogen atoms from the hydroxyl radicals to form H2 molecules. This pathway presents a large energy barrier of 1.39 eV and an overall exothermic energy of 2.56 eV. For the third pathway in Figure 10c, there are excess hydroxyl radicals near the vacancy site. The dissociation of H2O will result in a hydroxyl radical that occupies the vacancy site, and the formation of O-H-H structure. This reaction pathway is also exothermic by 1.98 eV and has an energy barrier of 0.53 eV [54].
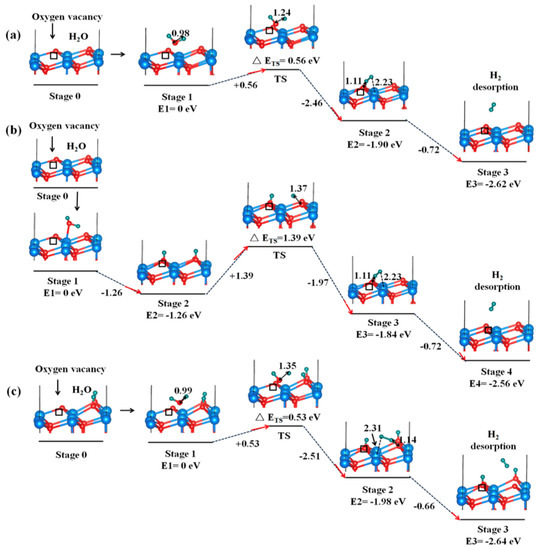
Figure 10.
H2O dissociation and H2 production on reduced UO2 (111) surface through three different reaction pathways (a–c) [54].
A comparative study of H2 formation on reduced ThO2, UO2, and PuO2 (111) surfaces has been performed to show the difference in the H2 formation across the AnO2 series [55]. It is found that the formation of H2 from catalytic splitting of H2O is endothermic for all three AnO2 (111) surfaces evaluated (Table 7). In contrast, H2O can readily dissociate on the reduced ThO2, UO2, and PuO2 (111) surfaces, while the thermodynamics of H2 production is significantly different [55]. On reduced ThO2 (111) and UO2 (111) surfaces, the H2O adsorption and H2 production is exothermic and releases 3.55 eV and 3.11 eV, respectively (Table 7). Although this process releases 0.31 eV on reduced PuO2 (111) surface, there is a larger energy barrier for the H2 production. This suggests that the protons of H2O will stay in the hydroxylated form on PuO2 (111) surface, instead of forming H2 molecule [55]. The high reactivity of H2O with reduced ThO2 surface is attributed to the lone electron pair at the vacancy site on ThO2 that can readily participate in the chemical reactions with H2O. On the contrary, the excess electrons are more tightly bound to the Pu 5f orbitals on reduced PuO2 surfaces, thus making it harder to participate in the reaction with H2O molecule.

Table 7.
The reaction energy of H2O splitting and H2 formation (H2O → O* + H2) on perfect ThO2, UO2, and PuO2 (111) surfaces and corresponding surfaces with oxygen vacancies. A negative/positive value represents the reaction is exothermic/endothermic, respectively.
