Water on Actinide Dioxide Surfaces: A Review of Recent Progress
Abstract
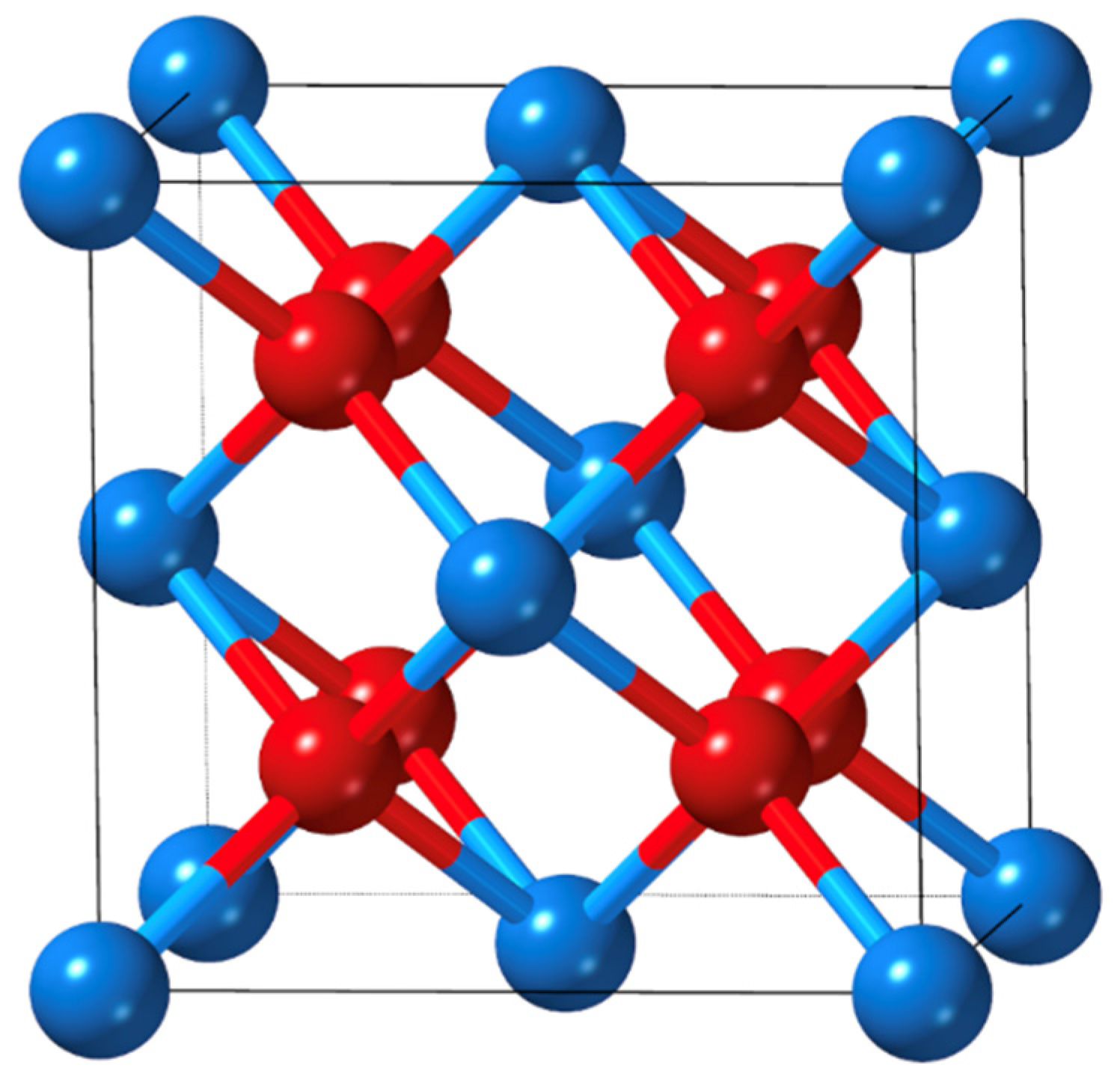
1. Introduction
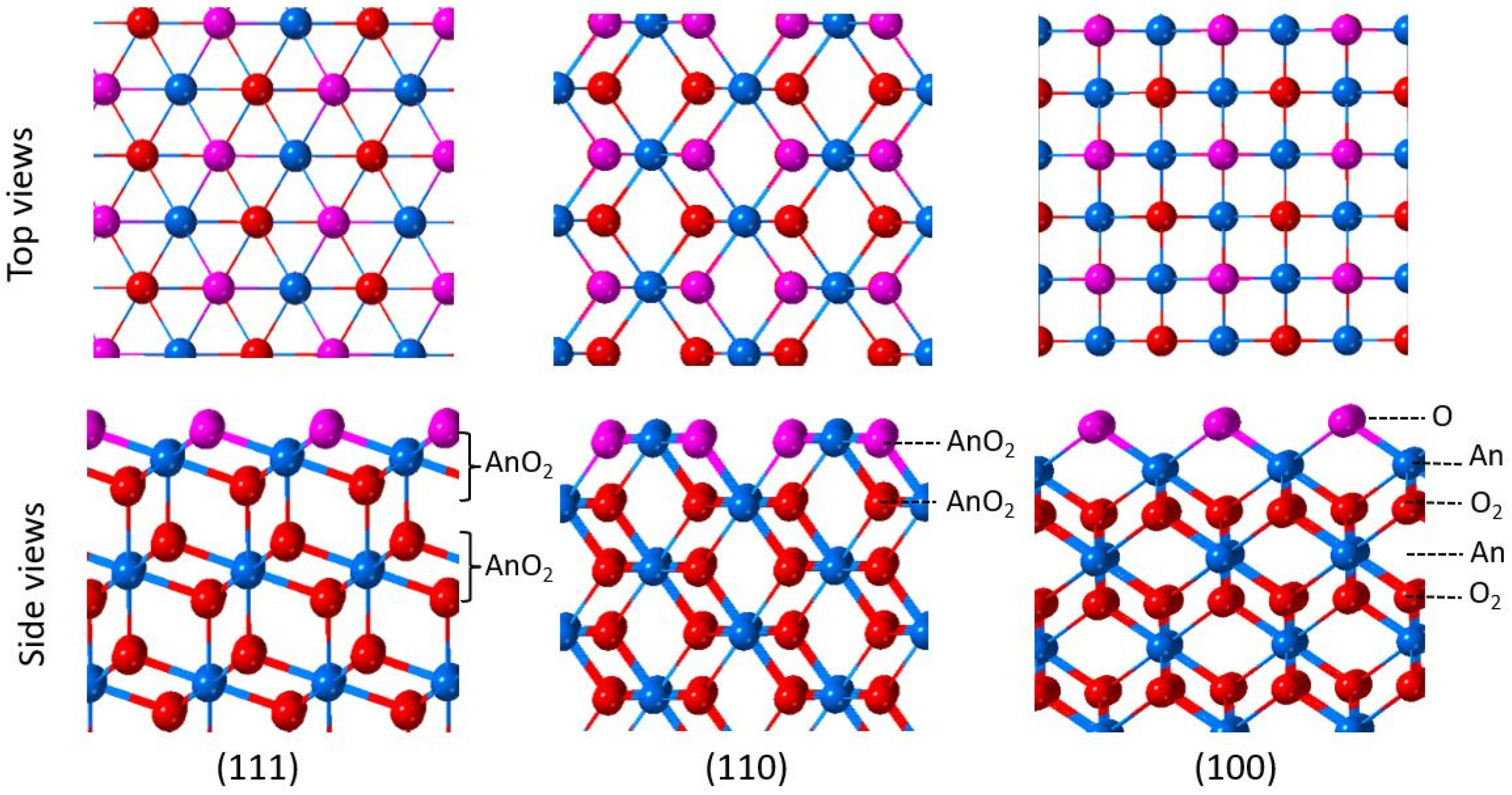
