Abstract
Indiscriminate overuse of liquid fertilizer and arsenic (As) contaminated soil by abandoned mines is one of the important environmental issues in Korea. This study was carried out to solve these two problems. Amendments (limestone, red mud and acid mine drainage sludge), liquid fertilizer and plant vegetation (Hairy vetch; Vicia villosa Roth) were simultaneously treated. Some soil chemical properties (pH, dissolved organic carbon, inorganic nitrogen content, and bioavailable As), soil respiration and enzyme activity (urease activity and dehydrogenase activity) were determined for chemical and biological assessment. Amendments decreased bioavailable As in soil, and acid mine drainage sludge had the best reduction efficiency in alkali soil. Liquid fertilizer affects not only soil chemical properties but also biological properties. Through multiple regression analysis, the rhizosphere effect through plant cultivation using specific root length index was reflected in the result of soil microbial and enzyme activity. In the reclamation of As-contaminated soil, the synergistic effect of multiple treatments could be confirmed. In particular, biological assessment indicators could be useful when evaluating the complex treatment of various restoration techniques, including the phytoremediation method. Based on these results, a long-term follow-up study on the field scale will be possible.
1. Introduction
In Korea, regarding the disturbance of the soil environment, two major issues that are still in progress and have many problems to be solved are the trace element contamination by abandoned mines and the exhaustion of surplus liquid fertilizer into soil.
Domestic livestock breeding scale is becoming commercialized and large-scale as meat consumption increases, and the amount of livestock manure is also increasing. More than 90% of the livestock manure is converted into compost and liquid fertilizer, and they are put into agricultural soil [1]. However, the area of agricultural soil to which compost and liquid fertilizer would be input is limited and the storage cost of liquid fertilizer has been accumulated. Thus, surplus liquid fertilizer is sprayed indiscriminately, contaminating the soil and ground water [2]. Intensive nutrient input generates a large amount of ammonia emission in a short time, which is a very problematic global issue, and of course, Korea is no exception recently [2,3,4]. Therefore, there is an urgent need for healthy and sustainable consumption of surplus fertilizer, and these issues may be global, not just one country’s.
More than 85% of the approximately 5400 mines distributed all over the country are poorly managed and abandoned, threatening the environment and the health of nearby residents [5]. There are three main pathways that cause health risks for residents in an abandoned mine area: (1) inhalation or skin adsorption of soil particles containing toxic trace elements, (2) drinking contaminated ground water, and (3) ingestion of contaminated food crops [6,7]. Among various remediation technologies, stabilization methods using amendments are being actively applied to reduce mobility, bioavailability and toxicity of trace elements in soil [8]. Because an abandoned mine area, including waste rock and mine tailing, is vulnerable to wind dispersal and water erosion, the revegetation method is also recommended. However, such an area has poor physicochemical properties of soil and a lack of nutrients, which makes it difficult to introduce vegetation [9]. For this reason, not only stabilizers that reduce trace elements toxicity but also fertilizers that increase mineral nutrient and soil fertility were all used in combination, and this is called aided-phytostabilization. To conserve limited resources and to maintain sustainable environmental management, the application of industrial and agricultural byproducts is highly recommended [10]. Chiang et al. [11] used liquid fertilizer from food waste for the reclamation of zinc-contaminated soil, resulting in increases in both soil organic matter and ammonium ion content. Kaimi et al. [12] also used liquid fertilizer for enhancement ryegrass growth in contaminated soil. Zhang et al. [13] reported that although it was not liquid fertilizer, solid type compost was treated within mine tailings with high lead and zinc concentrations to grow rye grass and to improve the physico-chemical properties of the tailings. These previous studies have confirmed that agricultural byproducts such as compost and liquid fertilizer could promote plant growth in heavy metal contaminated sites. In particular, considering the domestic situation that closed mines are distributed all over the country, and fertilizers originating from livestock manure are also produced all over the country, the applicability of agricultural byproducts to the remediation of abandoned mine areas can be examined.
Thus, the aim of this study is to evaluate the effects of incorporation of amendments (limestone, red mud, acid mine drainage sludge) and liquid fertilizer, into highly As-contaminated soil on the initial introduction of vegetation, and the effects of vegetation on soil microbiology.