4. Summary and Outlook
Understanding the surface chemistry of AnO2 is relevant to many stages in the nuclear industry. Significant efforts have been performed in the community to explore the interaction of H2O with AnO2 surfaces. These studies provide significant insights on the adsorption and dissociation of H2O, the vacancy formation, and the H2 production on the AnO2 surfaces owing to chemical reactions with H2O. There is still important work to be done in this area. (i) AnO2 surfaces beyond UO2: Previous experimental studies mostly focus on UO2 surfaces. The rest of the AnO2 surfaces have been hardly explored in experiments. Especially, controlled synthesis of high quality AnO2 films using advanced techniques, such as polymer assisted deposition (PAD), atomic vapor deposition, and crystal truncation rod (CTR), among others, is needed to study the surface chemistry of AnO2 surfaces beyond UO2. (ii) AnO2 surfaces with complicated surface structures: Compared with experiments that mostly focus on UO2 films, more theoretical simulations have been conducted on AnO2 surfaces beyond UO2. However, most of the simulations are on low indexed (111), (110), and (100) surfaces of AnO2, because the simulation cells are smaller and more accessible to computational methods than the high indexed ones. In fact, other surfaces with higher surface energy are expected to be more reactive, which might be more important for understanding the dissolution, reactivity, and environmental effects of nuclear fuels. Moreover, defected surfaces with only one oxygen vacancy have been investigated in earlier work, while defected surfaces with more complicated structures are yet to be explored both in experiments and in theory. (iii) Chemical reactions on the surfaces and reaction dynamics. Previous theoretical simulations are limited to small systems with a few water molecules on the surfaces and the very early stage of H2O interaction with the surfaces owing to the computational cost of DFT simulations. To this regard, developing methods that can deal with large systems and perform dynamics on a long time scale, such as density functional tight binding (DFTB) [71], is in urgent need to study the AnO2 surfaces, chemical reactions and dynamics on the surfaces, and their interactions with the environments.
Author Contributions
Writing—original draft preparation, G.W.; writing—review and editing, E.R.B. and P.Y.; supervision, E.R.B. and P.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Laboratory Directed Research and Development program of Los Alamos National Laboratory (LANL), grant number 20160604ECR, 20180007DR, and US DOE office of Basic Energy Science under the Heavy Element Chemistry program.
Acknowledgments
G.X.W. acknowledges the Director’s Postdoc Fellow from LANL. LANL, an affirmative action/equal opportunity employer, is managed by Triad National Security, LLC, for the National Nuclear Security Administration of the U.S. Department of Energy under Contract 89233218CNA000001.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haschke, J.M.; Allen, T.H.; Morales, L.A. Reaction of Plutonium Dioxide with Water: Formation and Properties of PuO2+x. Science 2000, 287, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Skomurski, F.; Shuller-Nickles, L.; Ewing, R.C.; Becker, U. Corrosion of UO2 and ThO2: A quantum-mechanical investigation. J. Nucl. Mater. 2008, 375, 290–310. [Google Scholar] [CrossRef]
