How Fishy Is Your Fish? Authentication, Provenance and Traceability in Fish and Seafood by Means of Vibrational Spectroscopy
Abstract
1. Introduction
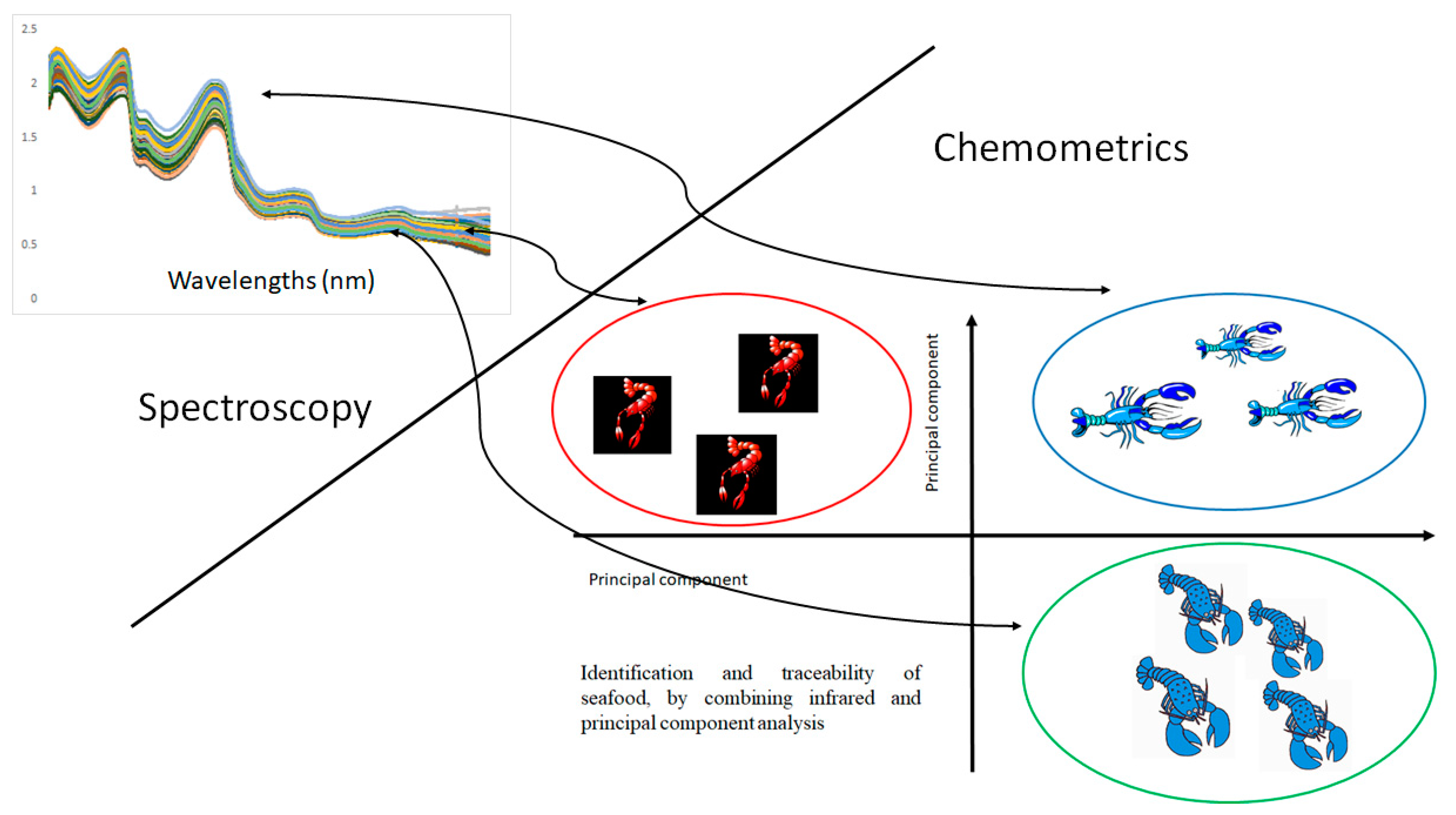
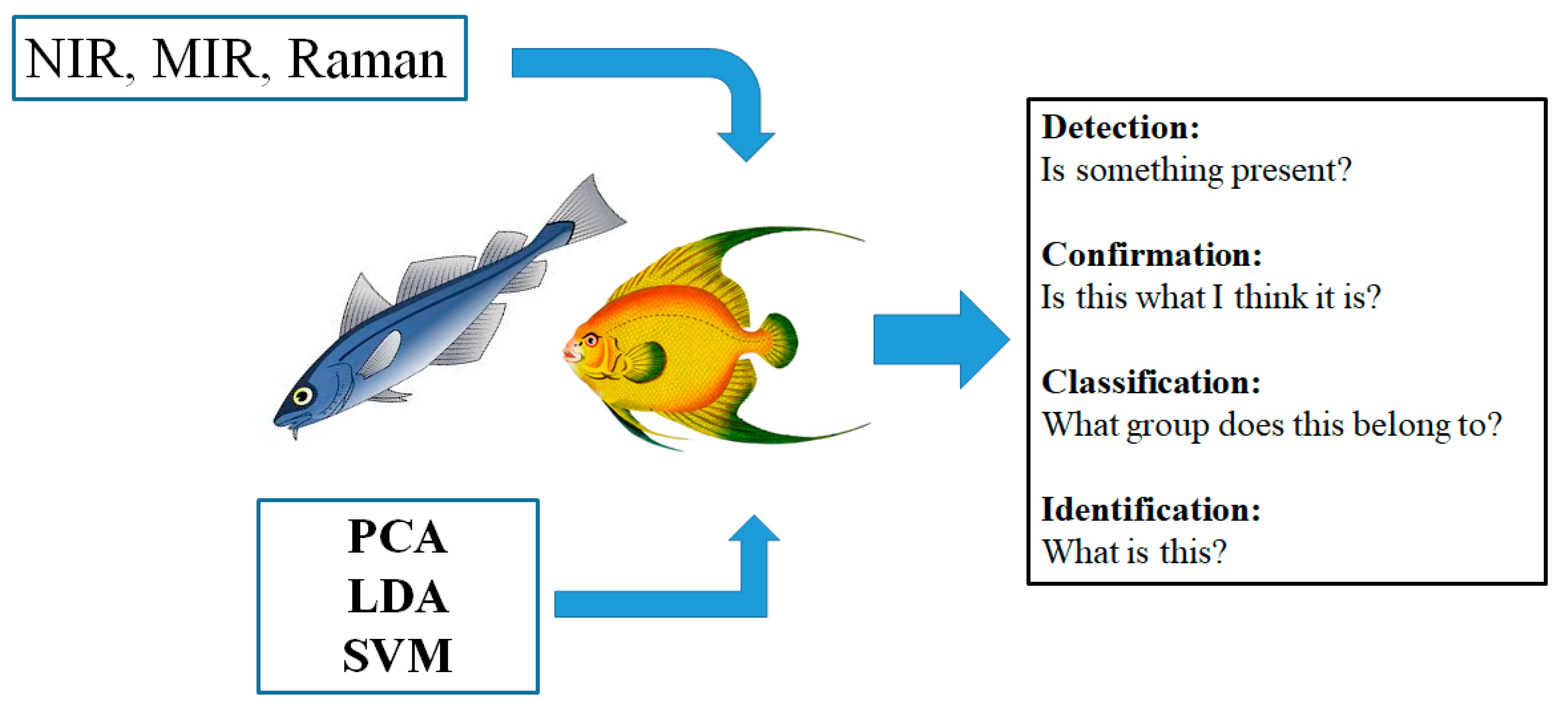
2. The Tools—Chemometrics and Vibrational Spectroscopy
2.1. Chemometrics
2.2. Vibrational Spectroscopy
2.3. Target and Non-Target Analysis
2.4. The Sample (Authentic Sample, Number and Representative Samples)
3. Examples of Chemometrics and Vibrational Spectroscopy Applications in Seafood
3.1. Authentication, Provenance and Traceability
3.2. Freshness, Spoilage and Storage
4. Summary (Present and Future Challenges)
Author Contributions
Funding
Conflicts of Interest
References
- Carcea, M.; Brereton, P.; Hsu, R.; Kelly, S.; Marmiroli, N.; Melini, F.; Soukoulis, C.; Wenping, D. Food authenticity assessment: Ensuring compliance with food legislation and traceability requirements. Qual. Assur. Saf. Crop. Foods 2009, 1, 93–100. [Google Scholar] [CrossRef]
- Gálvez, J.F.; Mejuto, J.; Simal-Gandara, J. Future challenges on the use of blockchain for food traceability analysis. TrAC Trends Anal. Chem. 2018, 107, 222–232. [Google Scholar] [CrossRef]
