Photodynamic Properties of CdSe/CdS Quantum Dots in Intracellular Media
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Quantum Dots CdSe/CdS
2.1.2. Bio-Cells
2.2. Cell Culture and In Vitro Experiment
2.3. Apparatus
3. Results and Discussion
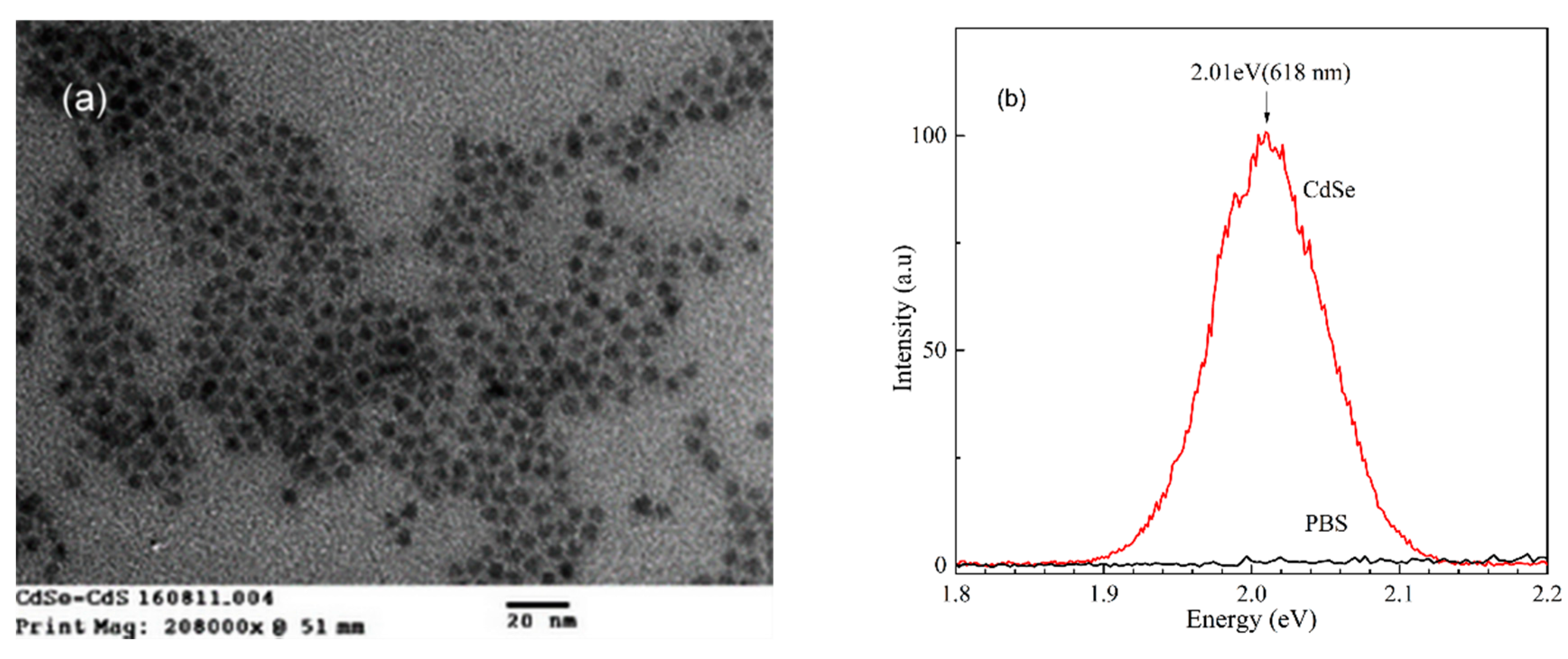
3.1. Characteristics of CdSe/CdS QDs
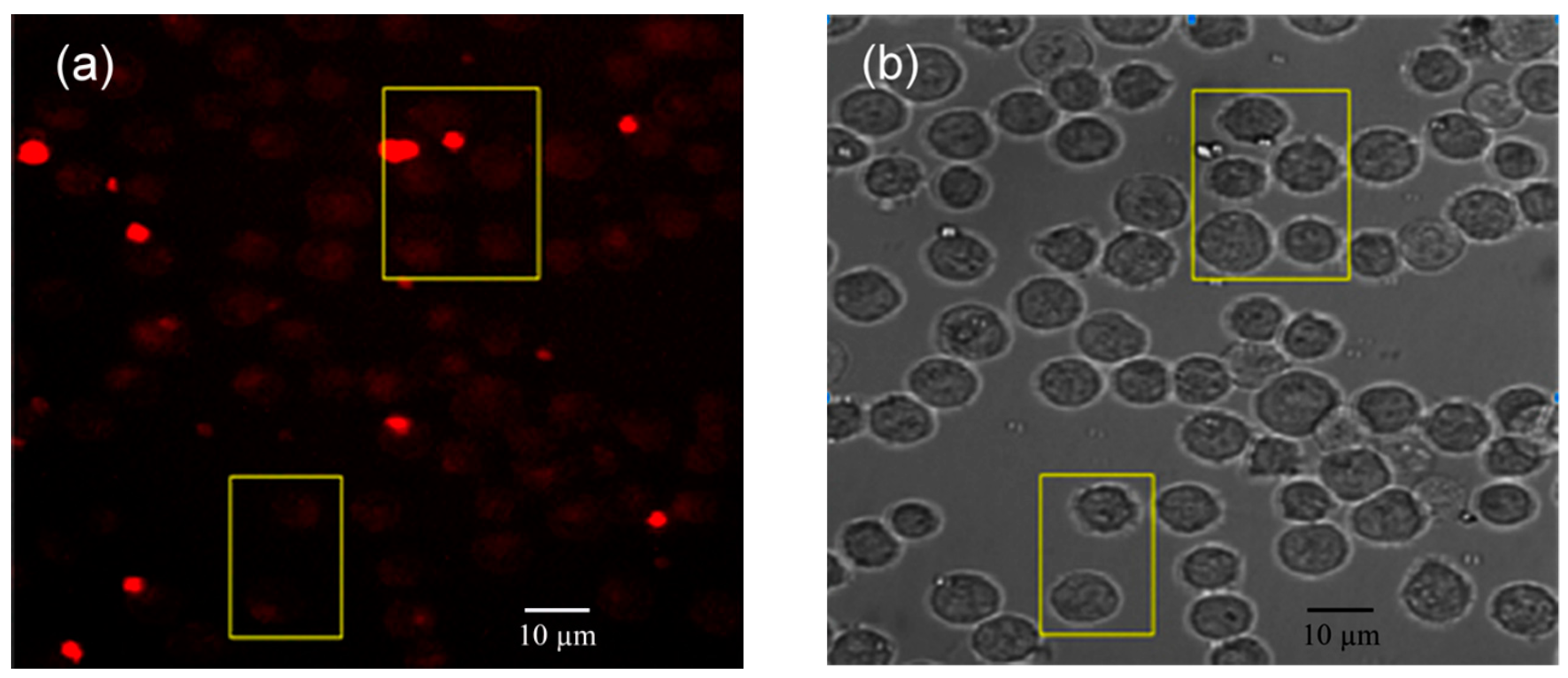
3.2. Fluorescence Microscopy Analysis
3.3. Fluorescence Spectra
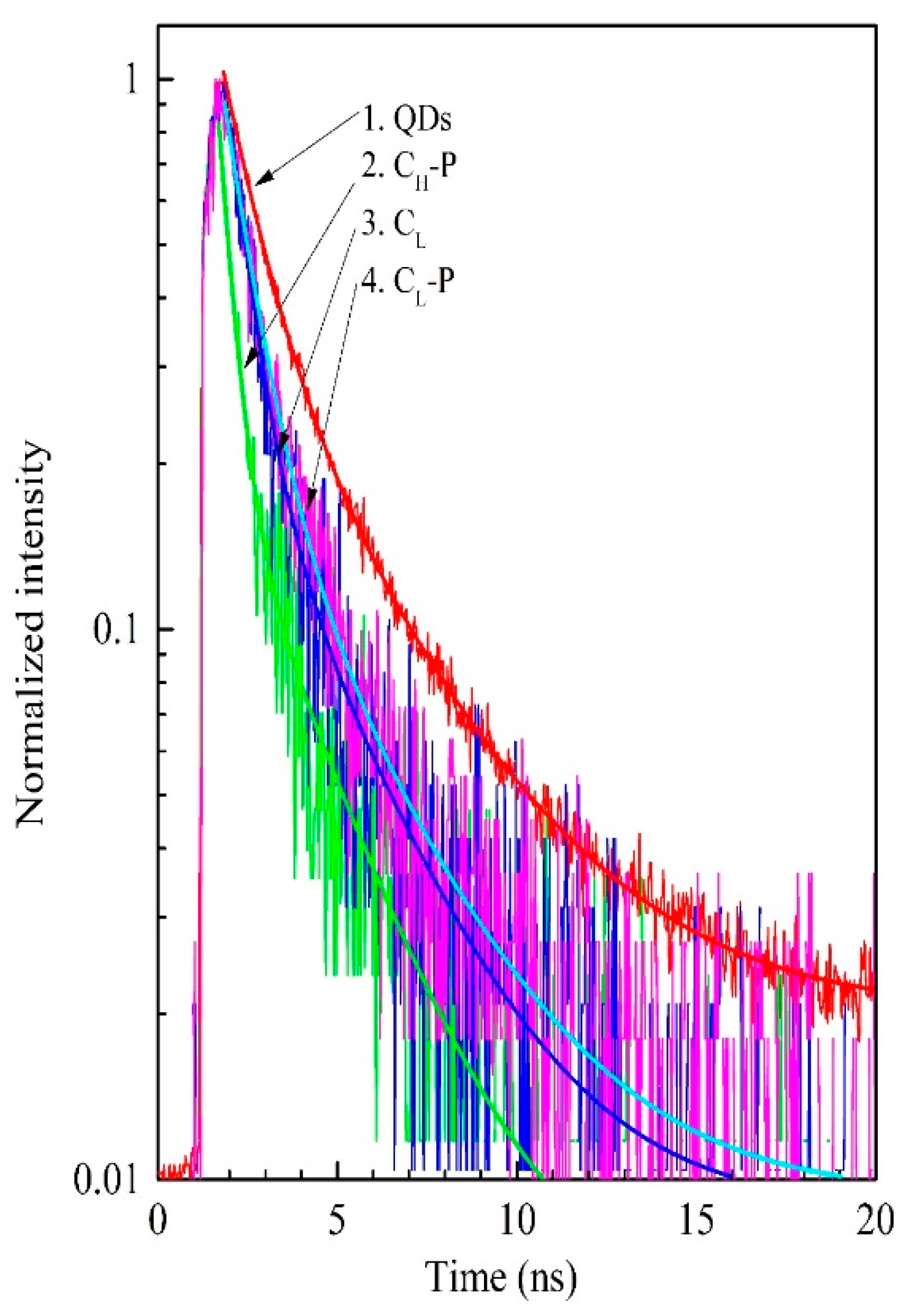
3.4. Fluorescence Lifetime
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallite. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Chu, V.H.; Nghiem, T.H.L.; Le, T.H.; Vu, D.L.; Tran, H.N.; Vu, T.K.L. Synthesis and optical properties of water soluble CdSe/CdS quantum dots for biological applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025017. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Liu, H.J.; Liu, J.Q.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.F.; Peale, F.; Bruchez, M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef]
- Han, M.Y.; Gao, X.H.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.D.T.; Pham, S.T.; Tran, T.K.C.; Dinh, D.K.; Nguyen Q, L. CdTe quantum dots for an application in the life sciences. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 045009. [Google Scholar]
- Nguyen, N.H.; Duong, T.G.; Hoang, V.N.; Pham, N.T.; Dao, T.C.; Pham, T.N. Synthesis and application of quantum dots-based biosensor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 015015. [Google Scholar] [CrossRef][Green Version]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Dimitrijevic, J.; Krapf, L.; Wolter, C.; Schmidtke, C.; Merkl, J.P.; Jochum, T.; Kornowski, A.; Schüth, A.; Gebert, A.; Hüttmann, G.; et al. CdSe/CdS-quantum Rods: Fluorescent Probes for in Vivo Two-Photon Laser Scanning Microscopy. Nanoscale 2014, 6, 10413–10422. [Google Scholar] [CrossRef]