- Jonsson, M.; Nielsen, F.; Roth, O.; Ekeroth, E.; Nilsson, S.; Hossain, M.M. Radiation Induced Spent Nuclear Fuel Dissolution under Deep Repository Conditions. Environ. Sci. Technol. 2007, 41, 7087–7093. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Low, J.; Spahiu, K. Environmental behaviors of spent nuclear fuel and canister materials. Energy Environ. Sci. 2011, 4, 2537. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Morss, L.R.; Fuger, J.; Morss, L.R. The Chemistry of the Actinide and Transactinide Elements; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Dorado, B.; Amadon, B.; Freyss, M.; Bertolus, M. DFT+U calculations of the ground state and metastable states of uranium dioxide. Phys. Rev. B 2009, 79, 235125. [Google Scholar] [CrossRef]
- Jomard, G.; Amadon, B.; Bottin, F.; Torrent, M. Structural, thermodynamic, and electronic properties of plutonium oxides from first principles. Phys. Rev. B 2008, 78, 075125. [Google Scholar] [CrossRef]
- Cococcioni, M.; de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band Theory and Mott Insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943. [Google Scholar] [CrossRef] [PubMed]
- Himmetoglu, B.; Floris, A.; de Gironcoli, S.; Cococcioni, M. Hubbard-corrected DFT energy functionals: The LDA+U description of correlated systems. Int. J. Quant. Chem. 2013, 114, 14–49. [Google Scholar] [CrossRef]
- Dorado, B.; Garcia, P.; Carlot, G.; Davoisne, C.; Fraczkiewicz, M.; Pasquet, B.; Freyss, M.; Valot, C.; Baldinozzi, G.; Simeone, D.; et al. First-principles calculation and experimental study of oxygen diffusion in uranium dioxide. Phys. Rev. B 2011, 83, 035126. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Andersson, D.; Uberuaga, B.P. First-principles DFT modeling of nuclear fuel materials. J. Mater. Sci. 2012, 47, 7367–7384. [Google Scholar] [CrossRef]
- Dorado, B.; Freyss, M.; Amadon, B.; Bertolus, M.; Jomard, G.; Garcia, P. Advances in first-principles modelling of point defects in UO2: F electron correlations and the issue of local energy minima. J. Phys. Condens. Matter 2013, 25, 333201. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.; Lezama, J.; Uberuaga, B.P.; Deo, C.; Conradson, S.D. Cooperativity among defect sites in AO2+x and A4O9 (A= U, Np, Pu): Density functional calculations. Phys. Rev. B 2009, 79, 024110. [Google Scholar] [CrossRef]
- Yu, J.; Devanathan, R.; Weber, W.J. First-principles study of defects and phase transition in UO2. J. Phys. Condens. Matter 2009, 21, 435401. [Google Scholar] [CrossRef] [PubMed]
- Dorado, B.; Jomard, G.; Freyss, M.; Bertolus, M. Stability of oxygen point defects in UO2 by first-principles DFT+U calculations: Occupation matrix control and Jahn-Teller distortion. Phys. Rev. B 2010, 82, 035114. [Google Scholar] [CrossRef]
- Allen, J.; Watson, G.W. Occupation matrix control of d- and f-electron localisations using DFT + U. Phys. Chem. Chem. Phys. 2014, 16, 21016–21031. [Google Scholar] [CrossRef]
- Meredig, B.; Thompson, A.; Hansen, H.A.; Wolverton, C.; van de Walle, A. Method for locating low-energy solutions withinDFT+U. Phys. Rev. B 2010, 82, 195128. [Google Scholar] [CrossRef]
- Rabone, J.; Krack, M. A procedure for bypassing metastable states in local basis set DFT+U calculations and its application to uranium dioxide surfaces. Comput. Mater. Sci. 2013, 71, 157–164. [Google Scholar] [CrossRef]
- Prodan, I.D.; Scuseria, G.E.; Martin, R.L. Covalency in the actinide dioxides: Systematic study of the electronic properties using screened hybrid density functional theory. Phys. Rev. B 2007, 76, 033101. [Google Scholar] [CrossRef]