- Hansen, H.; Onozaka, Y. When diseases hit aquaculture: An experimental study of spillover effects from negative publicity. Mar. Resour. Econ. 2011, 26, 281–291. [Google Scholar] [CrossRef]
- Käferstein, F.K. Foodborne diseases in developing countries: Aetiology, epidemiology and strategies for prevention. Int. J. Environ. Health Res. 2003, 13, S161–S168. [Google Scholar] [CrossRef]
- Raspor, P.; Jevšnik, M. Good nutritional practice from producer to consumer. Crit. Rev. Food Sci. Nutr. 2008, 48, 276–292. [Google Scholar] [CrossRef]
- Marshall, D. Food availability and the European consumer. In Food, People and Society; Springer: Berlin/Heidelberg, Germany, 2001; pp. 317–338. [Google Scholar]
- Muir, J. Managing to harvest? Perspectives on the potential of aquaculture. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 191–218. [Google Scholar] [CrossRef]
- Dabbene, F.; Gay, P.; Tortia, C. Traceability issues in food supply chain management: A review. Biosyst. Eng. 2014, 120, 65–80. [Google Scholar] [CrossRef]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Trienekens, J.; Zuurbier, P. Quality and safety standards in the food industry, developments and challenges. Int. J. Prod. Econ. 2008, 113, 107–122. [Google Scholar] [CrossRef]
- Manning, L.; Baines, R. Effective management of food safety and quality. Br. Food J. 2004, 106, 598–606. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Da Cruz, A.G.; Faria, J.A. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Ortea, I.; O’Connor, G.; Maquet, A. Review on proteomics for food authentication. J. Proteom. 2016, 147, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Ortea, I. Food authentication of seafood species. In Proteomics in Food Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 331–342. [Google Scholar]
- Björkstén, B.; Crevel, R.; Hischenhuber, C.; Løvik, M.; Samuels, F.; Strobel, S.; Taylor, S.; Wal, J.-M.; Ward, R. Criteria for identifying allergenic foods of public health importance. Regul. Toxicol. Pharmacol. 2008, 51, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Falci, K.J.; Gombas, K.L.; Elliot, E.L. Food allergen awareness: An FDA priority. Food Safety Magazine. February–March 2001. Available online: https://www.foodsafetymagazine.com/magazine-archive1/februarymarch-2001/food-allergen-awareness-an-fda-priority/ (accessed on 16 June 2020).