2. Materials and Methods
2.1. Experimental Set-Up
Arsenic (As) contaminated surface soil was collected near Gilgok mine at the Gangwon province, Republic of Korea (37°36′51.4″ N, 127°44′01.1″ E). The soil sampling site consisted of forest land adjacent to waste rock and mine tailing dump in an abandoned mining area and the soil sample was air-dried and passed through a 4 mm sieve. Three types of amendments were examined: limestone (LS), red mud (RM) and acid mine drainage sludge (AMDS). The LS, RM and AMDS were obtained from industrial plants and the sludge from an acid mine drainage treatment facility, respectively, at the Hamtae mine in Gangwon Province, Korea. All amendments were dried at 60 °C over 48 h, ground, and sieved to less than 0.5 mm. Livestock liquid fertilizer was collected from public manure resource facility in Gangwon province, Republic of Korea, and the collected sample was stored at −60 °C until used in experiments.
The experimental design and treatments are summarized in Table 1. In this study, three types of treatments were applied: amendments types, liquid fertilizer treatment option and hairy vetch cultivation treatment options. It could be broadly divided into 3 groups, each consisting of 3 amendment treatment and control. The amendments were applied to soil at a 3% w/w ratio and were mixed thoroughly for homogeneity; the samples were equilibrated for 4 weeks while maintaining the soil moisture at approximately 60% of the water holding capacity. After 4 weeks, liquid fertilizer was applied to soil at 200 kg-N ha−1 ratio in half of the amendments treated soils and aged for 1 week. After 1 week, seed of hairy vetch (Vicia villosa Roth, Leguminosae family) was planted directly into the treated soils (5 seeds per pot, 3 pots per treatment). The pot had a 1 kg capacity, 20 cm diameter, and 20 cm height. The seed germination and cultivation were conducted under a controlled growth chamber (16 h of daylight and 8 h of darkness per day with 23 ± 2 °C). After germination, 1 or 2 seedlings were removed to unify the number of seedlings into 3 per all pot. After 4 weeks of cultivation, all seedlings were harvested and washed with deionized water and root elongation was immediately measured using a desktop scanner (Epson Perfection V700 Photo, Sewashi, Japan,) and image analyzer program (WinRhizo 5.0a, Reagent, Canada). After determining the root elongation, the fresh weight was also measured.

Table 1.
Experimental design and process.
2.2. Soil, Amendments, Liquid Fertilizer Characteristics
For soil chemical analysis, soil samples were air dried and passed through a 2 mm sieve after hairy vetch cultivation. The pH and electrical conductivity (EC) of the soils were measured in a 1:5 suspension of solid:water using a combined pH-EC meter (Thermo Orion 920A, Thermo Fisher Scientific, Waltham, MA, USA). The total concentration of As was determined by digesting soil sample with aqua regia, a mixture of HCl and HNO3, in accordance with ISO 11466 [14]. The bioavailability of As was evaluated by extraction with a 0.5 M CaCl2 solution according to the procedure of Esnaola and Millan [15]. The As concentration in solution was determined using an inductively coupled plasma optical emission spectrometer (IPC-OES, Agilent, Santa Clara, CA, USA). Dissolved organic carbon (DOC) was analyzed using an automatic total organic carbon analyzer (Shimadzu, TOC-VCPH, Tokyo, Japan) after shaking 10 g of soil and 100 mL of deionized water for 6 h [16]. To confirm the changes in inorganic nitrogen content by liquid fertilizer and plant cultivation, ammonium and nitrate ion were determined separately using Kjeldahl distillation (Distillation Unit B-324, BÜCHI, Flawil, Switzerland) method after extraction with 2 M KCl solution [17]. The pH, EC and total As concentration of the amendments were measured in the same methods as the soil analysis method. The pH and EC of liquid fertilizer were also determined using the same pH-EC meter. Total nitrogen (TN) and total ammoniacal nitrogen (TAN) contents of liquid fertilizer were determined using a Kjeldahl digester (KjelDigester K-446, BÜCHI, Flawil, Switzerland), distillation and titration method.