- Bo, T.; Lan, J.-H.; Zhao, Y.; Zhang, Y.-J.; He, C.; Chai, Z.; Shi, W.-Q. First-principles study of water adsorption and dissociation on the UO2 (1 1 1), (1 1 0) and (1 0 0) surfaces. J. Nucl. Mater. 2014, 454, 446–454. [Google Scholar] [CrossRef]
- Muggelberg, C.; Castell, M.; Briggs, G.; Goddard, D. An STM study of the UO2(001) surface. Appl. Surf. Sci. 1999, 142, 124–128. [Google Scholar] [CrossRef]
- Taylor, T.; Ellis, W. Distorted surface oxygen structure on UO2 (100). Surf. Sci. 1981, 107, 249–262. [Google Scholar] [CrossRef]
- Tasker, P.W. The structure and properties of fluorite crystal surfaces. J. Phys. Colloq. 1980, 41, 6. [Google Scholar] [CrossRef]
- Chatzimichail, R.; Bebelis, S.; Nikolopoulos, P. Temperature Dependence of the Surface Energy of the Low Index Planes of UO2 and ThO2. J. Mater. Eng. Perform. 2016, 25, 1691–1696. [Google Scholar] [CrossRef]
- Abramowski, M.; Redfern, S.; Grimes, R.; Owens, S. Modification of UO2 crystal morphologies through hydroxylation. Surf. Sci. 2001, 490, 415–420. [Google Scholar] [CrossRef]
- Jomard, G.; Bottin, F. Thermodynamic stability of PuO 2 surfaces: Influence of electronic correlations. Phys. Rev. B 2011, 84, 195469. [Google Scholar] [CrossRef]
- Tan, A.H.; Grimes, R.W.; Owens, S. Structures of UO2 and PuO2 surfaces with hydroxide coverage. J. Nucl. Mater. 2005, 344, 13–16. [Google Scholar] [CrossRef]
- Jelea, A.; Colbert, M.; Ribeiro, F.; Tréglia, G.; Pellenq, R.-M. An atomistic modelling of the porosity impact on UO2 matrix macroscopic properties. J. Nucl. Mater. 2011, 415, 210–216. [Google Scholar] [CrossRef]
- Sattonnay, G.; Tétot, R. Bulk, surface, and point defect properties in UO2from a tight-binding variable-charge model. J. Phys. Condens. Matter 2013, 25, 125403. [Google Scholar] [CrossRef]
- Benson, G.C.; Freeman, P.J.; Dempsey, E. Calculation of Cohesive and Surface Energies of Thorium and Uranium Dioxides. J. Am. Ceram. Soc. 1963, 46, 43–47. [Google Scholar] [CrossRef]
- Boyarchenkov, A.; Potashnikov, S.; Nekrasov, K.; Kupryazhkin, A. Molecular dynamics simulation of UO2 nanocrystals surface. J. Nucl. Mater. 2012, 421, 1–8. [Google Scholar] [CrossRef]
- Wul, G. Zur Frage der Geschwindigkeit des Wachstums und der Auflosung der Kristall Achen. Z. Kristallogr. 1901, 34, 449–530. [Google Scholar]
- Wang, G.; Batista, E.R.; Yang, P. Ligand induced shape transformation of thorium dioxide nanocrystals. Phys. Chem. Chem. Phys. 2018, 20, 17563–17573. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.; Symington, A.R.; Tse, J.S.; Dawson, J.; Flitcroft, J.M.; Parker, S.C.; Cooke, D.J.; Harker, R.M.; Molinari, M. The energetics of carbonated PuO2 surfaces affects nanoparticle morphology: A DFT+U study. Phys. Chem. Chem. Phys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rák, Z.; Ewing, R.C.; Becker, U. Hydroxylation-induced surface stability of AnO2 (An=U, Np, Pu) from first principles. Surf. Sci. 2013, 608, 180–187. [Google Scholar] [CrossRef]
- Alexandrov, V.; Shvareva, T.Y.; Hayun, S.; Asta, M.; Navrotsky, A. Actinide Dioxides in Water: Interactions at the Interface. J. Phys. Chem. Lett. 2011, 2, 3130–3134. [Google Scholar] [CrossRef]
- Shields, A.E.; Santos-Carballal, D.; de Leeuw, N.H. A density functional theory study of uranium-doped thoria and uranium adatoms on the major surfaces of thorium dioxide. J. Nucl. Mater. 2016, 473, 99–111. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Bandura, A.V.; Blokhin, E. Surface modelling on heavy atom crystalline compounds: HfO2 and UO2 fluorite structures. Acta Mater. 2009, 57, 600–606. [Google Scholar] [CrossRef]
