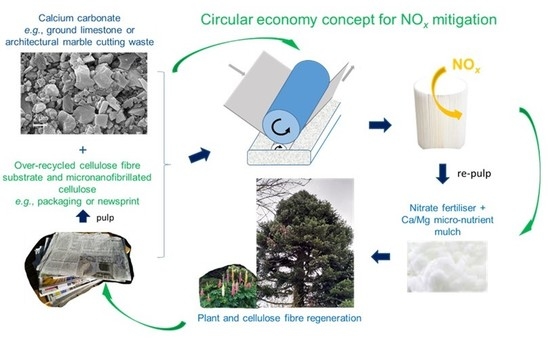
Unveiling a Recycling-Sourced Mineral-Biocellulose Fibre Composite for Use in Combustion-Generated NOx Mitigation Forming Plant Nutrient: Meeting Sustainability Development Goals in the Circular Economy
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Material Production
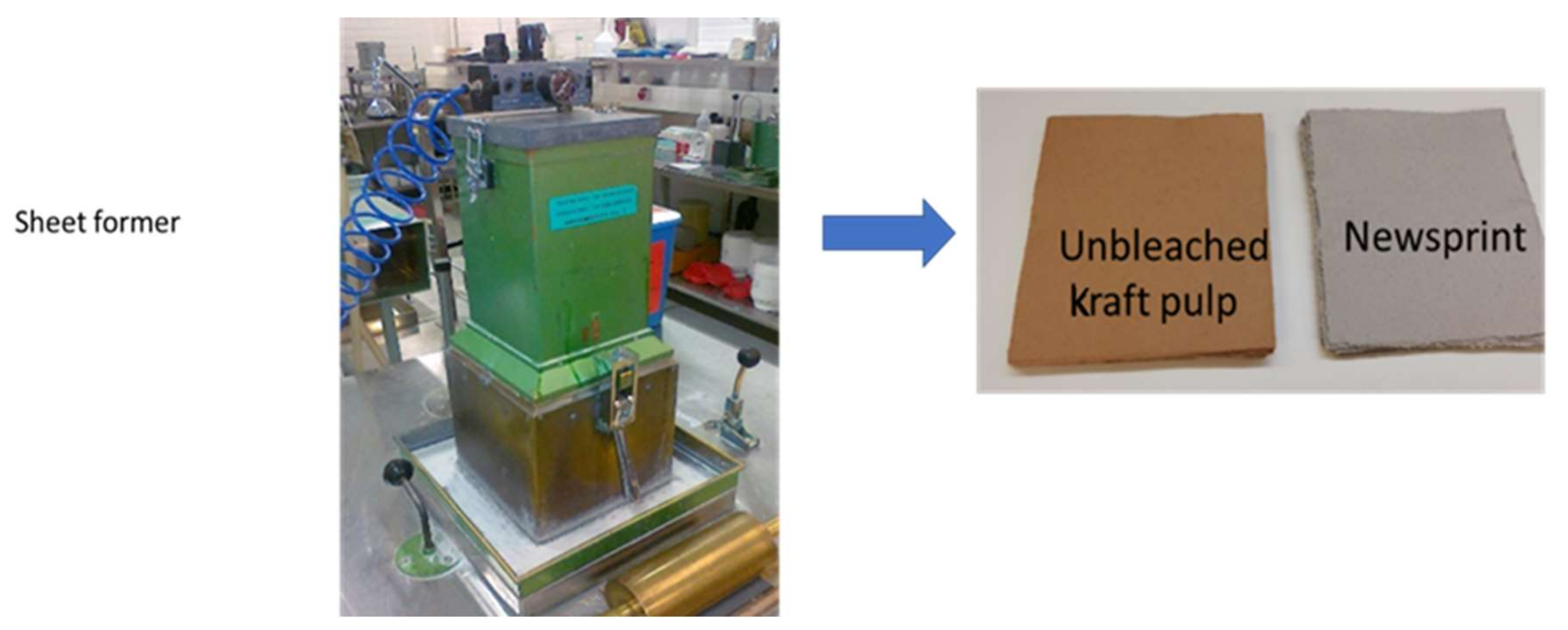