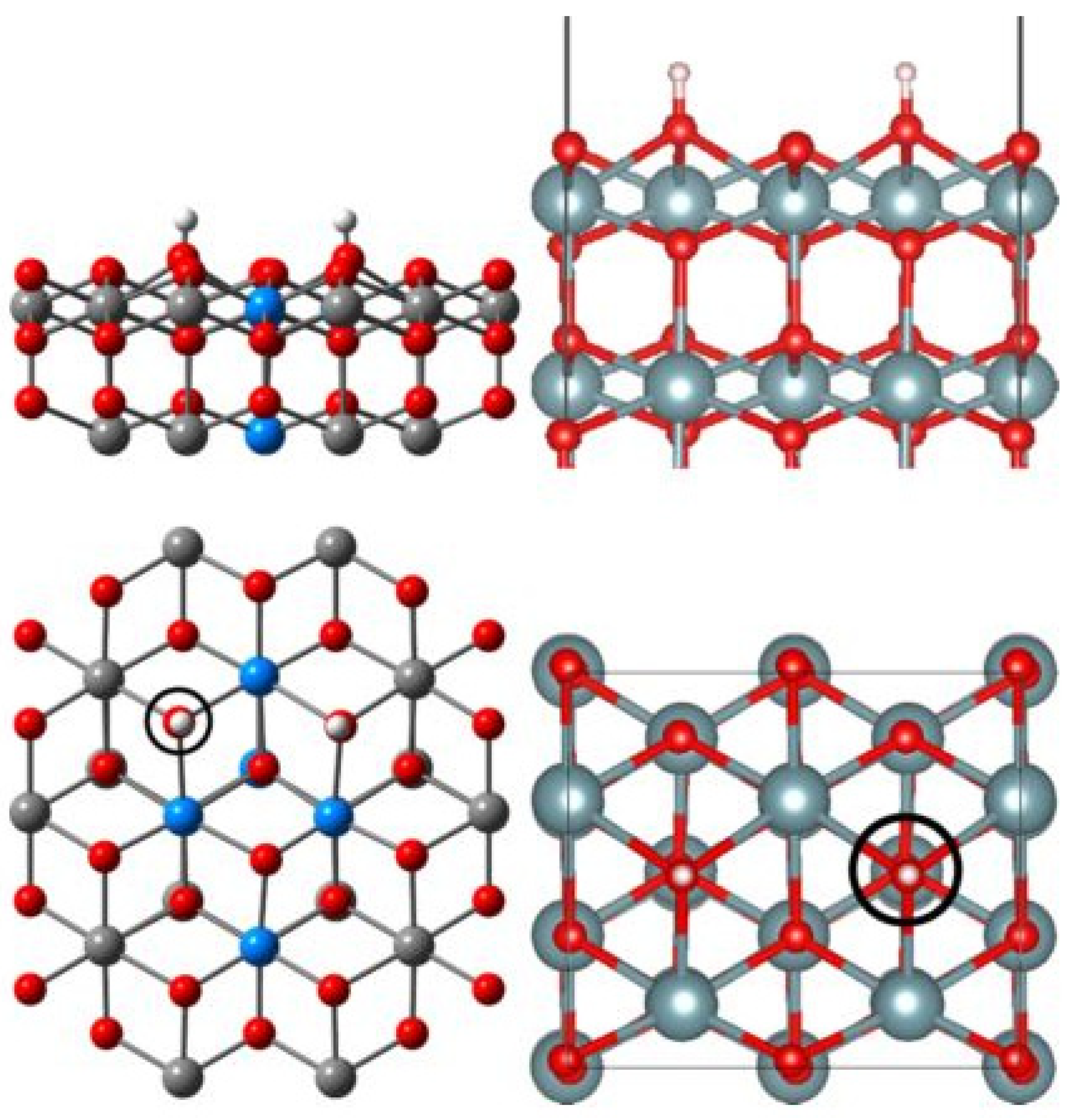
2. Oxygen Vacancies on AnO2 Surfaces
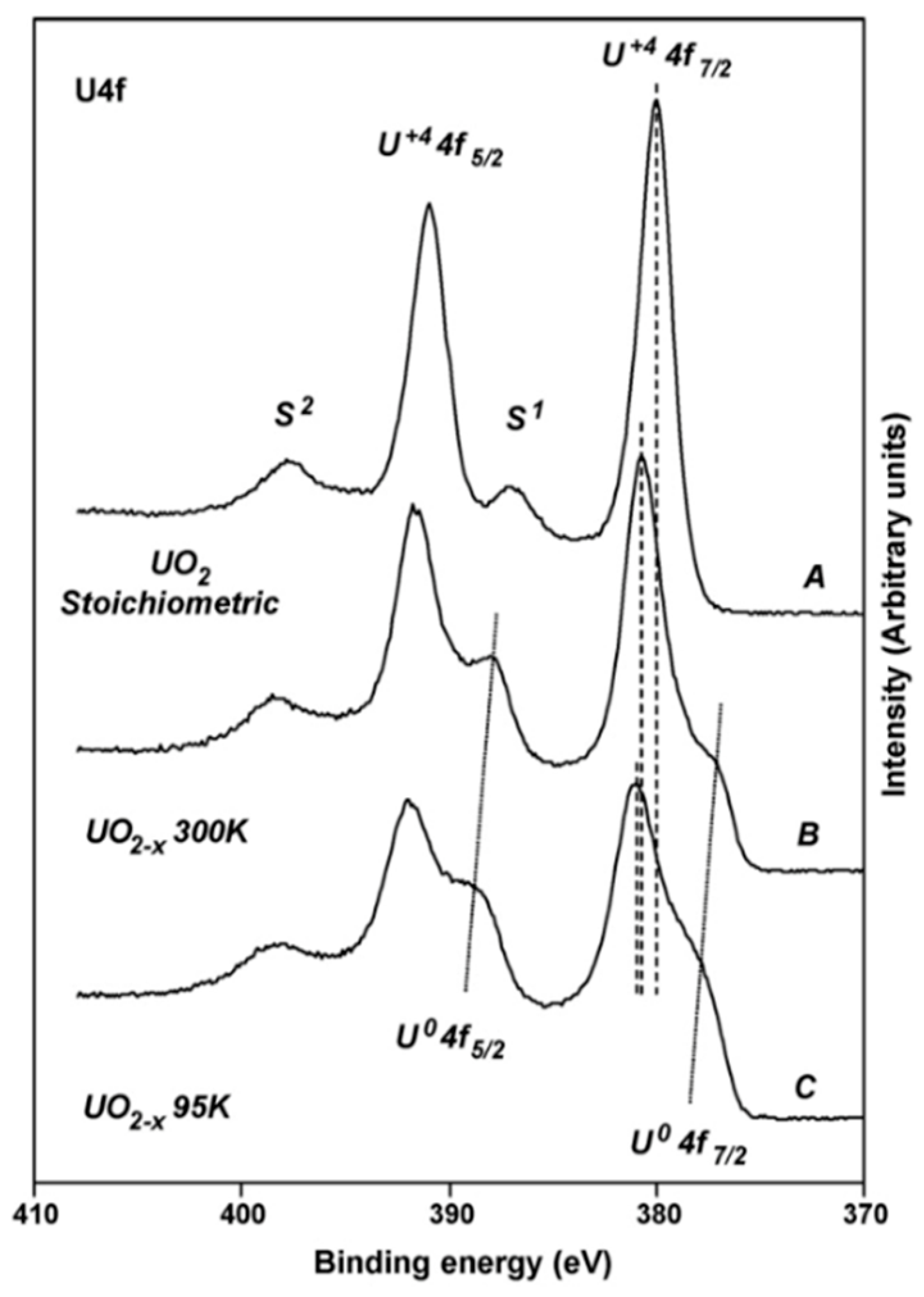
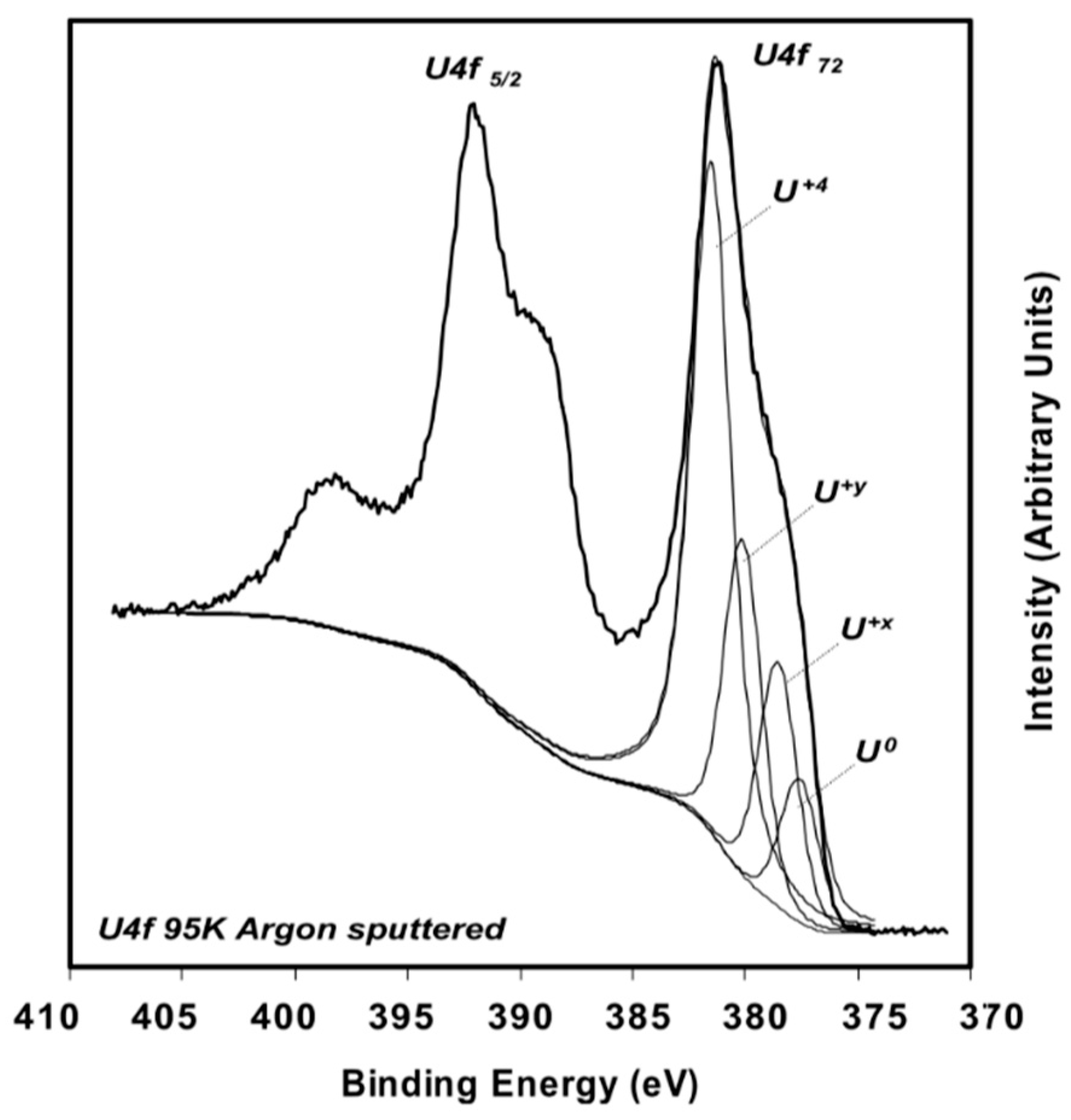
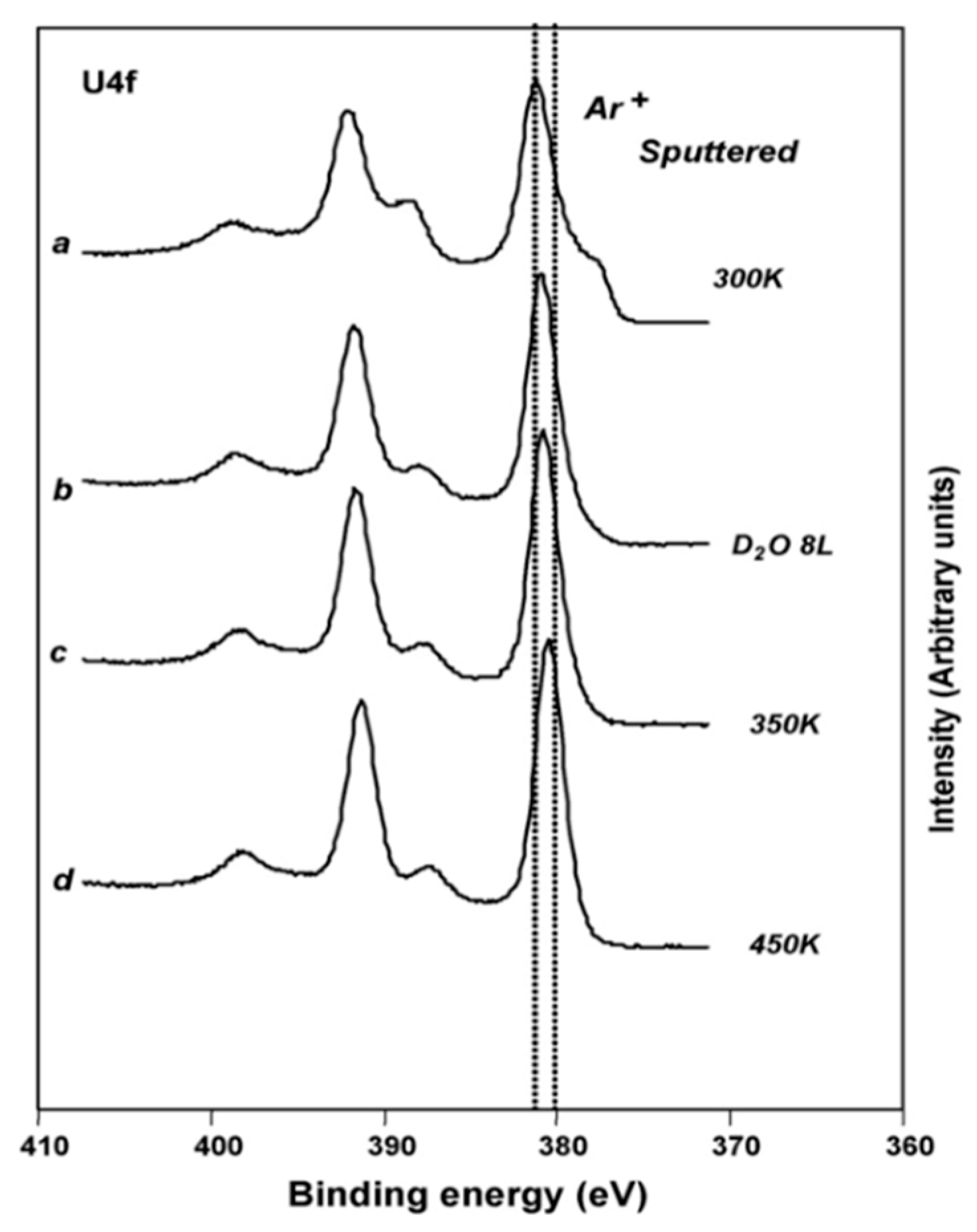