2.3. Soil Microbial and Enzyme Activity for Biological Assessment
To comprehensively evaluate the effects of the amendment, liquid fertilizer and plant cultivation on soil quality, biological assessments were also conducted. After hairy vetch cultivation, soil samples were kept moist at <4 °C. Just before the experiment, the soil samples were sieved through a 2 mm sieve and water content was then determined separately for the expression of activity based on dry weight. Soil total microbial activity was determined using the soil respiration method (SRA) by Jäggi [18]. Wet soil was placed in the center of the closed bottle (250 mL) containing 20 mL 0.05 M NaOH and incubated 24 h at 26 °C. After incubation, concentration of CO2 captured by NaOH was measured using 0.05 M HCl. Dehydrogenase activity (DHA) was assayed by the reduction of 2,3,5-triphenyltertrazolium chloride (TTC) to triphenylformazan (TPF) [19] and urease activity (URA) was assayed by the reduction of urea to ammonium [20]. Both enzyme activities were determined using a spectrophotometer (Shimadzu, UV-1650PC, Tokyo, Japan).
2.4. Statistical Analysis
All of the determinations were performed in triplicate for each sample. One-way analysis of variance (one-way ANOVA) was used to compare the means of the different treatments. Where significant p-values (p < 0.05) were obtained, the differences between the means were evaluated using Tukey’s test. The relationships among the experimental results were evaluated using stepwise multiple regression. The data were analyzed using the SAS program (SAS 9.4, SAS Institute Inc., Cary, NC, USA).
3. Results and Discussion
3.1. Basic Properties of Soil, Amendments and Liquid Fertilizer
The pH of soil, LS, RM, AMDS and liquid fertilizer (LF) were 8.3, 11.3, 12.4, 8.4 and 9.0, respectively. The EC of soil, LS, RM, AMDS and liquid fertilizer were 0.2, 8.8, 8.4, 3.8 and 23.5 ds·m−1, respectively. Total As concentration of soil was 2998.4 mg·kg−1, which was much higher than worrisome level and countermeasure level in Korean soil regulation (50 and 150 mg·kg−1, respectively, for forest land), and As was also detected only in LS as 6.8 mg·kg−1, not for RM and AMDS. The concentration TN and TAN of LF were 2087 and 1798 mg·L−1, respectively. Since the fraction of ammonium that is easily absorbed by plant root among TN is more than 85%, it seems that LF easily supplies available nitrogen to plants when treated in soil [21].
3.2. Effects of Treatments on the Soil Characteristics
For the non-cultivation and non-liquid fertilizer (NCNL) group, soil pH was significantly increased in LS and RM. All treatments significantly increased soil EC and inorganic nitrogen contents. For the cultivation and non-liquid fertilizer (CNL) group, soil pH was significantly increased in LS and RM and EC was increased by all types of amendments. For the cultivation and liquid fertilizer (CL) group, the tendency of changes in soil pH and EC was the same as that of NCNL and CNL groups. Both LS and RM were well known for their great effect of raising pH when they were applied to the soil [8,22]. In this study, the alkali characteristic of RM was very strong, and the pH of soil was increased to nearly 9. Significant increases in soil pH affect the solubility of soil organic matter [23], and DOC concentration was highest in the RM treatment in each group. Since DOC in soil affects the availability of As and copper, which have high affinity with organic adsorption colloid [24,25], it could be supposed that these increases in DOC also affect the As bioavailability. The bioavailability of As assessed by CaCl2 solution was significantly decreased by all types of treatments in all groups. There was no significant differences in stabilization efficiency between LS and RM. On the other hand, in the case of AMDS, the stabilization efficiency was highest in all groups. It is well known that the decreases in As bioavailability in alkali soil are highly dependent on the amorphous metal oxides, and similar results were found in this study [26]. The high efficiency of AMDS could be confirmed through several previous studies [27,28,29]. The content of inorganic nitrogen was significantly changed in some treatments, but it was difficult to find a certain trend depending on groups and the types of amendments.
3.3. Early Root Growth of Hairy Vetch
After immersion of hairy vetch seeds, it took a week to germinate, and the actual early root growth took three weeks. A total of 24 seedlings were cultivated and analyzed for a total of eight treatments in two groups (CNL and CL) (Figure 1). The minimum, maximum and median root lengths were 240, 1575 and 610 mm, respectively. Average and standard deviation of root lengths were 737 and 385 mm, respectively. The minimum, maximum and median root weights were 110, 782 and 209 mg, respectively. Average and standard deviation of root weights were 273 and 177 mm, respectively. Specific root length (SRL), which is root length divided by root weight, was developed as an index to express the nutrient absorption utility of roots and the cost of forming and maintaining new roots [30,31]. Considering sustainable rooting of plant in contaminated soil [32], previous data have been recalculated as SRL and the minimum, maximum and median of SRL were 1.44, 4.98 and 2.71 mm·mg−1, respectively. Average and standard deviation of SRL were 2.91 and 0.90 mm·mg−1, respectively. Since various types of treatment were incorporated, it was difficult to identify the trend of intuitive changes only by distribution of root length, root weight and SRL index.