- Spanjersberg, M.; Knulst, A.; Kruizinga, A.; Van Duijn, G.; Houben, G. Concentrations of undeclared allergens in food products can reach levels that are relevant for public health. Food Addit. Contam. Part A 2010, 27, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Allan, G.L. Fish as food: Aquaculture’s contribution. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef]
- Smith, M.D.; Roheim, C.A.; Crowder, L.B.; Halpern, B.S.; Turnipseed, M.; Anderson, J.L.; Asche, F.; Bourillón, L.; Guttormsen, A.G.; Khan, A.; et al. Sustainability and global seafood. Science 2010, 327, 784–786. [Google Scholar] [CrossRef]
- Hellberg, R.S.R.; Morrissey, M.T. Advances in DNA-based techniques for the detection of seafood species substitution on the commercial market. J. Lab. Autom. 2011, 16, 308–321. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, S.Y.; Hanner, R.; Levin, J.; Lu, X. Study of fish products in Metro Vancouver using DNA barcoding methods reveals fraudulent labeling. Food Control 2018, 94, 38–47. [Google Scholar] [CrossRef]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood traceability: Current needs, available tools, and biotechnological challenges for origin certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, J.; Pauly, D. Trade secrets: Renaming and mislabeling of seafood. Mar. Policy 2008, 32, 309–318. [Google Scholar] [CrossRef]
- Teletchea, F. Molecular identification methods of fish species: Reassessment and possible applications. Rev. Fish Biol. Fish. 2009, 19, 265–293. [Google Scholar] [CrossRef]
- Ward, R.D.; Hanner, R.; Hebert, P.D. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009, 74, 329–356. [Google Scholar] [CrossRef]
- Griffiths, A.M.; Sotelo, C.G.; Mendes, R.; Pérez-Martín, R.I.; Schröder, U.; Shorten, M.; Silva, H.; Verrez-Bagnis, V.; Mariani, S. Current methods for seafood authenticity testing in Europe: Is there a need for harmonisation? Food Control 2014, 45, 95–100. [Google Scholar] [CrossRef]
- Hallerman, E.M.; Kapuscinski, A.R. Transgenic fish and public policy: Regulatory concerns. Fisheries 1990, 15, 12–20. [Google Scholar] [CrossRef]
- Hooker, N.H. Food safety regulation and trade in food products. Food Policy 1999, 24, 653–668. [Google Scholar] [CrossRef]
- Echols, M.A. Food safety regulation in the European Union and the United States: Different cultures, different laws. Columbia J. Eur. Law 1998, 4, 525–544. [Google Scholar]
- D’Amico, P.; Armani, A.; Gianfaldoni, D.; Guidi, A. New provisions for the labelling of fishery and aquaculture products: Difficulties in the implementation of Regulation (EU) n. 1379/2013. Mar. Policy 2016, 71, 147–156. [Google Scholar] [CrossRef]
- Hofherr, J.; Martinsohn, J.; Cawthorn, D.; Rasco, B.; Naaum, A.M. Regulatory frameworks for seafood authenticity and traceability. Seaf. Authent. Traceability 2016, 47–82. [Google Scholar] [CrossRef]
- Nguyen, A.V.T.; Wilson, N.L. Effects of Food Safety Standards on Seafood Exports to US, EU and Japan. In Proceedings of the 2009 Annual Meeting, Atlanta, GA, USA, 31 January–3 February 2009. [Google Scholar]
- Regulation, E. No 1379/2013 of the European Parliament and of the Council of 11 December 2013 on the common organisation of the markets in fishery and aquaculture products, amending Council Regulations (EC) No 1184/2006 and (EC) No 1224/2009 and repealing Council Regulation (EC) No 104/2000. Off. J. Eur. Union 2013, 354, 12–14. [Google Scholar]
- Amigo, J.M.; Martí, I.; Gowen, A. Hyperspectral imaging and chemometrics: A perfect combination for the analysis of food structure, composition and quality. Data Handl. Sci. Technol. 2013, 28, 343–370. [Google Scholar]