2.1. Experimental Work
2.2. Theoretical Calculations
3. Water on AnO2 Surfaces
3.1. Experiments
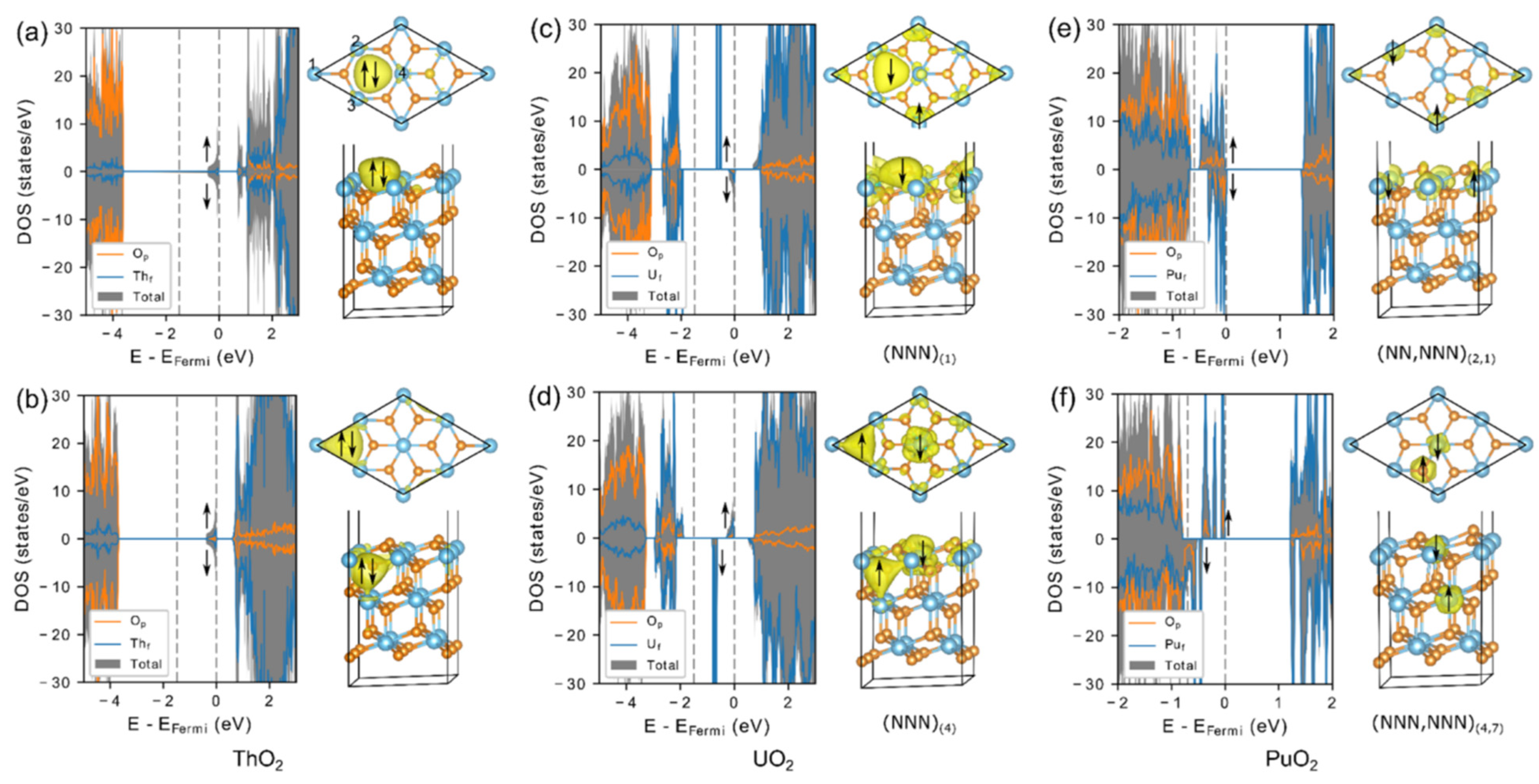
3.2. Theoretical Calculations