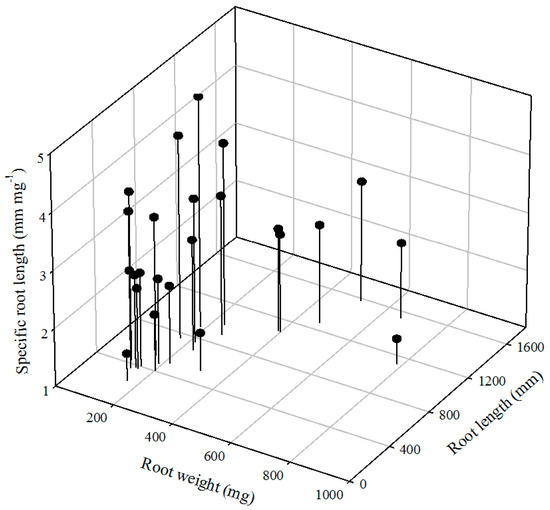
Figure 1.
Distribution of root length, root weight and specific root length of hairy vetch after 4 weeks cultivation.
3.4. Effects of Hairy Vetch Cultivation and Amendment Types on Soil Microbial and Enzyme Activity
Although chemical assessment has been widely used, it has a limitation that it only shows indirectly what the actual organism has affected [33]. So to compensate for that limitation, soil microbial activity and soil enzyme activity were analyzed for all groups and treatments based on when the hairy vetch were cultivated and harvested (Figure 2). All three types of amendments decreased SRA in NCNL and CNL groups, significantly. When liquid fertilizer was not applied, there was no significant difference in the changes in SRA due to hairy vetch cultivation. However, SRA increased nearly four times when liquid fertilizer and cultivation were incorporated (CL group). SRA also increased by LS and AMDS amendments, significantly. SRA is one of the oldest and most frequently used indexes for quantifying microbial activities by aerobic microorganisms [34]. Therefore, since aerobic microorganisms in LF flowed into and settled in soil, SRA appears to be very high in the CL group. The LF used in this study was made through an aerobic aeration process of livestock manure and was sufficiently fermented by aerobic microorganisms. Lim et al. [35] examined total aerobic bacteria in domestic commercial LF (n = 33) and it ranged from 2.8 to 24.3 × 104 CFU mL−1. However, because LF contains not only aerobic microorganisms but also harmful pathogens, it should be used on the site after safety is secured.
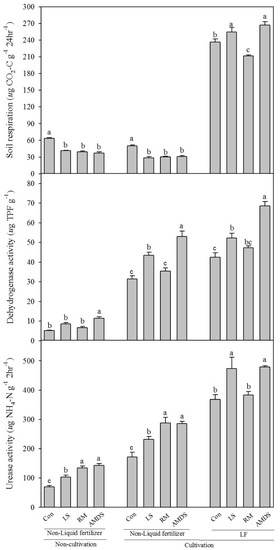
Figure 2.
Changes in soil microbial and enzyme activity according to various combinations of treatments (Con, control; LS, limestone; RM, red mud; AMDS, acid mine drainage sludge). Different letters indicate significant differences at the 5% level according to Tukey’s test between amendments.
In the NCNL group, only AMDS increased DHA. When cultivation was performed, DHA was increased in all treatment and groups. In each group, the highest DHA was in the AMDS treatment, decreasing in the order of LS, RM and control. In addition, the synergistic effect of the increase in DHA was increased when liquid fertilizer and cultivation were simultaneously incorporated than when only cultivation was performed. The tendency of DHA results was similar in URA. When liquid fertilizer was not treated, the effect of cultivation on URA was markedly different and the tendency to change according to the type of amendments was the same (AMDS = RM > LS > control). DHA and URA are enzymes that facilitate the oxidation/reduction reactions of substrates and catalyze the hydrolysis of urea [36,37]. Since two enzyme activity act only on a specific substrate, unlike SRA, the results are more specific. In particular when comparing NCNL and CNL, the difference due to plant cultivation was clearly observed, and the difference due to the presence or absence of LF was also apparent when comparing CNL and CL. Synergistic effects of various complex treatments could not be confirmed through chemical assessment (Table 2), but could be confirmed through enzyme activity evaluation, biological assessment. Krżyzak et al. [38] showed that phytostabilization could improve soil biological properties (substrate induced respiration and nitrification activity). Son et al. [39] and Pérez-Palacios et al. [40] evaluated the efficiency of phytostabilization using liquid peroxidation, reactive oxygen species-scavenging enzyme. These series of results reinforced the usefulness and clarity of biological assessment in a mixture of various types of treatments and will continue to be used in further study.