- Chaka, A.M.; Oxford, G.A.; Stubbs, J.E.; Eng, P.J.; Bargar, J.R. Density-functional theory investigation of oxidative corrosion of UO2. Comput. Theor. Chem. 2012, 987, 90–102. [Google Scholar] [CrossRef]
- Weck, P.F.; Jove-Colon, C.F.; Sassani, D.C.; Kim, E. On the role of strong electron correlations in the surface properties and chemistry of uranium dioxide. Dalton Trans. 2013, 42, 4570. [Google Scholar] [CrossRef]
- Bottin, F.; Geneste, G.; Jomard, G. Thermodynamic stability of the UO2 surfaces: Interplay between over-stoichiometry and polarity compensation. Phys. Rev. B 2016, 93, 115438. [Google Scholar] [CrossRef]
- Sun, B.; Liu, H.; Song, H.; Zhang, G.; Zheng, H.; Zhao, X.; Zhang, P. First-principles study of surface properties of PuO2: Effects of thickness and O-vacancy on surface stability and chemical activity. J. Nucl. Mater. 2012, 426, 139–147. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Waterhouse, G.I.N.; Chan, A.; Madey, T.; Mullins, D.; Idriss, H. The reactions of water vapour on the surfaces of stoichiometric and reduced uranium dioxide: A high resolution XPS study. Catal. Today 2007, 120, 151–157. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Waterhouse, G.I.N.; Chan, A.S.Y.; Madey, T.E.; Mullins, D.R.; Idriss, H. Probing Surface Oxidation of Reduced Uranium Dioxide Thin Film Using Synchrotron Radiation. J. Phys. Chem. C 2007, 111, 7963–7970. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; da Silva, J.L.F.; Sauer, J. Density-Functional Calculations of the Structure of Near-Surface Oxygen Vacancies and Electron Localization onCeO2(111). Phys. Rev. Lett. 2009, 102, 026101. [Google Scholar] [CrossRef] [PubMed]
- Calaza, F.C.; Xu, Y.; Mullins, D.R.; Overbury, S.H. Oxygen Vacancy-Assisted Coupling and Enolization of Acetaldehyde on CeO2(111). J. Am. Chem. Soc. 2012, 134, 18034–18045. [Google Scholar] [CrossRef]
- Idriss, H. Surface reactions of uranium oxide powder, thin films, and single crystals. Surf. Sci. Rep. 2010, 65, 67–109. [Google Scholar] [CrossRef]
- Schlereth, T.W.; Hedhili, M.N.; Yakshinskiy, B.V.; Gouder, T.; Madey, T.E. Adsorption and Reaction of SO2with a Polycrystalline UO2Film: Promotion of S−O Bond Cleavage by Creation of O-Defects and Na or Ca Coadsorption. J. Phys. Chem. B 2005, 109, 20895–20905. [Google Scholar] [CrossRef]
- Manner, W.L.; Lloyd, J.A.; Paffett, M.T. Reexamination of the fundamental interactions of water with uranium. J. Nucl. Mater. 1999, 275, 37–46. [Google Scholar] [CrossRef]
- Konno, H. X-ray Photoelectron Spectroscopy, in Materials Science and Engineering of Carbon; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–171. [Google Scholar]
- Wellington, J.P. Oxygen Vacancy Formation and Water Adsorption on Reduced AnO2 {111},{110}, and {100} Surfaces (An = U, Pu): A Computational Study. J. Phys. Chem. C 2018, 122, 7149–7165. [Google Scholar] [CrossRef]
- Bo, T.; Lan, J.-H.; Wang, C.-Z.; Zhao, Y.; He, C.-H.; Zhang, Y.-J.; Chai, Z.-F.; Shi, W.-Q. First-Principles Study of Water Reaction and H2 Formation on UO2 (111) and (110) Single Crystal Surfaces. J. Phys. Chem. C 2014, 118, 21935–21944. [Google Scholar] [CrossRef]
- Wang, G.; Batista, E.R.; Yang, P. Excess Electrons on Reduced AnO2 (111) Surfaces (An = Th, U, Pu) and Their Impacts on Catalytic Water Splitting. J. Phys. Chem. C 2019, 123, 30245–30251. [Google Scholar] [CrossRef]