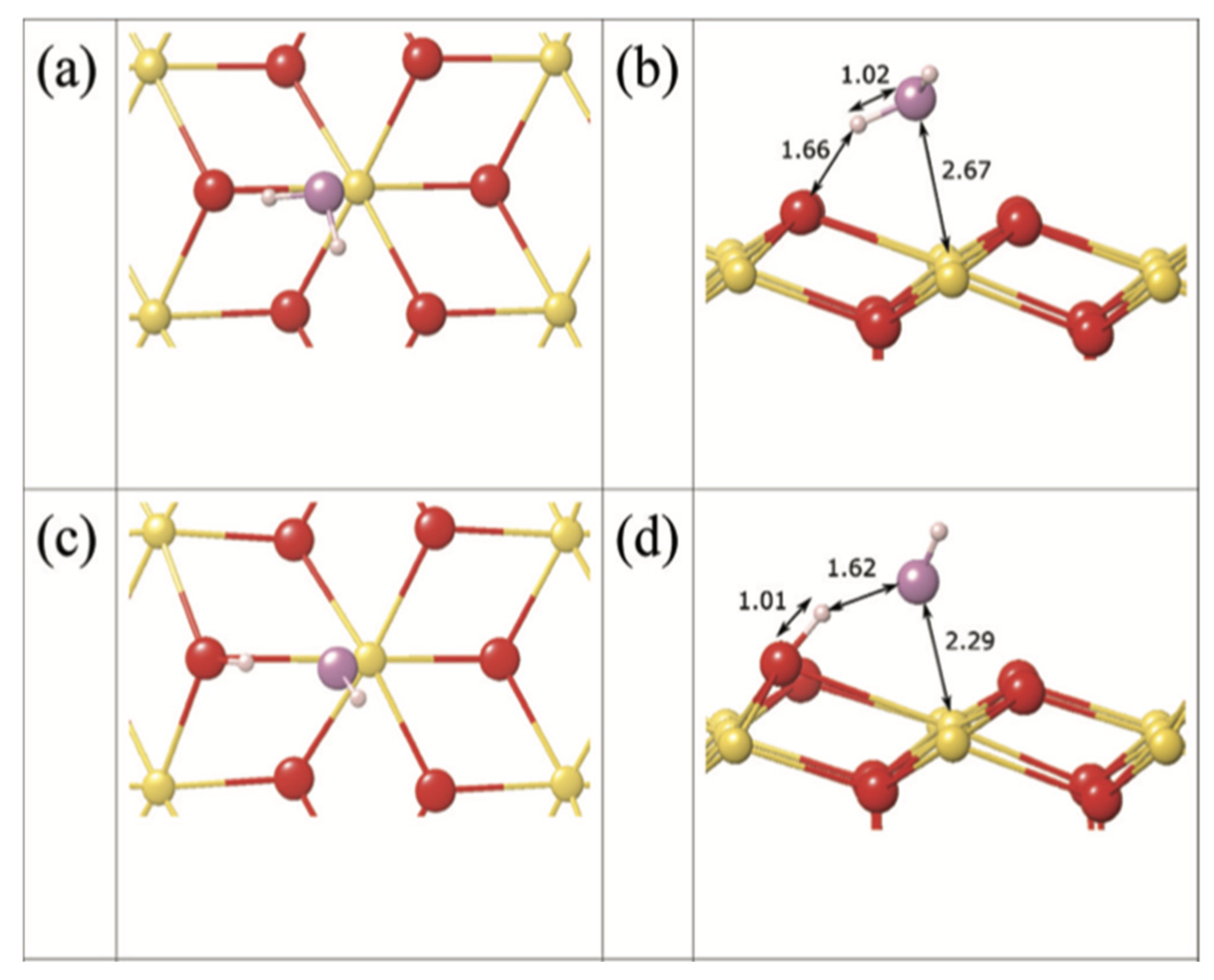
3.2.1. Adsorption and dissociation of H2O on AnO2 surfaces
- (i)
- A mixture of molecular and dissociated adsorption of H2O occurs on the (111) surface of AnO2.