Table 2.
Effects of treatments on soil chemical properties.
3.5. Impact of Changed Soil Characteristics on Soil Microbial and Enzyme Activity
As can be seen from the previous results, amendment treatment, liquid fertilizer treatment and hairy vetch cultivation changed soil characteristics. The relationships between soil biological characteristics and experimental results are summarized in Table 3. Both SRA and URA were mainly negatively correlated with soil pH and As availability and positively correlated with DOC and SRL. In the case of DHA, ammoniacal nitrogen had a negative correlation and SRL had a positive correlation. Particularly, SRL was found to have a significant positive effect on all soil biological characteristics.

Table 3.
Contribution of various experimental results on soil biological characteristics using stepwise multiple regression analysis.
It is known that the sensitivity of SRA varies depending on the soil pH. In low soil pH, SRA was not sensitive to pH change [41]. In addition, the effect of pH on SRA is not significant with the pH range of 6 to 8 [42]. However, in alkali conditions, the increase in soil pH has a negative effect on SRA via biochemical reactions [42,43,44]. The soil used in this study had a pH of 8 or higher in all treated soils. Therefore, the SRA tends to decrease with increasing pH according to the treatment group, and it is shown that the pH constant is negative in the regression equation. It is known that the tendency of enzyme activity to be inhibited by high pH in alkaline soils is similar in SRA as well as in URA [45]. Not only pH but also bioavailable As has a negative effect on SRA and URA. The presence of bioavailable As in soil decreased the production of soil enzymes, resulting in a decrease in soil enzyme activity [46]. Also, the greater bioavailable As in the soil, the greater the exposure and absorption into microorganisms. Thus, the high concentration of As generated a large amount of reactive oxygen species (ROS), and when the ROS exceed the microbial unique antioxidant mechanism (including enzymatic and non-enzymatic), it causes oxidative stress resulting in the inhibition of soil organisms [47,48]. Therefore, both SRA and URA were significantly and negatively correlated with bioavailable As. On the other hand, both DOC and SRL have a positive effect on SRA and URA and it seems to be caused by LF treatment and the rhizosphere effect. The LF used in the experiment contained a high concentration of organic matter, and the root also produced root exudates and were released into the soil [49,50]. It could be summarized that LF treatment has a positive effect on SRL and DOC, and strengthened SRL increased DOC again. For DHA, ammonium ion content was found to have a negative correlation (Table 3). Nitrogen is one of the essential macro elements of plant growth, and plant roots could absorb ammonium and nitrate ions in particular, so their concentration in soil would have a positive effect on plant growth [51]. However, this is not necessarily the case with soil microorganisms and enzyme activities. Rogers and Li [52], Li and Zhao [53], and Anghinoni et al. [54] have reported that ammonium ion above a certain level inhibited the negative effect of ammonium ion on DHA through disruption of biological decarbonation, nitrification and oxidation. Nevertheless, the rhizosphere effect by SRL has a positive correlation with DHA as in the case of SRA and URA. Liu et al. [55] have evaluated the efficiency of phytoremediation using five types of plants including Medicago sativa L. (Leguminosae family) in organic pollutant-contaminated soil using DHA and URA. Also, Kim et al. [40] verified the usefulness of enzyme activity index for evaluating restoration efficiency using Vicia villosa Roth and Trifolium pretense L. belonging to the Leguminosae family. Taken together, soil enzyme activity has been shown to be influenced by various biotic (SRL) and abiotic factors (pH, DOC, bioavailable As and ammonium ion) and is considered to be a suitable indicator for evaluating the effect of plant cultivation, phytoremediation.