- Brown, S.D.; Blank, T.B.; Sum, S.T.; Weyer, L.G. Chemometrics. Anal. Chem. 1994, 66, 315–359. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Karoui, R. Analytical methods coupled with chemometric tools for determining the authenticity and detecting the adulteration of dairy products: A review. Trends Food Sci. Technol. 2015, 46, 27–48. [Google Scholar] [CrossRef]
- Workman, J.J.; Mobley, P.R.; Kowalski, B.R.; Bro, R. Review of chemometrics applied to spectroscopy: 1985-95, Part I. Appl. Spectrosc. Rev. 1996, 31, 73–124. [Google Scholar] [CrossRef]
- Cozzolino, D.; Daniel, C. Foodomics and infrared spectroscopy: From compounds to functionality. Curr. Opin. Food Sci. 2015, 4, 39–43. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I. A review on the application of infrared technologies to determine and monitor composition and other quality characteristics in raw fish, fish products, and seafood. Appl. Spectrosc. Rev. 2012, 47, 207–218. [Google Scholar] [CrossRef]
- Cozzolino, D.; Power, A.; Chapman, J. Interpreting and reporting principal component analysis in food science analysis and beyond. Food Anal. Methods 2019, 12, 2469–2473. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Defernez, M.; Kemsley, E.K. The use and misuse of chemometrics for treating classification problems. TrAC Trends Anal. Chem. 1997, 16, 216–221. [Google Scholar] [CrossRef]
- Szymanska, E.; Gerretzen, J.; Engel, J.; Geurts, B.; Blanchet, L.; Buydens, L.M. Chemometrics and qualitative analysis have a vibrant relationship. TrAC Trends Anal. Chem. 2015, 69, 34–51. [Google Scholar] [CrossRef]
- Cheng, J.; Dai, Q.; Sun, D.; Zeng, X.; Liu, D.; Pu, H.-B. Applications of non-destructive spectroscopic techniques for fish quality and safety evaluation and inspection. Trends Food Sci. Technol. 2013, 34, 18–31. [Google Scholar] [CrossRef]
- Ding, R.; Huang, X.; Han, F.; Dai, H.; Teye, E.; Xu, F. Rapid and nondestructive evaluation of fish freshness by near infrared reflectance spectroscopy combined with chemometrics analysis. Anal. Methods 2014, 6, 9675–9683. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.M.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Almaraz-Abarca, N.; Castañeda-Pérez, E. Application of MIR-FTIR spectroscopy and chemometrics to the rapid prediction of fish fillet quality. CyTA-J. Food 2014, 12, 369–377. [Google Scholar] [CrossRef]
- Karoui, R.; Downey, G.; Blecker, C. Mid-infrared spectroscopy coupled with chemometrics: A tool for the analysis of intact food systems and the exploration of their molecular structure—Quality relationships—A review. Chem. Rev. 2010, 110, 6144–6168. [Google Scholar] [CrossRef]
- Weeranantanaphan, J.; Downey, G.; Allen, P.; Sun, D. A review of near infrared spectroscopy in muscle food analysis: 2005–2010. J. Near Infrared Spectrosc. 2011, 19, 61–104. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.; Sun, D. NIR spectroscopy and imaging techniques for evaluation of fish quality—A review. Appl. Spectrosc. Rev. 2013, 48, 609–628. [Google Scholar] [CrossRef]
- Nilsen, H.A.; Esaiassen, M.; Heia, K.; Sigernes, F. Visible/near-infrared spectroscopy: A new tool for the evaluation of fish freshness? J. Food Sci. 2002, 67, 1821–1826. [Google Scholar] [CrossRef]
- Nilsen, H.A.; Heia, K. VIS/NIR spectroscopy. Fish. Prod. 2009, 2009, 89–104. [Google Scholar] [CrossRef]
- Ellis, D.I.; Eccles, R.; Xu, Y.; Griffen, J.; Muhamadali, H.; Matousek, P.; Goodall, I.; Goodacre, R. Through-container, extremely low concentration detection of multiple chemical markers of counterfeit alcohol using a handheld SORS device. Sci. Rep. 2017, 7, 12082. [Google Scholar] [CrossRef]
- Zheng, J.; He, L. Surface-enhanced raman spectroscopy for the chemical analysis of food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 317–328. [Google Scholar] [CrossRef]