- Chen, J.-L.; Kaltsoyannis, N. Computational Study of the Bulk and Surface Properties of the Minor Actinide Dioxides MAnO2 (Man = Np, Am, Cm); Water Adsorption on Stoichiometric and Reduced {111},{110} and {100} Surfaces. J. Phys. Chem. C 2019, 15540–15550. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, H.-F.; Gong, X.-Q.; Guo, Y.-L.; Guo, Y.; Lu, G.; Hu, P. Multiple configurations of the two excess4felectrons on defectiveCeO2(111): Origin and implications. Phys. Rev. B 2009, 79, 193401. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Idriss, H. Water reactions over stoichiometric and reduced UO2 (111) single crystal surfaces. Surf. Sci. 2004, 563, 135–144. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Rousseau, R.; Colegrave, D.; Idriss, H. The reaction of water on polycrystalline UO2: Pathways to surface and bulk oxidation. J. Nucl. Mater. 2005, 342, 179–187. [Google Scholar] [CrossRef]
- Seibert, A.; Gouder, T.; Huber, F. Interaction of PuO2thin films with water. Radiochim. Acta 2010, 98, 647–657. [Google Scholar] [CrossRef]
- Gouder, T.; Shick, A.; Huber, F. Surface Interaction of PuO2, UO2+x and UO3 with Water Ice. Top. Catal. 2013, 56, 1112–1120. [Google Scholar] [CrossRef]
- Haschke, J.M.; Allen, T.H.; Morales, L.A. Reactions of plutonium dioxide with water and hydrogen–oxygen mixtures: Mechanisms for corrosion of uranium and plutonium. J. Alloy. Compd. 2001, 314, 78–91. [Google Scholar] [CrossRef]
- Haschke, J.M.; Ricketts, T.E. Adsorption of water on plutonium dioxide. J. Alloy. Compd. 1997, 252, 148–156. [Google Scholar] [CrossRef]
- Tian, X.-F.; Wang, H.; Xiao, H.; Gao, T. Adsorption of water on UO2 (111) surface: Density functional theory calculations. Comput. Mater. Sci. 2014, 91, 364–371. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, B.; Zhang, Q.; Liu, H.; Liu, K.; Song, H. Polaron modulation mechanism of H2O and CO2 adsorption on PuO2 (111) surface. Appl. Surf. Sci. 2020, 146164. [Google Scholar] [CrossRef]
- Wellington, J.P.; Kerridge, A.; Austin, J.; Kaltsoyannis, N. Electronic structure of bulk AnO 2 (An = U, Np, Pu) and water adsorption on the (111) and (110) surfaces of UO 2 and PuO 2 from hybrid density functional theory within the periodic electrostatic embedded cluster method. J. Nucl. Mater. 2016, 482, 124–134. [Google Scholar] [CrossRef]
- Tegner, B.E.; Molinari, M.; Kerridge, A.; Parker, S.C.; Kaltsoyannis, N. Water adsorption on AnO2 {111},{110}, and {100} surfaces (An = U and Pu): A density functional theory+ U study. J. Phys. Chem. C 2017, 121, 1675–1682. [Google Scholar] [CrossRef]
- Tegner, B.E.; Kaltsoyannis, N. Multiple water layers on AnO2 {111},{110}, and {100} surfaces (An= U, Pu): A computational study. J. Vacuum Sci. Technol. A Vacuum Surf. Films 2018, 36, 041402. [Google Scholar] [CrossRef]
- Maldonado, P.; Evins, L.Z.; Oppeneer, P.M. Ab Initio Atomistic Thermodynamics of Water Reacting with Uranium Dioxide Surfaces. J. Phys. Chem. C 2014, 118, 8491–8500. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Zhang, P. Dissociation Mechanism of Water Molecules on the PuO2(110) Surface: An Ab Initio Molecular Dynamics Study. J. Phys. Chem. C 2017, 122, 371–376. [Google Scholar] [CrossRef]
- Carlson, R.K.; Cawkwell, M.J.; Batista, E.R.; Yang, P. Tight-Binding Modeling of Uranium in an Aqueous Environment. J. Chem. Theory Comput. 2020, 16, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).