4. Conclusions
In this study, industrial byproduct (amendments), agricultural byproducts (livestock liquid fertilizer) and plant cultivation (phytostabilization) were incorporated into highly As-contaminated soil near an abandoned mine area for sustainable environmental management. It was confirmed that biological assessment indicators could be utilized to evaluate the complex treatment of various restoration techniques, including the phytoremedation method. However, it is difficult to determine the clear impacts due to the short-term cultivation period and lab-scale conditions; future research needs long-term cultivation studies at the on-site scale.
Author Contributions
Writing—original draft preparation, M.-S.K.; methodology, H.-G.M.; writing—review and editing, J.-G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF) (2019R1I1A1A01043684) and partly supported by Korea University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yoon, Y.M.; Lee, S.E.; Chung, D.Y.; Cho, G.Y.; Kim, J.D.; Kim, C.H. The analysis of environmental loads and material recycling of the nutrients by the livestock wastewater originating from imported feeds. J. Kor. Grassl. Forage. Sci. 2008, 28, 139–154. [Google Scholar]
- Hasse, M.; Rösch, C.; Ulrici, O. Feasibility study on the processing of surplus livestock manure into an organic fertilizer by thermal concentration–the case study of Les Plenesses in Wallonia. J. Clean. Prod. 2017, 161, 896–907. [Google Scholar] [CrossRef]
- Shin, D.W.; Joo, H.S.; Seo, E.; Kim, C.Y. Management Strategies to Reduce PM-2.5 emission: Emphasis-Ammonia; Korea Environment Institute: Sejong City, Korea, 2017. [Google Scholar]
- Svanbäck, A.; McCrackin, M.L.; Swaney, D.P.; Linefur, H.; Gustafsson, B.G.; Howarth, R.W.; Humborg, C. Reducing agricultural nutrient surpluses in a large catchment–links to livestock density. Sci. Total Environ. 2019, 648, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, S.H.; Kim, J.G. Assessment of fraction and mobility of arsenic in soil near the mine waste dam. Sustainability 2020, 12, 1480. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, K.H.; Yan, X.; Chen, S.J.; Hu, G.C.; Peng, X.W.; Yuan, J.G.; Mai, B.X.; Yang, Z.Y. Heavy metals in food, house dust, and water from an e-waste recycling area in South China and the potential risk to human health. Ecotox. Environ. Safe. 2013, 96, 205–212. [Google Scholar] [CrossRef]
- Zhuang, P.; Lu, H.; Li, Z.; Zou, B. McBride, M.B. Multiple exposure and effects assessment of heavy metals in the population near mining area in South China. PLoS ONE 2014, 9, e94484. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.Y.; Park, H.; Yun, J.; Kim, J.G. In situ stabilization of arsenic and metal-contaminated agricultural soil using industrial by-products. Geoderma 2011, 161, 1–7. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Pourmohammadi, H.; Rahimi, M.; Dessouky, M. Sustainable reverse logistic for distribution of industrial waste/byproducts: A joint optimization of operation and environmental cost. Supply Chain Forum Int. J. 2008, 9, 2–17. [Google Scholar] [CrossRef]
- Chiang, P.N.; Tong, O.Y.; Chiou, C.S.; Lin, Y.A.; Wang, M.K. Reclamation of zinc-contaminated soil using a dissolved organic carbon solution prepared using liquid fertilizer from food-waste composting. J. Hazard. Mater. 2016, 301, 100–105. [Google Scholar] [CrossRef]
- Kaimi, E.; Mukaidani, T.; Miyoshi, S.; Tamaki, M. Ryegrass enhancement of biodegradation in diesel-contaminated soil. Environ. Exp. Bot. 2006, 55, 110–119. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, S.X.; Duan, C.; Liu, F.; Li, F.M. Amelioration of lead-zinc tailings by spent mushroom compost: Effects on growth of Lolium perenne L. and physico-chemical properties of tailings. J. Agro. Environ. Sci. 2014, 33, 526–531. [Google Scholar]
- ISO. Soil Quality–Extraction of Trace Element Soluble in Aqua Regia; ISO 11466; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Esnaola, M.V.; Millán, E. Evaluation of heavy metal lability in polluted soils by a cation exchange bath procedure. Environ. Pollut. 1998, 99, 79–86. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Shiralipour, A. Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator, Pteris vittata L. Environ. Pollut. 2003, 126, 157–167. [Google Scholar] [CrossRef]