- Ellis, D.I.; Muhamadali, H.; Haughey, S.A.; Elliott Ch, T.; Goodacre, R. Point-and-shoot: Rapid quantitative detection methods for on-site food fraud analysis—Moving out of the laboratory and into the food supply chain. Anal. Methods 2015, 7, 9401–9414. [Google Scholar] [CrossRef]
- Ozaki, Y.; Sasic, S. Introduction to raman spectroscopy. In Pharmaceutical Applications of Raman Spectroscopy; Sasic, S., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 1–28. [Google Scholar]
- Alamprese, C.; Casiraghi, E. Application of FT-NIR and FT-IR spectroscopy to fish fillet authentication. LWT Food Sci. Technol. 2015, 63, 720–725. [Google Scholar] [CrossRef]
- Esslinger, S.; Riedl, J.; Fauhl-Hassek, C. Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Res. Int. 2014, 60, 189–204. [Google Scholar] [CrossRef]
- McGrath, T.F.; Haughey, S.; Patterson, J.; Fauhl-Hassek, C.; Donarski, J.; Alewijn, M.; Van Ruth, S.M.; Elliott, C.T. What are the scientific challenges in moving from targeted to non-targeted methods for food fraud testing and how can they be addressed?—Spectroscopy case study. Trends Food Sci. Technol. 2018, 76, 38–55. [Google Scholar] [CrossRef]
- Buck, E.H. Seafood Marketing: Combating Fraud and Deception 2007; Congressional Research Service; Library of Congress: Washington, DC, USA, 2007. [Google Scholar]
- Cawthorn, D.-M.; Steinman, H.A.; Hoffman, L.C. A high incidence of species substitution and mislabelling detected in meat products sold in South Africa. Food Control 2013, 32, 440–449. [Google Scholar] [CrossRef]
- Everstine, K.; Spink, J.; Kennedy, S. Economically Motivated Adulteration (EMA) of food: Common characteristics of EMA incidents. J. Food Prot. 2013, 76, 723–735. [Google Scholar] [CrossRef]
- Reilly, A. Overview of food fraud in the fisheries sector. FAO Fish. Aquac. Circ. 2018, C1165, I-21. [Google Scholar]
- Gayo, J. Species Authenticity and Detection of Economic Adulteration of Atlantic Blue Crab Meat Using VIS/NIR Spectroscopy. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2006. [Google Scholar]
- Gayo, J.; Hale, S.A. Detection and quantification of species authenticity and adulteration in crabmeat using visible and near-infrared spectroscopy. J. Agric. Food Chem. 2007, 55, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Gayo, J.; Hale, S.A.; Blanchard, S.M. Quantitative analysis and detection of adulteration in crab meat using visible and near-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, K.; Bremner, H. Exploration of the use of NIR reflectance spectroscopy to distinguish and measure attributes of conditioned and cooked shrimp (Pandalus borealis). LWT Food Sci. Technol. 2001, 34, 533–541. [Google Scholar] [CrossRef]
- Qu, J.-H.; Cheng, J.; Sun, D.; Pu, H.; Wang, Q.-J.; Ma, J. Discrimination of shelled shrimp (Metapenaeus ensis) among fresh, frozen-thawed and cold-stored by hyperspectral imaging technique. LWT Food Sci. Technol. 2015, 62, 202–209. [Google Scholar] [CrossRef]
- Wu, D.; Shi, H.; He, Y.; Yu, X.; Bao, Y. Potential of hyperspectral imaging and multivariate analysis for rapid and non-invasive detection of gelatin adulteration in prawn. J. Food Eng. 2013, 119, 680–686. [Google Scholar] [CrossRef]
- Ottavian, M.; Facco, P.; Fasolatoa, L.; Novelli, E.; Mirisola, M.; Perini, M.; Barolo, M. Use of near-infrared spectroscopy for fast fraud detection in seafood: Application to the authentication of wild European Sea Bass (Dicentrarchus labrax). J. Agric. Food Chem. 2012, 60, 639–648. [Google Scholar] [CrossRef]
- Lv, H.; Xu, W.; You, J.; Xiong, S. Classification of freshwater fish species by linear discriminant analysis based on near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 54–62. [Google Scholar] [CrossRef]
- Grassi, S.; Casiraghi, E.; Alamprese, C. Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chem. 2018, 243, 382–388. [Google Scholar] [CrossRef]