- (ii)
- Dissociative adsorption of H2O preferentially occurs on the (110) and (100) surfaces of AnO2.
- (iii)
- The adsorption energies of H2O are correlated with the surface energies; a stronger adsorption energy is expected on the surface with a higher surface energy.
- (iv)
- The presence of oxygen vacancies on the surface of AnO2 favors the dissociative adsorption mechanism. The distribution of excess electrons on reduced AnO2 surfaces is different upon the adsorption of H2O.
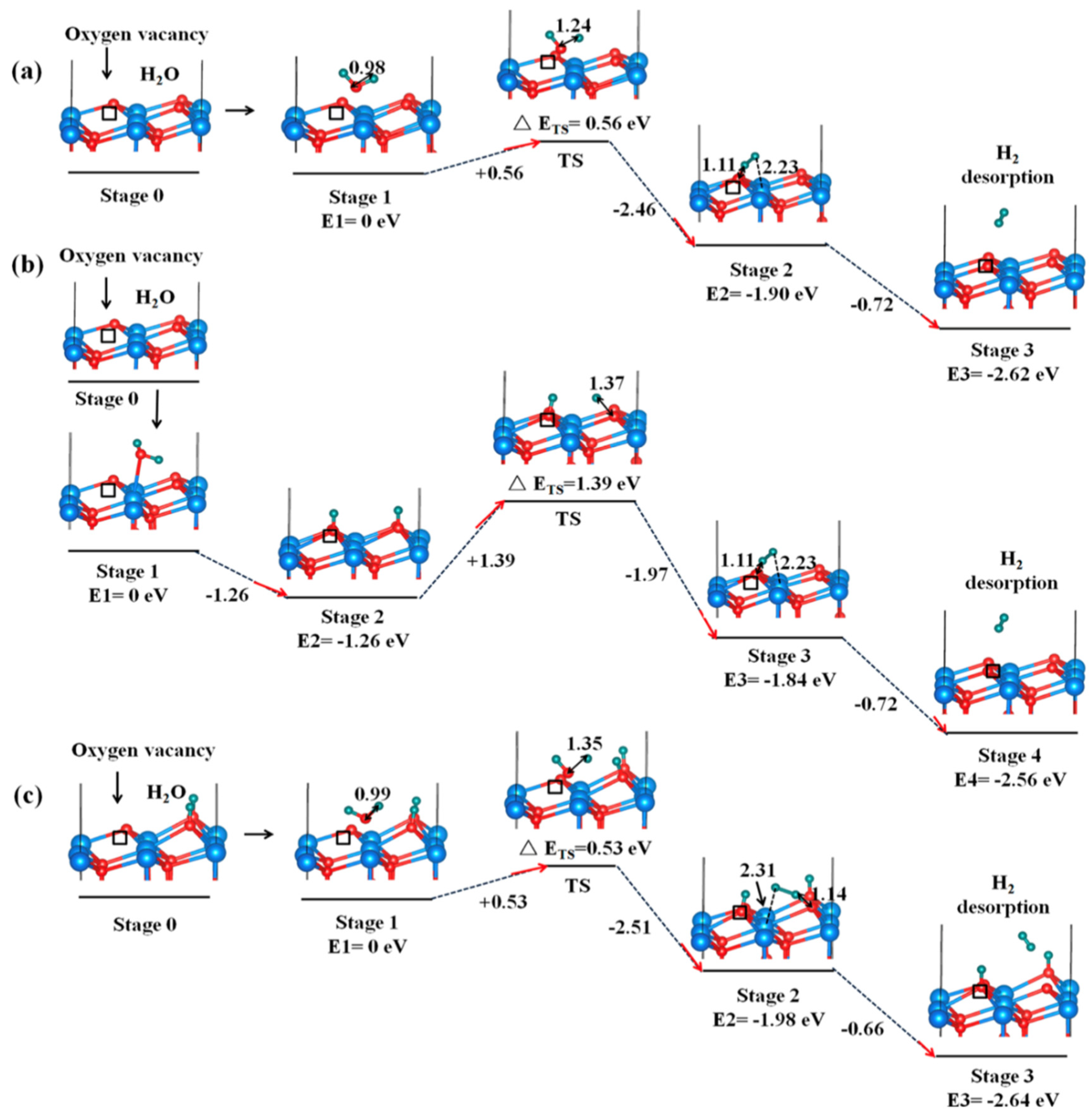
3.2.2. H2 production from H2O splitting
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haschke, J.M.; Allen, T.H.; Morales, L.A. Reaction of Plutonium Dioxide with Water: Formation and Properties of PuO2+x. Science 2000, 287, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Skomurski, F.; Shuller-Nickles, L.; Ewing, R.C.; Becker, U. Corrosion of UO2 and ThO2: A quantum-mechanical investigation. J. Nucl. Mater. 2008, 375, 290–310. [Google Scholar] [CrossRef]
- Jonsson, M.; Nielsen, F.; Roth, O.; Ekeroth, E.; Nilsson, S.; Hossain, M.M. Radiation Induced Spent Nuclear Fuel Dissolution under Deep Repository Conditions. Environ. Sci. Technol. 2007, 41, 7087–7093. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Low, J.; Spahiu, K. Environmental behaviors of spent nuclear fuel and canister materials. Energy Environ. Sci. 2011, 4, 2537. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Morss, L.R.; Fuger, J.; Morss, L.R. The Chemistry of the Actinide and Transactinide Elements; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Dorado, B.; Amadon, B.; Freyss, M.; Bertolus, M. DFT+U calculations of the ground state and metastable states of uranium dioxide. Phys. Rev. B 2009, 79, 235125. [Google Scholar] [CrossRef]
- Jomard, G.; Amadon, B.; Bottin, F.; Torrent, M. Structural, thermodynamic, and electronic properties of plutonium oxides from first principles. Phys. Rev. B 2008, 78, 075125. [Google Scholar] [CrossRef]
- Cococcioni, M.; de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band Theory and Mott Insulators: Hubbard U instead of Stoner I. Phys. Rev. B 1991, 44, 943. [Google Scholar] [CrossRef] [PubMed]
- Himmetoglu, B.; Floris, A.; de Gironcoli, S.; Cococcioni, M. Hubbard-corrected DFT energy functionals: The LDA+U description of correlated systems. Int. J. Quant. Chem. 2013, 114, 14–49. [Google Scholar] [CrossRef]
- Dorado, B.; Garcia, P.; Carlot, G.; Davoisne, C.; Fraczkiewicz, M.; Pasquet, B.; Freyss, M.; Valot, C.; Baldinozzi, G.; Simeone, D.; et al. First-principles calculation and experimental study of oxygen diffusion in uranium dioxide. Phys. Rev. B 2011, 83, 035126. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Andersson, D.; Uberuaga, B.P. First-principles DFT modeling of nuclear fuel materials. J. Mater. Sci. 2012, 47, 7367–7384. [Google Scholar] [CrossRef]
- Dorado, B.; Freyss, M.; Amadon, B.; Bertolus, M.; Jomard, G.; Garcia, P. Advances in first-principles modelling of point defects in UO2: F electron correlations and the issue of local energy minima. J. Phys. Condens. Matter 2013, 25, 333201. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.; Lezama, J.; Uberuaga, B.P.; Deo, C.; Conradson, S.D. Cooperativity among defect sites in AO2+x and A4O9 (A= U, Np, Pu): Density functional calculations. Phys. Rev. B 2009, 79, 024110. [Google Scholar] [CrossRef]
- Yu, J.; Devanathan, R.; Weber, W.J. First-principles study of defects and phase transition in UO2. J. Phys. Condens. Matter 2009, 21, 435401. [Google Scholar] [CrossRef] [PubMed]
- Dorado, B.; Jomard, G.; Freyss, M.; Bertolus, M. Stability of oxygen point defects in UO2 by first-principles DFT+U calculations: Occupation matrix control and Jahn-Teller distortion. Phys. Rev. B 2010, 82, 035114. [Google Scholar] [CrossRef]
- Allen, J.; Watson, G.W. Occupation matrix control of d- and f-electron localisations using DFT + U. Phys. Chem. Chem. Phys. 2014, 16, 21016–21031. [Google Scholar] [CrossRef]
- Meredig, B.; Thompson, A.; Hansen, H.A.; Wolverton, C.; van de Walle, A. Method for locating low-energy solutions withinDFT+U. Phys. Rev. B 2010, 82, 195128. [Google Scholar] [CrossRef]
- Rabone, J.; Krack, M. A procedure for bypassing metastable states in local basis set DFT+U calculations and its application to uranium dioxide surfaces. Comput. Mater. Sci. 2013, 71, 157–164. [Google Scholar] [CrossRef]
- Prodan, I.D.; Scuseria, G.E.; Martin, R.L. Covalency in the actinide dioxides: Systematic study of the electronic properties using screened hybrid density functional theory. Phys. Rev. B 2007, 76, 033101. [Google Scholar] [CrossRef]