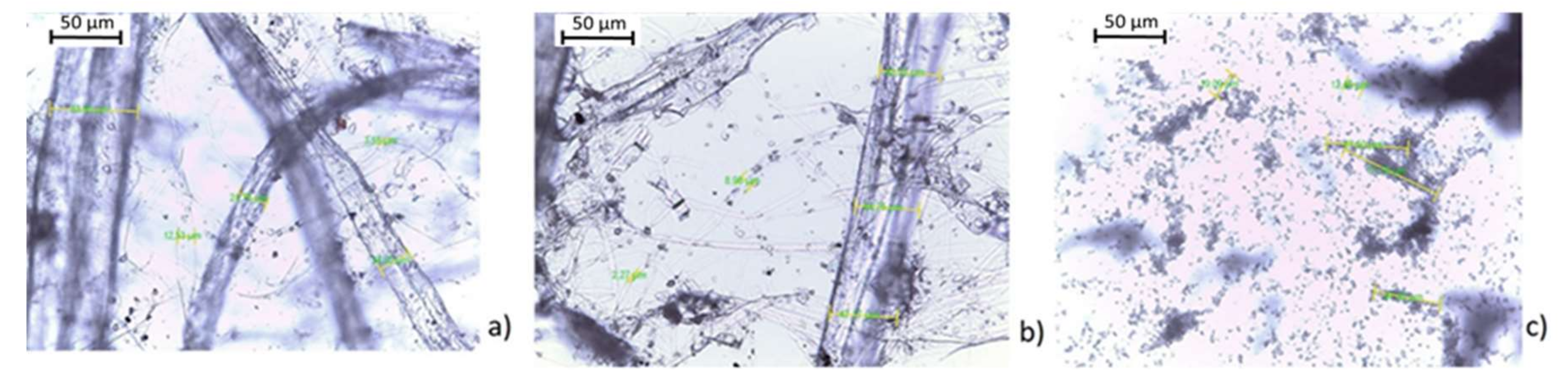
2.1. Substrate
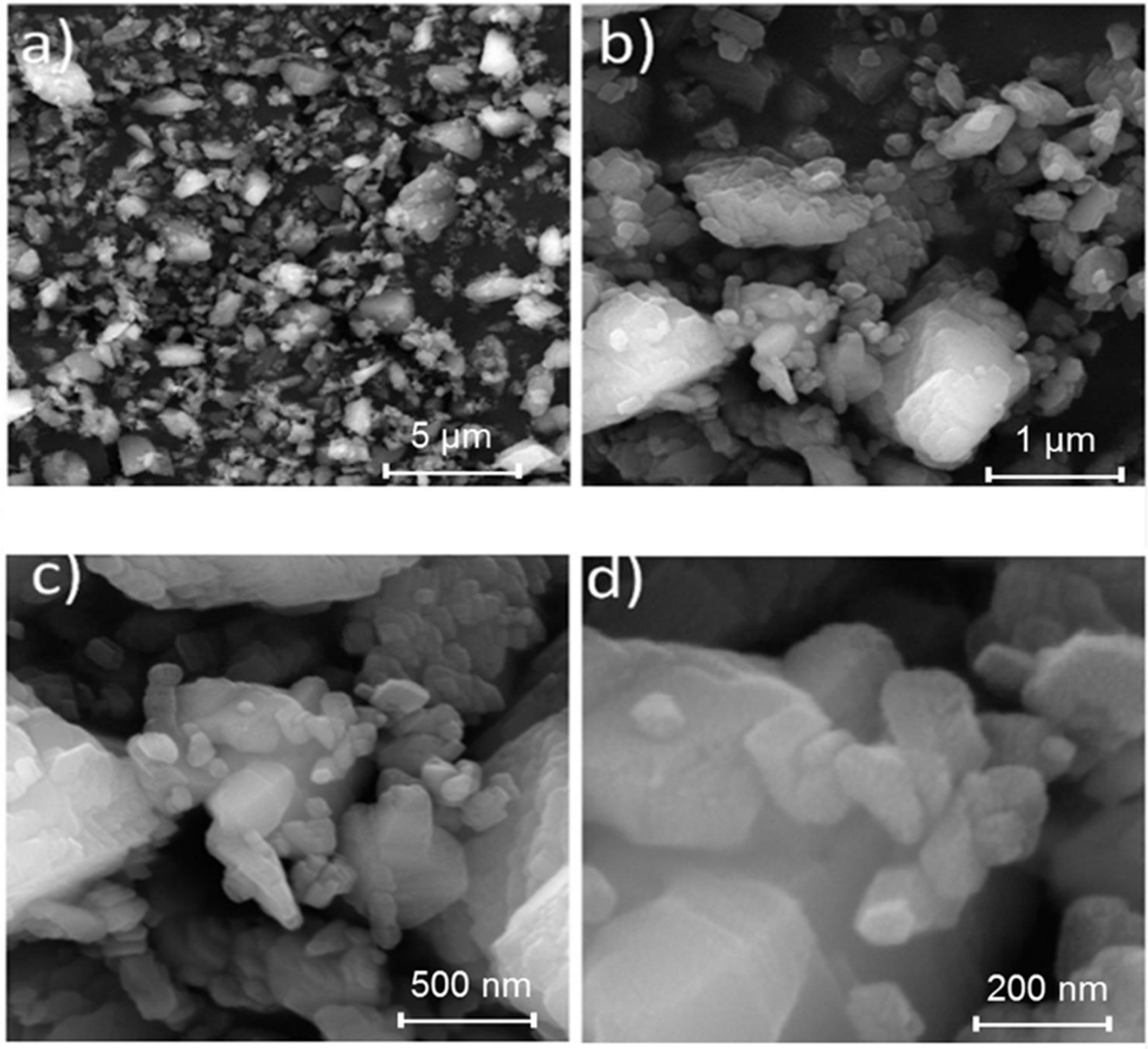
2.2. Reactive Coating