- National Institute of Agricultural Science and Technology. Method of Soil and Plant Analysis; Rural Development Administration: Suwon, Korea, 2000. [Google Scholar]
- Jäggi, W. Estimation of soil respiration with closed bottles. In Method in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1976; pp. 216–217. [Google Scholar]
- Friedel, J.K.; Mölter, K.; Fischer, W.R. Comparisonand improvement of methods for determining soil dehydrogenase activity by using triphenyltetrazolium chloride and iodonitrotetrazolium chloride. Biol. Fert. Soil. 1994, 18, 291–296. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colormetric determination of ammonium. Biol. Fert. Soil. 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Gale, E.S.; Sullivan, D.M.; Cogger, G.G.; Bary, A.I.; Hemphill, D.D.; Myhre, E.A. Estimating plant available nitrogen release from manures, composts, and specialty products. J. Environ. Qual. 2006, 35, 2321–2332. [Google Scholar] [CrossRef]
- Gray, C.W.; Dunham, S.J.; Dennis, P.G.; Zhao, F.J.; McGrath, S.P. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 142, 530–539. [Google Scholar] [CrossRef]
- Oste, L.A.; Temminghoff, E.J.M.; Riemsdifk, W.H.V. Soild-solution partitioning of organic matter in soils as influenced by an increase in pH or Ca concentration. Environ. Sci. Technol. 2002, 36, 208–214. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Naidu, R. Heavy metal distribution, bioaccessibility and phytoavailability in long-term contaminated soils from Lake Macquarie, Australia. Aust. J. Soil Res. 2009, 47, 166–176. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behavior in soil-plant system: Biogeochemical reactions and chemical speciation influences. In Enhancing Cleanup of Environmental Pollutants; Springer: Cham, Switzerland, 2017; pp. 97–140. [Google Scholar]
- Lee, H.K.; Kim, D.H.; Kim, J.S.; Ji, M.K.; Han, Y.S.; Park, Y.T.; Yun, H.S.; Choi, J. As(III) and As(V) removal from the aqueous phase via adsorption onto acid mine drainage sludge (AMDS) alginate beads and goethite alginate beads. J. Hazard. Mater. 2015, 292, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.S.; Kim, J.Y.; Park, H.S.; Kim, K.W. Field assessment of arsenic immobilization in soil amended with iron rich acid mine drainage sludge. J. Clean. Product. 2015, 108, 1073–1080. [Google Scholar] [CrossRef]
- Kim, M.S.; Min, H.G.; Lee, S.H.; Kim, J.G. The effects of various amendments on trace element stabilization in acidic, neutral and alkali soil with similar pollution index. PLoS ONE 2016, 11, e0166335. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.M.; Júnior, V.V.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; de Souza, T.C. Copper toxicity on photosynthetic responses and root morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut. 2018, 229, 138. [Google Scholar] [CrossRef]
- Fitter, A.H. Effects of nutrient supply and competition from other species on root growth of Lolium perenne in soil. Plant. Soil 1976, 45, 177–189. [Google Scholar] [CrossRef]
- Ryser, P. The mysterious root length. Plant. Soil 2006, 286, 1–6. [Google Scholar] [CrossRef]
- Brunner, I.; Luster, J.; Günthardt-Goerg, M.S.; Frey, B. Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environ. Pollut. 2008, 152, 559–568. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Choi, Y.J.; Kim, J.G. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 2009, 77, 1069–1075. [Google Scholar] [CrossRef]
- Kieft, T.L.; Rosacker, L.L. Application of respiration- and adenylate-based soil microbiological assays to deep subsurface terrestrial sediments. Soil Biol. Biochem. 1991, 23, 563–568. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, J.H.; Go, W.R.; Kunhikrishnan, A.; Kim, W.I. Monitoring of microorganisms in commercial liquid pig manures in Korea. Kor. J. Soil Sci. Fert. 2011, 44, 1181–1184. [Google Scholar] [CrossRef][Green Version]
- Klose, S.; Tabatabai, M.A. Urease activity of microbial biomass in soils as affected by cropping systems. Biol. Fert. Soil. 2000, 31, 191–199. [Google Scholar] [CrossRef]