- Qi, L.; Gao, X. Emerging application of quantum dots for drug delivery and therapy. Expert Opin. Drug Deliv. 2008, 5, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yee, D.; Wang, C. Quantum dots for cancer diagnosis and therapy: Biological and clinical perspectives. Nanomedicine 2008, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Medintz, I.L.; Algar, W.R. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed]
- Boeneman, K.; Delehanty, J.B.; Blanco-Canosa, J.B.; Susumu, K.; Stewart, M.H.; Oh, E.; Huston, A.L.; Dawson, G.; Ingale, S.; Walters, R.; et al. Selecting improved peptidyl motifs for cytosolic delivery of disparate protein and nanoparticle materials. ACS Nano 2013, 7, 3778–3796. [Google Scholar] [CrossRef]
- Spillmann, C.M.; Ancona, M.G.; Buckhout-White, S.; Algar, W.R.; Stewart, M.H.; Susumu, K.; Huston, A.L.; Goldman, E.R.; Medintz, I.L. Achieving effective terminal exciton delivery in quantum dot antenna-sensitized multistep DNA photonic wires. ACS Nano 2013, 7, 7101–7118. [Google Scholar] [CrossRef]
- Medintz, I.L.; Farrell, D.; Susumu, K.; Trammell, S.A.; Deschamps, J.R.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H.M. Multiplex charge-transfer interactions between quantum dots and peptide-bridged ruthenium complexes. Anal. Chem. 2009, 81, 4831–4839. [Google Scholar] [CrossRef]
- Claussen, J.C.; Algar, W.R.; Hildebrandt, N.; Susumu, K.; Ancona, M.G.; Medintz, I.L. Biophotonic logic devices based on quantum dots and temporally-staggered Förster energy transfer relays. Nanoscale 3013, 5, 12156–12170. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Boeneman, K.; Bradburne, C.E.; Robertson, K.; Medintz, I.L. Quantum dots: A powerful tool for understanding the intricacies of nanoparticle-mediated drug delivery. Exp. Opin. Drug Deliv. 2009, 6, 1091–1112. [Google Scholar] [CrossRef]
- Medintz, I.L.; Mattoussi, H. Quantum dots-based resonance energy transfer and its growing application in biology. Phys. Chem. Chem. Phys. 2009, 11, 17–45. [Google Scholar] [CrossRef]