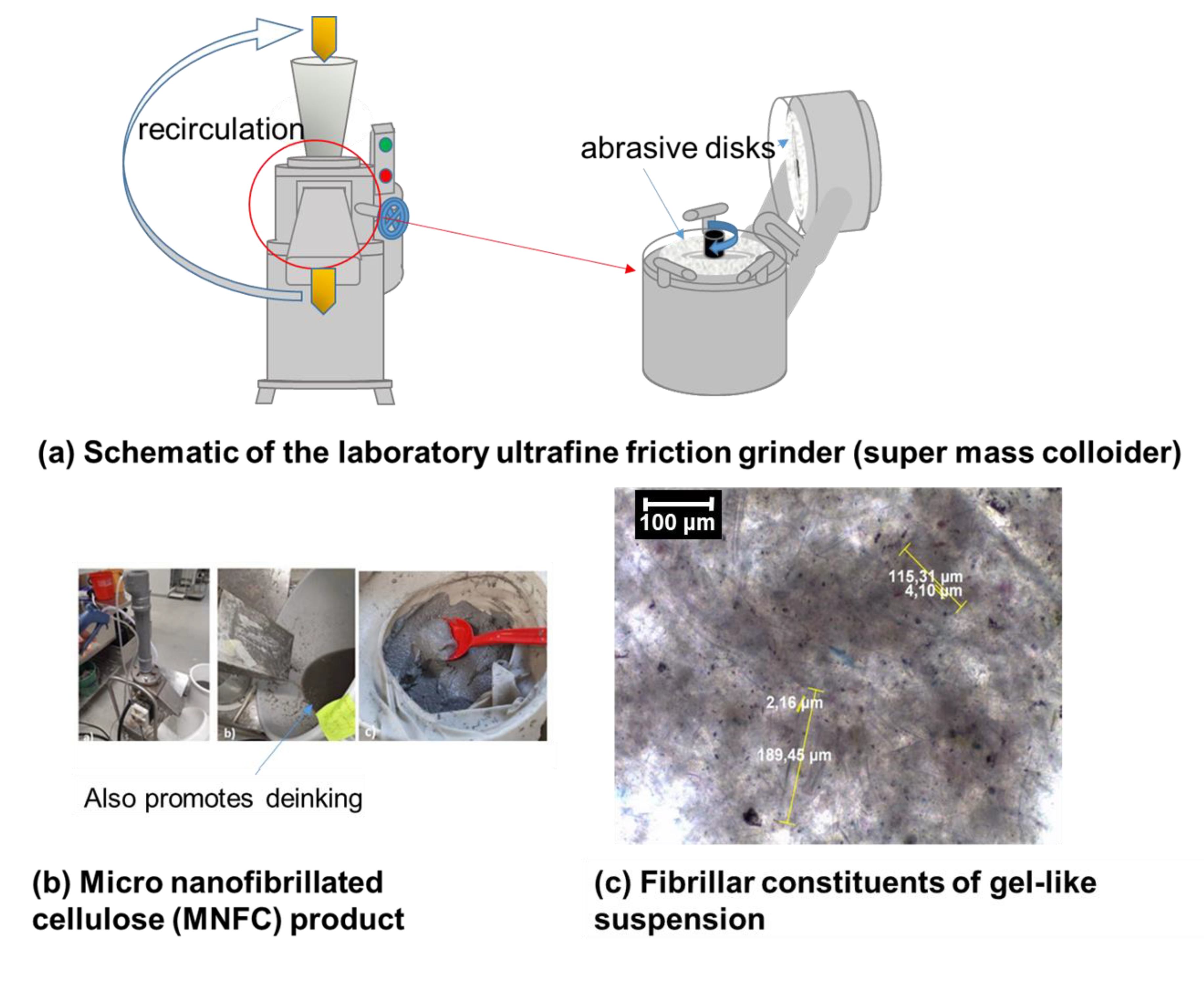
2.3. Nanocellulose (Binder + Humectant)
2.4. Coating Formulation
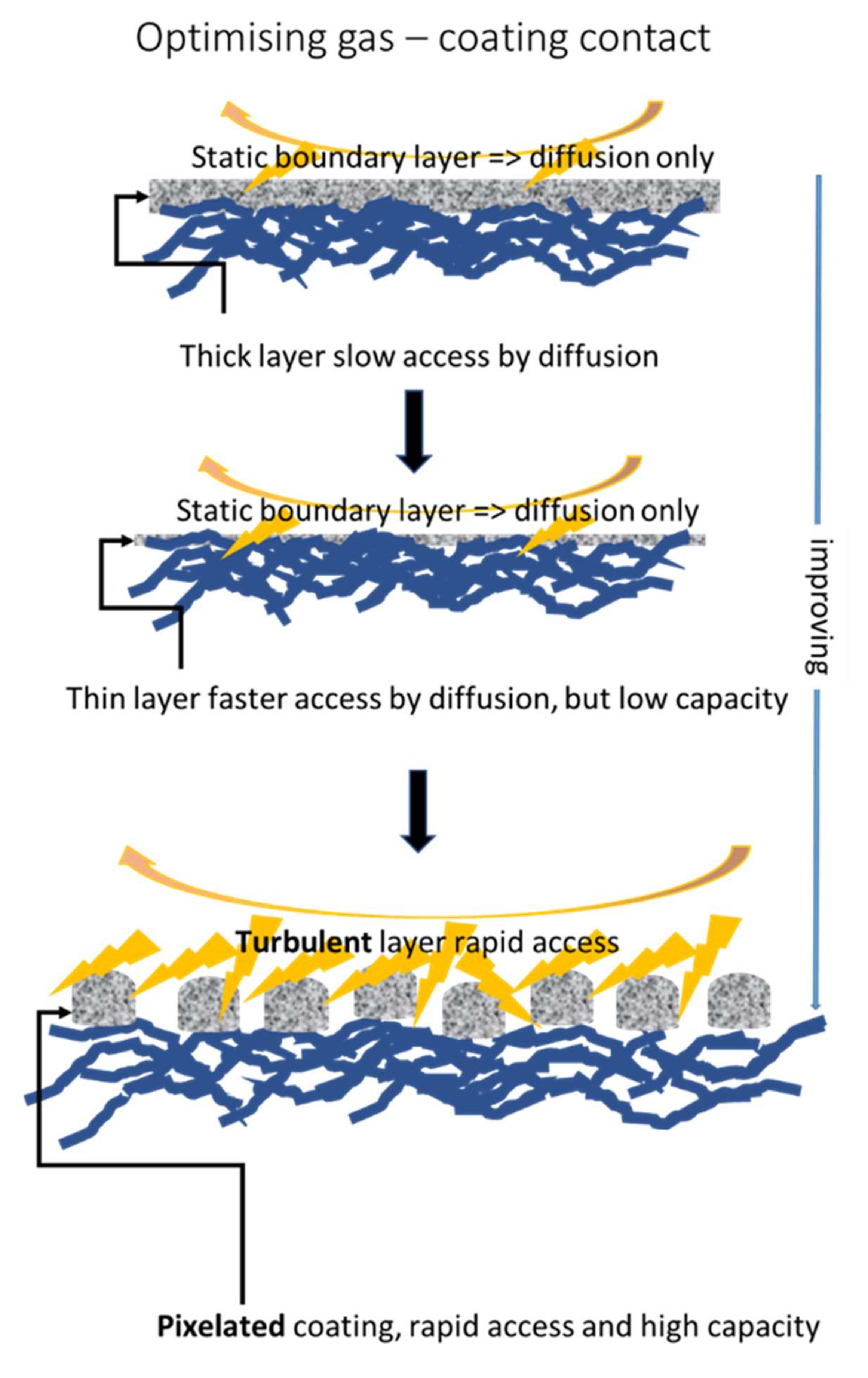