- Chaperon, S.; Sauvé, S. Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biol. Biochem. 2007, 39, 2329–2338. [Google Scholar] [CrossRef]
- Krżyzak, J.; Plaza, G.; Margesin, R.; Wasilkowski, D.; Mrozik, A. Microbial parameters as bioindicators of soil quality during aided phytostabilization of metal contaminated soil. Environ. Engineer. Manag. J. 2012, 11, 1775–1782. [Google Scholar]
- Son, K.H.; Kim, D.Y.; Koo, N.; Kim, K.R.; Kim, J.G.; Owens, G. Detoxification through phytochelatin synthesis in Oenothera odorata exposed to Cd solution. Environ. Exp. Bot. 2012, 75, 9–15. [Google Scholar] [CrossRef]
- Pérez-Palacios, P.; Romero-Aguilar, A.; Delgadillo, J.; Doukkali, B.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Pajuelo, E. Double genetically modified symbiotic system for improved Cu phytostabilization in legume roots. Environ. Sci. Pollut. Res. 2017, 24, 14910–14923. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Mclntosh, M. Changes in soil biological activities under reduced soil pH during Thlaspi caerulescens phytoextraction. Soil Biol. Biochem. 2006, 38, 1451–1461. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Y.; Yang, K.; Wang, S.; Zeng, H.; Baumann, F.; Kuehn, P.; Scholten, T.; He, J.S. Soil respiration on Tibetan alpine grasslands: Belowground biomass and soil moisture, but not soil temperature, best explain the large-scale patterns. PLoS ONE 2012, 7, e34968. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, T.; Hatano, R.; Yajima, T.; Takahashi, K.; Isaev, A.P. Soil respiration in Siberian taiga ecosystems with different histories of forest fire. Soil Sci. Plant. Nutr. 2000, 46, 31–42. [Google Scholar] [CrossRef]
- Lai, L.; Zhao, X.; Jiang, L.; Wang, Y.; Luo, L.; Zheng, Y.; Chen, X.; Rimmington, G.M. Soil respiration in different agricultural and natural ecosystems in an arid region. PLoS ONE 2012, 7, e48011. [Google Scholar] [CrossRef]
- Rao, D.L.N.; Chai, S.K. Urease and dehydrogenase activity of alkali and reclaimed soil. Aust. J. Soil Res. 1985, 23, 661–665. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bhattacharyya, P.; Pal, R. Effect of arsenic contamination on microbial biomass and its activities in arsenic contaminated soils of Gangetic West Bengal, India. Environ. Int. 2004, 30, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal–stressed plants a little easier. Funct. Plant. Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Ai, X.; Wang, X.; Robin, P.; Cavanagh, J.; Matthew, C.; Qiu, J. Antioxidant and bdhavior responses of earthworms after introduction to a stimulated vermifilter environment. Ecol. Envineer. 2015, 81, 218–227. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How Plant Root Exudates Shape the Nitrogen Cycle. Trends Plant. Sci. 2017, 22, 661–673. [Google Scholar]
- Kang, T.W.; Halder, J.N.; Kim, S.R.; Yoon, Y.M.; Lee, M.G. Nutrient composition and heavy metal contents of matured livestock liquid fertilizer in Korea. J. Kor. Org. Resour. Recyc. Assoc. 2017, 25, 31–39. [Google Scholar]
- Yu, K.W.; Gao, W.Y.; Hahn, E.J.; Paek, K.Y. Effects of macro elements and nitrogen source on adventitious root growth and ginsenoside production in ginseng (Panax ginseng C.A. Meyer). J. Plant. Biol. 2001, 44, 179–184. [Google Scholar] [CrossRef]
- Rogers, J.E.; Li, S.W. Effect of metals and other inorganic ions on soil microbial activity: Soil dehydrogenase assay as a simple toxicity test. Bullet. Environ. Contam. Toxicol. 1985, 34, 858–865. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhao, Q.L. Inhibition of microbial activity of activated sludge by ammonia in leachate. Environ. Int. 1999, 25, 961–968. [Google Scholar] [CrossRef]
- Anghinoni, I.; Magalhãs, J.R.; Barber, S.A. Enzyme activity, nitrogen uptake and corn growth as affected by ammonium concentration in soil solution. J. Plant. Nutr. 2008, 11, 131–144. [Google Scholar] [CrossRef]
- Liu, R.; Dai, Y.; Sun, L. Effect of rhizosphere enzymes on phytoremediation in PAH-contaminated soil using five plant species. PLoS ONE 2015, 10, e0120369. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