- Shan, J.; Yanxi, H.; Zhanjun, G.; Lei, L.; Hai-Chen, W. Application of Quantum Dots in Biological Imaging. J. Nanomater. 2011, 2011, 834139. [Google Scholar]
- Mazumder, S.; Dey, R.; Mitra, M.K.; Mukherjee, S.; Das, G.C. Biofunctionalized quantum dots in biology and medicine. J. Nanomater. 2009, 2009, 815734. [Google Scholar] [CrossRef]
- Sakmann, B.; Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu. Rev. Physiol. 1984, 46, 455–472. [Google Scholar] [CrossRef]
- Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology; Wiley: London, UK, 2003. [Google Scholar]
- Hai, A.; Shappir, J.; Spira, M.E. In-cell recordings by extracellular microelectrodes. Nat. Methods 2010, 7, 200–202. [Google Scholar] [CrossRef]
- Schrlau, M.G.; Dun, N.J.; Bau, H.H. Cell electrophysiology with carbon nanopipettes. ACS Nano 2009, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- De Asis, E.D.; Leung, J.; Wood, S.; Nguyen, C.V. High spatial resolution single multiwalled carbon nanotube electrode for stimulation, recording, and whole cell voltage clamping of electrically active cells. Appl. Phys. Lett. 2009, 95, 153701. [Google Scholar] [CrossRef]
- Huang, J.-A.; Caprettini, V.; Zhao, Y.; Melle, G.; Maccaferri, N.; Deleye, L.; Zambrana-Puyalto, X.; Ardini, M.; Tantussi, F.; Dipalo, F.; et al. On-Demand Intracellular Delivery of Single Particles in Single Cells by 3D Hollow Nanoelectrodes. Nano Lett. 2019, 19, 722–731. [Google Scholar] [CrossRef]
- Duan, X.; Gao, R.; Xie, P.; Cohen-Karni, T.; Qing, Q.; Choe, H.S.; Tian, B.; Jiang, X.; Lieber, C.M. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nature Nanotech. 2012, 7, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Caprettini, V.; Huang, J.-A.; Moia, F.; Jacassi, A.; Gonano, C.A.; Maccaferri, N.; Capozza, R.; Dipalo, M.; Angelis, F.D. Enhanced Raman Investigation of Cell Membrane and Intracellular Compounds by 3D Plasmonic Nanoelectrode Arrays. Adv. Sci. 2018, 5, 1800560. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 38–44. [Google Scholar] [CrossRef]