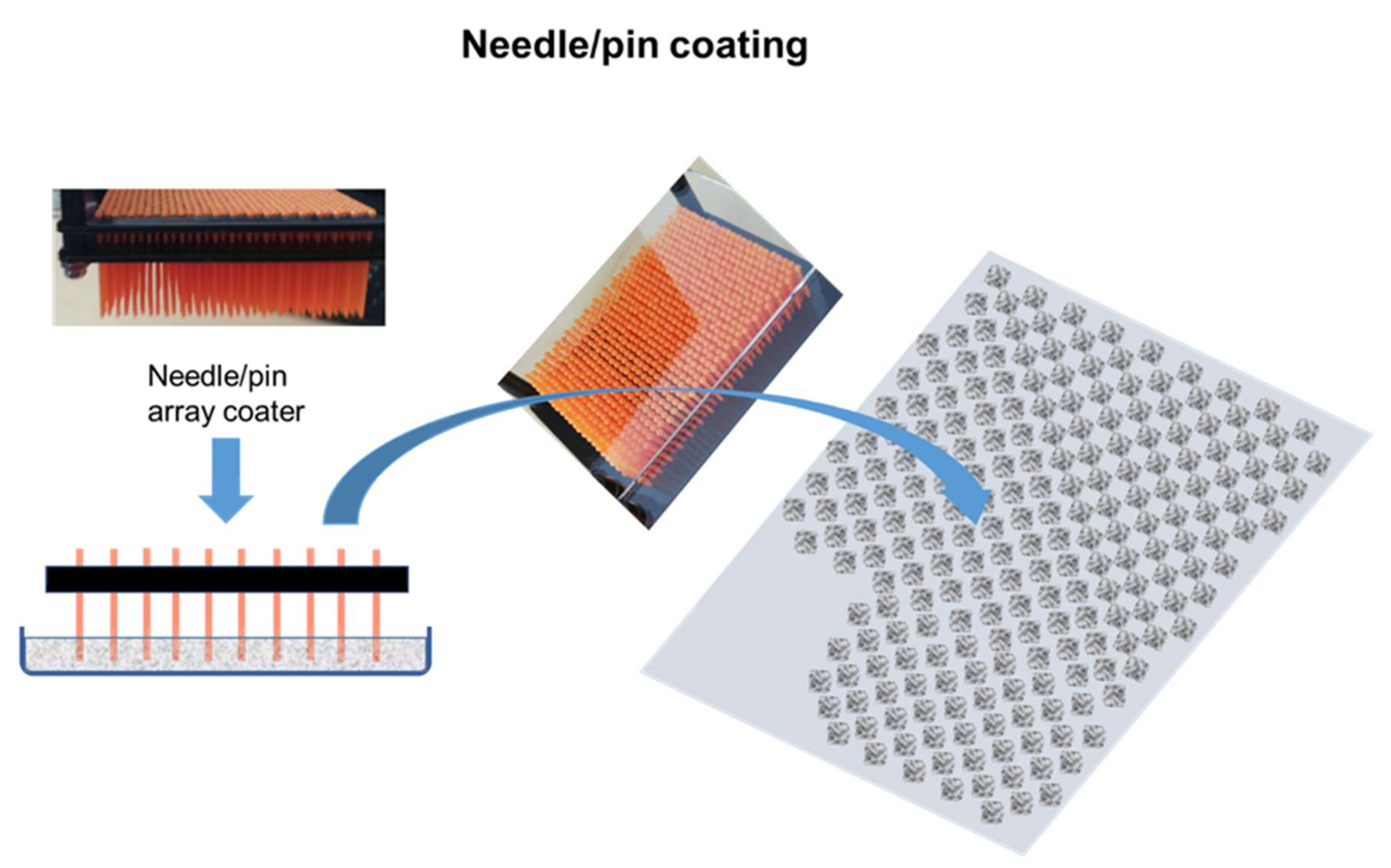
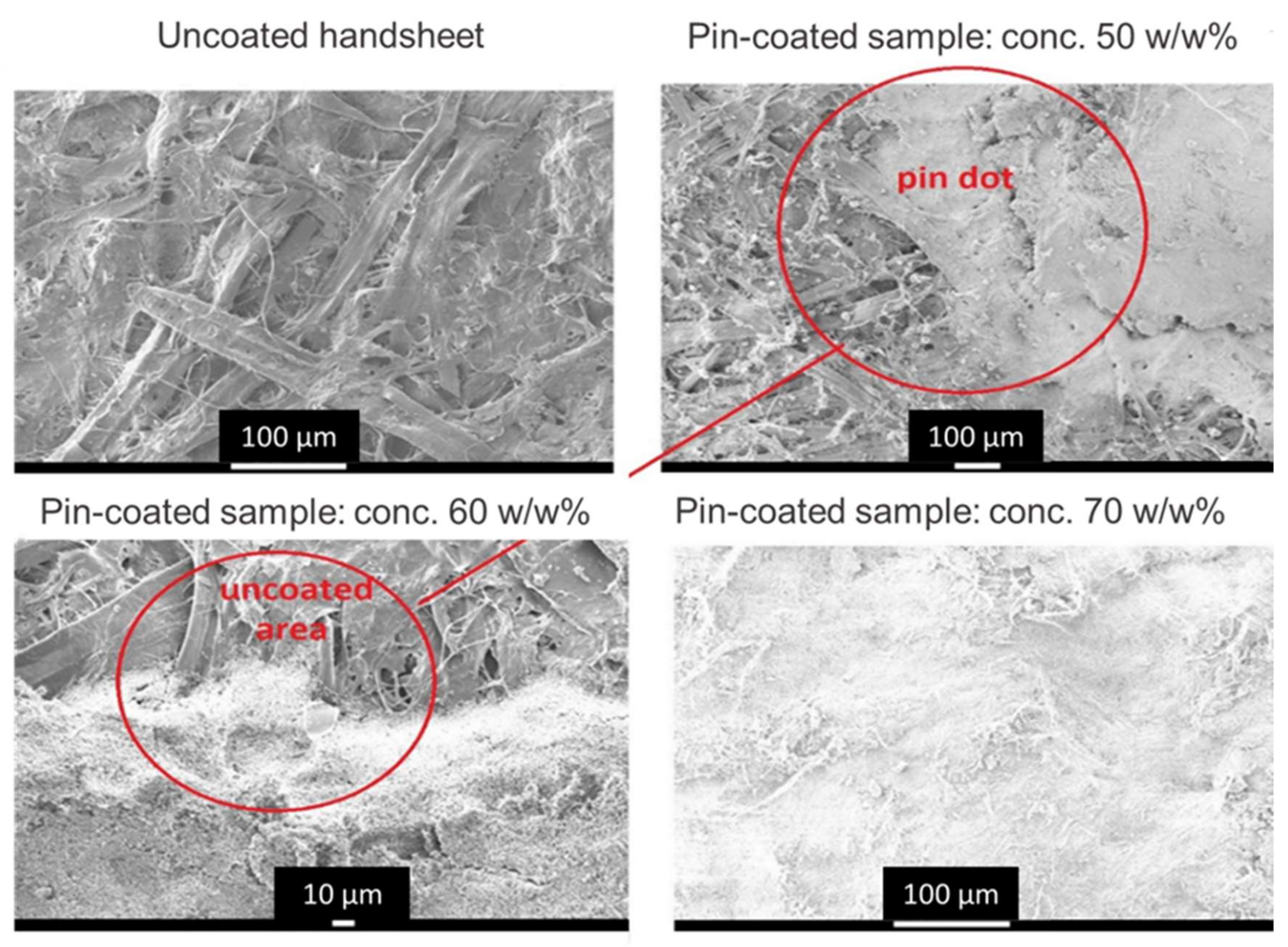
2.5. Coating Application