- Bo, T.; Lan, J.-H.; Zhao, Y.; Zhang, Y.-J.; He, C.; Chai, Z.; Shi, W.-Q. First-principles study of water adsorption and dissociation on the UO2 (1 1 1), (1 1 0) and (1 0 0) surfaces. J. Nucl. Mater. 2014, 454, 446–454. [Google Scholar] [CrossRef]
- Muggelberg, C.; Castell, M.; Briggs, G.; Goddard, D. An STM study of the UO2(001) surface. Appl. Surf. Sci. 1999, 142, 124–128. [Google Scholar] [CrossRef]
- Taylor, T.; Ellis, W. Distorted surface oxygen structure on UO2 (100). Surf. Sci. 1981, 107, 249–262. [Google Scholar] [CrossRef]
- Tasker, P.W. The structure and properties of fluorite crystal surfaces. J. Phys. Colloq. 1980, 41, 6. [Google Scholar] [CrossRef]
- Chatzimichail, R.; Bebelis, S.; Nikolopoulos, P. Temperature Dependence of the Surface Energy of the Low Index Planes of UO2 and ThO2. J. Mater. Eng. Perform. 2016, 25, 1691–1696. [Google Scholar] [CrossRef]
- Abramowski, M.; Redfern, S.; Grimes, R.; Owens, S. Modification of UO2 crystal morphologies through hydroxylation. Surf. Sci. 2001, 490, 415–420. [Google Scholar] [CrossRef]
- Jomard, G.; Bottin, F. Thermodynamic stability of PuO 2 surfaces: Influence of electronic correlations. Phys. Rev. B 2011, 84, 195469. [Google Scholar] [CrossRef]
- Tan, A.H.; Grimes, R.W.; Owens, S. Structures of UO2 and PuO2 surfaces with hydroxide coverage. J. Nucl. Mater. 2005, 344, 13–16. [Google Scholar] [CrossRef]
- Jelea, A.; Colbert, M.; Ribeiro, F.; Tréglia, G.; Pellenq, R.-M. An atomistic modelling of the porosity impact on UO2 matrix macroscopic properties. J. Nucl. Mater. 2011, 415, 210–216. [Google Scholar] [CrossRef]
- Sattonnay, G.; Tétot, R. Bulk, surface, and point defect properties in UO2from a tight-binding variable-charge model. J. Phys. Condens. Matter 2013, 25, 125403. [Google Scholar] [CrossRef]
- Benson, G.C.; Freeman, P.J.; Dempsey, E. Calculation of Cohesive and Surface Energies of Thorium and Uranium Dioxides. J. Am. Ceram. Soc. 1963, 46, 43–47. [Google Scholar] [CrossRef]
- Boyarchenkov, A.; Potashnikov, S.; Nekrasov, K.; Kupryazhkin, A. Molecular dynamics simulation of UO2 nanocrystals surface. J. Nucl. Mater. 2012, 421, 1–8. [Google Scholar] [CrossRef]
- Wul, G. Zur Frage der Geschwindigkeit des Wachstums und der Auflosung der Kristall Achen. Z. Kristallogr. 1901, 34, 449–530. [Google Scholar]
- Wang, G.; Batista, E.R.; Yang, P. Ligand induced shape transformation of thorium dioxide nanocrystals. Phys. Chem. Chem. Phys. 2018, 20, 17563–17573. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.; Symington, A.R.; Tse, J.S.; Dawson, J.; Flitcroft, J.M.; Parker, S.C.; Cooke, D.J.; Harker, R.M.; Molinari, M. The energetics of carbonated PuO2 surfaces affects nanoparticle morphology: A DFT+U study. Phys. Chem. Chem. Phys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rák, Z.; Ewing, R.C.; Becker, U. Hydroxylation-induced surface stability of AnO2 (An=U, Np, Pu) from first principles. Surf. Sci. 2013, 608, 180–187. [Google Scholar] [CrossRef]
- Alexandrov, V.; Shvareva, T.Y.; Hayun, S.; Asta, M.; Navrotsky, A. Actinide Dioxides in Water: Interactions at the Interface. J. Phys. Chem. Lett. 2011, 2, 3130–3134. [Google Scholar] [CrossRef]
- Shields, A.E.; Santos-Carballal, D.; de Leeuw, N.H. A density functional theory study of uranium-doped thoria and uranium adatoms on the major surfaces of thorium dioxide. J. Nucl. Mater. 2016, 473, 99–111. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Bandura, A.V.; Blokhin, E. Surface modelling on heavy atom crystalline compounds: HfO2 and UO2 fluorite structures. Acta Mater. 2009, 57, 600–606. [Google Scholar] [CrossRef]
- Chaka, A.M.; Oxford, G.A.; Stubbs, J.E.; Eng, P.J.; Bargar, J.R. Density-functional theory investigation of oxidative corrosion of UO2. Comput. Theor. Chem. 2012, 987, 90–102. [Google Scholar] [CrossRef]
- Weck, P.F.; Jove-Colon, C.F.; Sassani, D.C.; Kim, E. On the role of strong electron correlations in the surface properties and chemistry of uranium dioxide. Dalton Trans. 2013, 42, 4570. [Google Scholar] [CrossRef]
- Bottin, F.; Geneste, G.; Jomard, G. Thermodynamic stability of the UO2 surfaces: Interplay between over-stoichiometry and polarity compensation. Phys. Rev. B 2016, 93, 115438. [Google Scholar] [CrossRef]
- Sun, B.; Liu, H.; Song, H.; Zhang, G.; Zheng, H.; Zhao, X.; Zhang, P. First-principles study of surface properties of PuO2: Effects of thickness and O-vacancy on surface stability and chemical activity. J. Nucl. Mater. 2012, 426, 139–147. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Waterhouse, G.I.N.; Chan, A.; Madey, T.; Mullins, D.; Idriss, H. The reactions of water vapour on the surfaces of stoichiometric and reduced uranium dioxide: A high resolution XPS study. Catal. Today 2007, 120, 151–157. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Waterhouse, G.I.N.; Chan, A.S.Y.; Madey, T.E.; Mullins, D.R.; Idriss, H. Probing Surface Oxidation of Reduced Uranium Dioxide Thin Film Using Synchrotron Radiation. J. Phys. Chem. C 2007, 111, 7963–7970. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; da Silva, J.L.F.; Sauer, J. Density-Functional Calculations of the Structure of Near-Surface Oxygen Vacancies and Electron Localization onCeO2(111). Phys. Rev. Lett. 2009, 102, 026101. [Google Scholar] [CrossRef] [PubMed]