- O’Brien, N.; Hulse, C.A.; Pfeifer, F.; Siesler, H.W. Near infrared spectroscopic authentication of seafood. J. Near Infrared Spectrosc. 2013, 21, 299–305. [Google Scholar] [CrossRef]
- Wu, T.; Zhong, N.; Yang, L. Identification of adulterated and non-adulterated norwegian salmon using FTIR and an improved PLS-DA method. Food Anal. Methods 2017, 11, 1501–1509. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Pezoa, J.E.E. Spectral vision system for discriminating small pelagic species caught by small-scale fishing. In Proceedings of the Conference on Infrared Sensors, Devices, and Applications VIII, San Diego, CA, USA, 22–23 August 2018. [Google Scholar]
- Rašković, B.; Heinke, R.; Rösch, P.; Popp, J. The potential of Raman spectroscopy for the classification of fish fillets. Food Anal. Methods 2015, 9, 1301–1306. [Google Scholar] [CrossRef]
- Dai, Q.; Cheng, J.; Sun, D.; Pu, H.; Zeng, X.; Xiong, Z. Potential of visible/near-infrared hyperspectral imaging for rapid detection of freshness in unfrozen and frozen prawns. J. Food Eng. 2015, 149, 97–104. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Chen, Z.; You, J.; Xiong, S. Evaluation of freshness in freshwater fish based on near infrared reflectance spectroscopy and chemometrics. LWT 2019, 106, 145–150. [Google Scholar] [CrossRef]
- Saraiva, C.; Vasconcelos, H.; De Almeida, J. A chemometrics approach applied to Fourier transform infrared spectroscopy (FTIR) for monitoring the spoilage of fresh salmon (Salmo salar) stored under modified atmospheres. Int. J. Food Microbiol. 2017, 241, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Washburn, N.K.; Stormo, S.K.; Skjelvareid, M.H.; Hei, K. On-invasive assessment of packaged cod freeze-thaw history by hyperspectral imaging. J. Eng. 2017, 205, 64–73. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, D.; He, Y.; Liu, F.; Sun, D.-W. Application of visible and near infrared hyperspectral imaging to differentiate between fresh and frozen–thawed fish fillets. Food Bioprocess Technol. 2013, 6, 2931–2937. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Chu, B.-Q.; Ye, Q.; Liu, X.-M.; Luo, W. Classification of Fishness Based on Hyperspectra. Imaging Technol. Spectrosc. Spectr. Anal. 2018, 38, 559–563. [Google Scholar]
- He, H.-J.; Wu, D.; Sun, D.-W. Nondestructive spectroscopic and imaging techniques for quality evaluation and assessment of fish and fish products. Crit. Rev. Food Sci. Nutr. 2015, 55, 864–886. [Google Scholar] [CrossRef]
- Velioğlua, H.M.; Temiz, H.T.; Boyaci, I.H. Differentiation of fresh and frozen-thawed fish samples using Raman spectroscopy coupled with chemometric analysis. Food Chem. 2015, 172, 283–290. [Google Scholar] [CrossRef]
- Bateman, T.S.; Mann, M.E. The supply of climate leaders must grow. Nat. Clim. Chang. 2016, 6, 1052–1054. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Power, A.; Cozzolino, D. How Fishy Is Your Fish? Authentication, Provenance and Traceability in Fish and Seafood by Means of Vibrational Spectroscopy. Appl. Sci. 2020, 10, 4150. https://doi.org/10.3390/app10124150
Power A, Cozzolino D. How Fishy Is Your Fish? Authentication, Provenance and Traceability in Fish and Seafood by Means of Vibrational Spectroscopy. Applied Sciences. 2020; 10(12):4150. https://doi.org/10.3390/app10124150
Chicago/Turabian StylePower, Aoife, and Daniel Cozzolino. 2020. "How Fishy Is Your Fish? Authentication, Provenance and Traceability in Fish and Seafood by Means of Vibrational Spectroscopy" Applied Sciences 10, no. 12: 4150. https://doi.org/10.3390/app10124150
APA StylePower, A., & Cozzolino, D. (2020). How Fishy Is Your Fish? Authentication, Provenance and Traceability in Fish and Seafood by Means of Vibrational Spectroscopy. Applied Sciences, 10(12), 4150. https://doi.org/10.3390/app10124150