3. Experimental
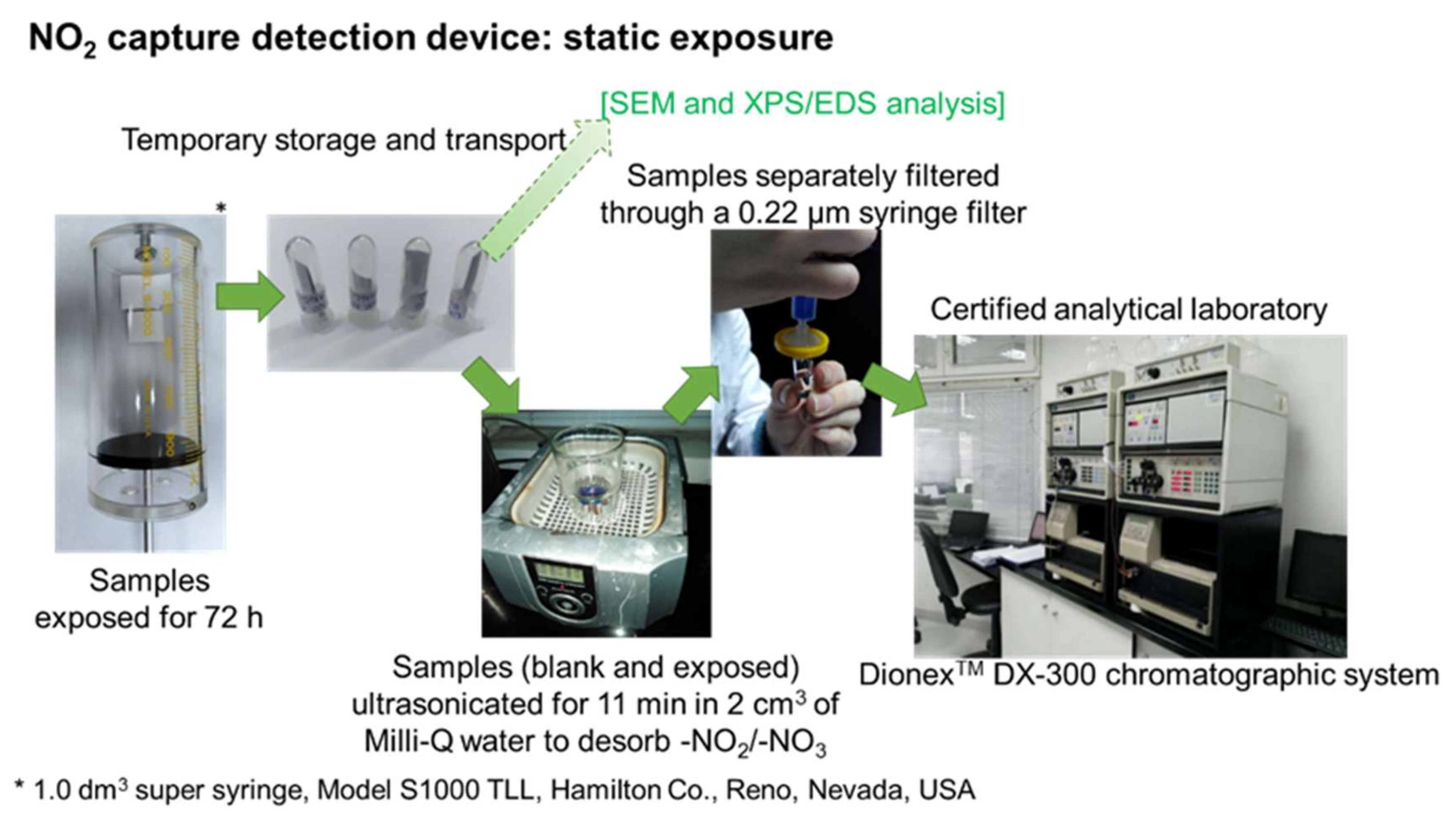
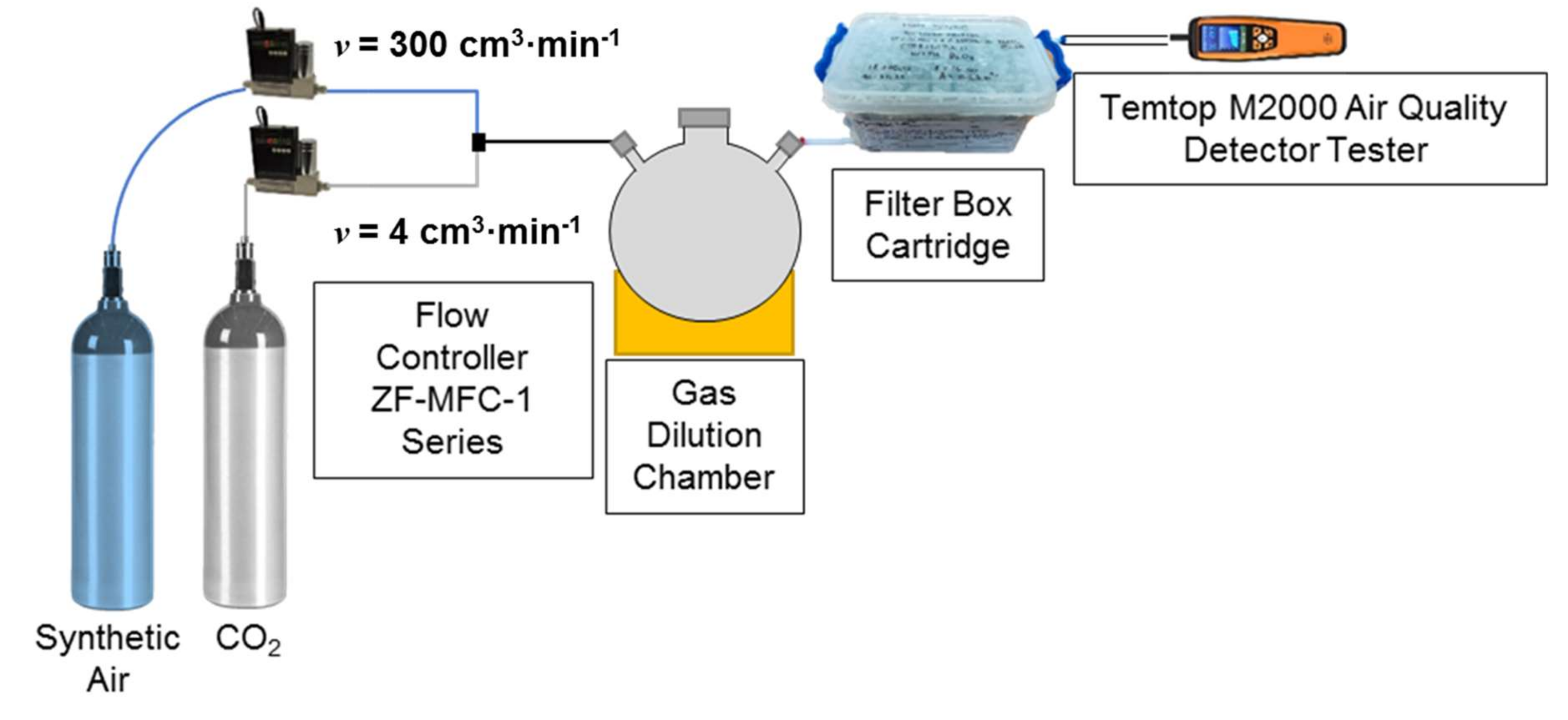