- Calaza, F.C.; Xu, Y.; Mullins, D.R.; Overbury, S.H. Oxygen Vacancy-Assisted Coupling and Enolization of Acetaldehyde on CeO2(111). J. Am. Chem. Soc. 2012, 134, 18034–18045. [Google Scholar] [CrossRef]
- Idriss, H. Surface reactions of uranium oxide powder, thin films, and single crystals. Surf. Sci. Rep. 2010, 65, 67–109. [Google Scholar] [CrossRef]
- Schlereth, T.W.; Hedhili, M.N.; Yakshinskiy, B.V.; Gouder, T.; Madey, T.E. Adsorption and Reaction of SO2with a Polycrystalline UO2Film: Promotion of S−O Bond Cleavage by Creation of O-Defects and Na or Ca Coadsorption. J. Phys. Chem. B 2005, 109, 20895–20905. [Google Scholar] [CrossRef]
- Manner, W.L.; Lloyd, J.A.; Paffett, M.T. Reexamination of the fundamental interactions of water with uranium. J. Nucl. Mater. 1999, 275, 37–46. [Google Scholar] [CrossRef]
- Konno, H. X-ray Photoelectron Spectroscopy, in Materials Science and Engineering of Carbon; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–171. [Google Scholar]
- Wellington, J.P. Oxygen Vacancy Formation and Water Adsorption on Reduced AnO2 {111},{110}, and {100} Surfaces (An = U, Pu): A Computational Study. J. Phys. Chem. C 2018, 122, 7149–7165. [Google Scholar] [CrossRef]
- Bo, T.; Lan, J.-H.; Wang, C.-Z.; Zhao, Y.; He, C.-H.; Zhang, Y.-J.; Chai, Z.-F.; Shi, W.-Q. First-Principles Study of Water Reaction and H2 Formation on UO2 (111) and (110) Single Crystal Surfaces. J. Phys. Chem. C 2014, 118, 21935–21944. [Google Scholar] [CrossRef]
- Wang, G.; Batista, E.R.; Yang, P. Excess Electrons on Reduced AnO2 (111) Surfaces (An = Th, U, Pu) and Their Impacts on Catalytic Water Splitting. J. Phys. Chem. C 2019, 123, 30245–30251. [Google Scholar] [CrossRef]
- Chen, J.-L.; Kaltsoyannis, N. Computational Study of the Bulk and Surface Properties of the Minor Actinide Dioxides MAnO2 (Man = Np, Am, Cm); Water Adsorption on Stoichiometric and Reduced {111},{110} and {100} Surfaces. J. Phys. Chem. C 2019, 15540–15550. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, H.-F.; Gong, X.-Q.; Guo, Y.-L.; Guo, Y.; Lu, G.; Hu, P. Multiple configurations of the two excess4felectrons on defectiveCeO2(111): Origin and implications. Phys. Rev. B 2009, 79, 193401. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Idriss, H. Water reactions over stoichiometric and reduced UO2 (111) single crystal surfaces. Surf. Sci. 2004, 563, 135–144. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Rousseau, R.; Colegrave, D.; Idriss, H. The reaction of water on polycrystalline UO2: Pathways to surface and bulk oxidation. J. Nucl. Mater. 2005, 342, 179–187. [Google Scholar] [CrossRef]
- Seibert, A.; Gouder, T.; Huber, F. Interaction of PuO2thin films with water. Radiochim. Acta 2010, 98, 647–657. [Google Scholar] [CrossRef]
- Gouder, T.; Shick, A.; Huber, F. Surface Interaction of PuO2, UO2+x and UO3 with Water Ice. Top. Catal. 2013, 56, 1112–1120. [Google Scholar] [CrossRef]
- Haschke, J.M.; Allen, T.H.; Morales, L.A. Reactions of plutonium dioxide with water and hydrogen–oxygen mixtures: Mechanisms for corrosion of uranium and plutonium. J. Alloy. Compd. 2001, 314, 78–91. [Google Scholar] [CrossRef]
- Haschke, J.M.; Ricketts, T.E. Adsorption of water on plutonium dioxide. J. Alloy. Compd. 1997, 252, 148–156. [Google Scholar] [CrossRef]
- Tian, X.-F.; Wang, H.; Xiao, H.; Gao, T. Adsorption of water on UO2 (111) surface: Density functional theory calculations. Comput. Mater. Sci. 2014, 91, 364–371. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, B.; Zhang, Q.; Liu, H.; Liu, K.; Song, H. Polaron modulation mechanism of H2O and CO2 adsorption on PuO2 (111) surface. Appl. Surf. Sci. 2020, 146164. [Google Scholar] [CrossRef]
- Wellington, J.P.; Kerridge, A.; Austin, J.; Kaltsoyannis, N. Electronic structure of bulk AnO 2 (An = U, Np, Pu) and water adsorption on the (111) and (110) surfaces of UO 2 and PuO 2 from hybrid density functional theory within the periodic electrostatic embedded cluster method. J. Nucl. Mater. 2016, 482, 124–134. [Google Scholar] [CrossRef]
- Tegner, B.E.; Molinari, M.; Kerridge, A.; Parker, S.C.; Kaltsoyannis, N. Water adsorption on AnO2 {111},{110}, and {100} surfaces (An = U and Pu): A density functional theory+ U study. J. Phys. Chem. C 2017, 121, 1675–1682. [Google Scholar] [CrossRef]