- Ardini, M.; Huang, J.; Sánchez, C.S.; Mousavi, M.Z.; Caprettini, V.; Maccaferri, N.; Melle, G.; Bruno, G.; Pasquale, L.; Garoli, D.; et al. Live Intracellular Biorthogonal Imaging by Surface Enhanced Raman Spectroscopy using Alkyne-Silver Nanoparticles Clusters. Sci Rep. 2018, 8, 12652. [Google Scholar] [CrossRef]
- Eberhardt, K.; Stiebing, C.; Matthäus, C.; Schmitt, M.; Popp, J. Advantages and limitations of Raman spectroscopy for molecular diagnostics: An update. Expert Rev. Mol. Diagn. 2015, 15, 773–787. [Google Scholar] [CrossRef]
- Zambrana-Puyalto, X.; Maccaferri, N.; Ponzellini, P.; Giovannini, G.; Angelisa, F.D.; Garoli, D. Site-selective functionalization of plasmonic nanopores for enhanced fluorescence emission rate and Forster resonance energy transfer. Nanoscale Adv. 2019, 1, 2454. [Google Scholar] [CrossRef]
- Biju, V.; Itoh, T.; Ishikawa, M. Delivering quantum dots to cells: Bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010, 39, 3031–3056. [Google Scholar] [CrossRef] [PubMed]
- Breger, J.; Delehanty, J.B.; Medintz, I.L. Continuing progress toward controlled intracellular delivery of semiconductor quantum dots. Wiley Interdiscip. Rev Nanomed Nanobiotechnol. 2015, 7, 131–151. [Google Scholar] [CrossRef]
- Tan, S.J.; Jana, N.R.; Gao, S.; Patra, P.K.; Ying, J.Y. Surface-ligand-dependent cellular interaction, subcellular localization, and cytotoxicity of polymer-coated quantum dots. Chem. Mater. 2010, 22, 2239–2247. [Google Scholar] [CrossRef]
- Medintz, I.L.; Clapp, A.R.; Mattoussi, H.; Goldman, E.R.; Fisher, B.; Mauro, J.M. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat. Mater. 2003, 2, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.L.; Clarke, S.J.; Khatchadourian, R.K.; Bahcheli, D.M.; Bradforth, S.E. Mechanisms of Quantum Dot Interactions with Biological Cells. In Proceedings of the SPIE International Society for Optical Engineering, Torrance, CA, USA, 12 October 2005. [Google Scholar] [CrossRef]
- Medintz, I.L.; Goldman, E.R.; Lassman, M.E.; Mauro, J.M. A fluorescence resonance energy transfer sensor based on maltose binding protein. Bioconjug. Chem. 2003, 14, 909–918. [Google Scholar] [CrossRef]
- Abraham, B.G.; Tkachenko, N.V.; Santala, V.; Lemmetyinen, H.; Karp, M. Bidirectional Fluorescence Resonance Energy Transfer (FRET) in Mutated and Chemically Modified Yellow Fluorescent Protein (YFP). Bioconjug. Chem. 2011, 22, 227–234. [Google Scholar] [CrossRef]

| Sample Name | Cell Concentration (cell/mL) | PEG |
|---|---|---|
| CL | No | |
| CL-P | Yes | |
| CH | No | |
| CH-P | Yes |
| Sample | (ns) |
|---|---|
| QDs in water | 1.92 |
| CL | 1.06 |
| CL-P | 1.21 |
| CH | // |
| CH-P | 0.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.B.; Vu, T.B.; Nguyen, D.C.; Do, T.T.; Pham, H.M.; Cadatal-Raduban, M. Photodynamic Properties of CdSe/CdS Quantum Dots in Intracellular Media. Appl. Sci. 2020, 10, 3988. https://doi.org/10.3390/app10113988
Nguyen TB, Vu TB, Nguyen DC, Do TT, Pham HM, Cadatal-Raduban M. Photodynamic Properties of CdSe/CdS Quantum Dots in Intracellular Media. Applied Sciences. 2020; 10(11):3988. https://doi.org/10.3390/app10113988
Chicago/Turabian StyleNguyen, Thanh Binh, Thi Bich Vu, Dinh Cong Nguyen, Thi Thao Do, Hong Minh Pham, and Marilou Cadatal-Raduban. 2020. "Photodynamic Properties of CdSe/CdS Quantum Dots in Intracellular Media" Applied Sciences 10, no. 11: 3988. https://doi.org/10.3390/app10113988
APA StyleNguyen, T. B., Vu, T. B., Nguyen, D. C., Do, T. T., Pham, H. M., & Cadatal-Raduban, M. (2020). Photodynamic Properties of CdSe/CdS Quantum Dots in Intracellular Media. Applied Sciences, 10(11), 3988. https://doi.org/10.3390/app10113988