- Tegner, B.E.; Kaltsoyannis, N. Multiple water layers on AnO2 {111},{110}, and {100} surfaces (An= U, Pu): A computational study. J. Vacuum Sci. Technol. A Vacuum Surf. Films 2018, 36, 041402. [Google Scholar] [CrossRef]
- Maldonado, P.; Evins, L.Z.; Oppeneer, P.M. Ab Initio Atomistic Thermodynamics of Water Reacting with Uranium Dioxide Surfaces. J. Phys. Chem. C 2014, 118, 8491–8500. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Zhang, P. Dissociation Mechanism of Water Molecules on the PuO2(110) Surface: An Ab Initio Molecular Dynamics Study. J. Phys. Chem. C 2017, 122, 371–376. [Google Scholar] [CrossRef]
- Carlson, R.K.; Cawkwell, M.J.; Batista, E.R.; Yang, P. Tight-Binding Modeling of Uranium in an Aqueous Environment. J. Chem. Theory Comput. 2020, 16, 3073–3083. [Google Scholar] [CrossRef] [PubMed]

| References | Methods | ||||
|---|---|---|---|---|---|
| Rak et al. [37] | DFT | GGA + U (U = 4 eV) | 0.79 | 1.18 | - |
| Alexandrov et al. [38] | GGA | 0.8 | 1.10 | 1.6 | |
| Skomurski et al. [2] | GGA | 0.72–0.81 | 0.98–1.30 | 1.55–1.75 | |
| Shields et al. [39] | GGA | 0.50 | 0.75 | 1.30 | |
| Wang et al. [35] | GGA | 0.801 | 1.202 | 1.762 | |
| Tasker et al. [25] | Classical models | Ionic shell model | 1.016 | 1.451 | - |
| Chatzimichail et al. [26] | Thermodynamic model | 0.973 | 1.297 | 1.382 | |
| References | Methods | ||||
|---|---|---|---|---|---|
| Skomurski et al. [2] | DFT | GGA | 0.59–0.33 | 1.01–0.83 | 1.21–1.07 |
| Rak et al. [37] | GGA + U (U = 4 eV) | 0.78 | 1.05 | 1.47 | |
| Evarestov et al. [40] | Hybrid functional | 0.94 | - | - | |
| Chaka et al. [41] | GGA | 0.888 | - | - | |
| Weck et al. [42] | GGA + U (U = 0 to 4 eV) | 0.76–0.78 | - | - | |
| Bo et al. [22] | GGA + U (U = 4 eV) | 0.71 | 1.08 | 1.49 | |
| Bottin et al. [43] | GGA + U (U = 4 eV) | 0.73 | 1.16 | 1.46 | |
| Rabone et al. [20] | GGA + U (U = 3.96 eV) | 0.48 | 0.77 | 1.25 | |
| Tasker et al. [25] | Classical models | Ionic shell model | 1.064 | 1.561 | - |
| Abramowski et al. [27] | Born model | 1.27 | 2.0 | 2.72 | |
| Tan et al. [29] | Pair potentials | 1.29 | 2.04 | 2.45 | |
| Jelea et al. [30] | Ionic crystal interatomic potentials | 1.24 | - | 2.22 | |
| Sattonay et al. [31] | Tight-binding variable-charge model | 1.07 | 1.72 | 2.03 | |
| Benson et al. [32] | Born–Mayer model | 1.030 | - | - | |
| Chatzimichail et al. [26] | Thermodynamic model | 0.95 | 1.266 | 1.333 | |
| Boyarchenkov et al. [33] | Pair potentials | 1.14 | - | 1.60 | |
| References | Methods | ||||
|---|---|---|---|---|---|
| Sun et al. [44] | DFT | LDA/GGA + U (U = 4 eV) | 0.72–1.04 | 1.20–1.44 | 1.52–1.84 |
| Jomard et al. [28] | GGA + U (U = 3.3 eV) | 0.72–0.74 | 1.10–1.13 | 1.64–1.69 | |
| Rak et al. [37] | GGA + U (U = 4 eV) | 0.74 | 1.15 | 1.76 | |
| In Units of eV | ThO2 | UO2 | PuO2 | |
|---|---|---|---|---|
| Wellington et al. [53] | Top | - | 6.45 | 3.35 |
| Sub | - | 6.14 | 3.40 | |
| Bo et al. [54] | Top | - | 5.95 | - |
| Sub | - | 6.08 | - | |
| Sun et al. [44] | Top | - | - | 2.85 |
| Sub | - | - | 2.89 | |
| Wang et al. [55] | Top | 6.95 | 5.21 | 2.81 |
| Sub | 6.58 | 4.98 | 2.43 | |
| In Units of eV | (111) | (110) | (100) |
|---|---|---|---|
| Bo et al. [22,54] | 0.61 0.68 | 0.62 1.27 | 1.02 1.71 |
| Tegner et al. [67] | 0.53 0.50 | 0.93 1.39 | 0.97 1.55 |
| Wang et al. [55] | 0.62 | - | - |
| In Units of eV | Perfect | Reduced | ||||
|---|---|---|---|---|---|---|
| ThO2 | UO2 | PuO2 | ThO2 | UO2 | PuO2 | |
| Tegner et al. [67] | - | 0.53 0.50 | 0.40 0.32 | - | - | - |
| Wellington et al. [53] | - | - | - | - | 2.23 | 2.10 |
| Bo et al. [54] | - | 0.61 0.68 | - | - | 2.20 | - |
| Alexandrov et al. [38] | 0.57 0.67 | - | - | - | - | - |
| Tian et al. [64] | - | 1.08 0.68 | - | - | 1.88 | - |
| Weck et al. [42] | - | 0.85 0.60 | - | - | - | - |
| Wang et al. [55] | 0.56 | 0.62 | 0.58 | 2.63 | 1.90 | 2.44 |
| ΔH (eV) | ThO2 | UO2 | PuO2 |
|---|---|---|---|
| Perfect surface | +1.72 | +1.85 | +4.11 |
| Oxygen vacancy | −3.55 | −3.11 | −0.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Batista, E.R.; Yang, P. Water on Actinide Dioxide Surfaces: A Review of Recent Progress. Appl. Sci. 2020, 10, 4655. https://doi.org/10.3390/app10134655
Wang G, Batista ER, Yang P. Water on Actinide Dioxide Surfaces: A Review of Recent Progress. Applied Sciences. 2020; 10(13):4655. https://doi.org/10.3390/app10134655
Chicago/Turabian StyleWang, Gaoxue, Enrique R. Batista, and Ping Yang. 2020. "Water on Actinide Dioxide Surfaces: A Review of Recent Progress" Applied Sciences 10, no. 13: 4655. https://doi.org/10.3390/app10134655
APA StyleWang, G., Batista, E. R., & Yang, P. (2020). Water on Actinide Dioxide Surfaces: A Review of Recent Progress. Applied Sciences, 10(13), 4655. https://doi.org/10.3390/app10134655