3.1. Filter Development, Test Methods, and Results
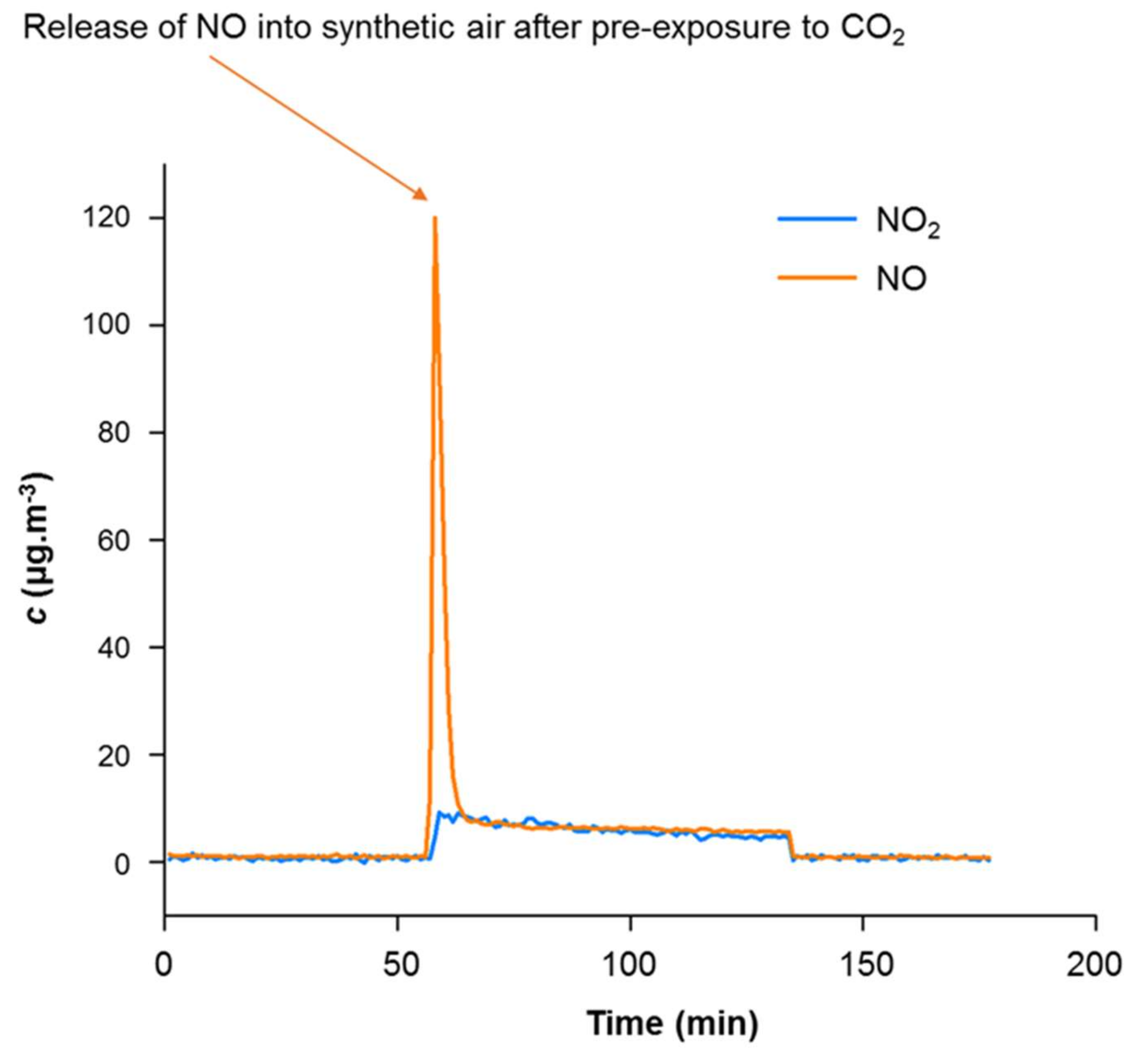
3.1.1. Confirmation of the CaCO3-NOx Reaction
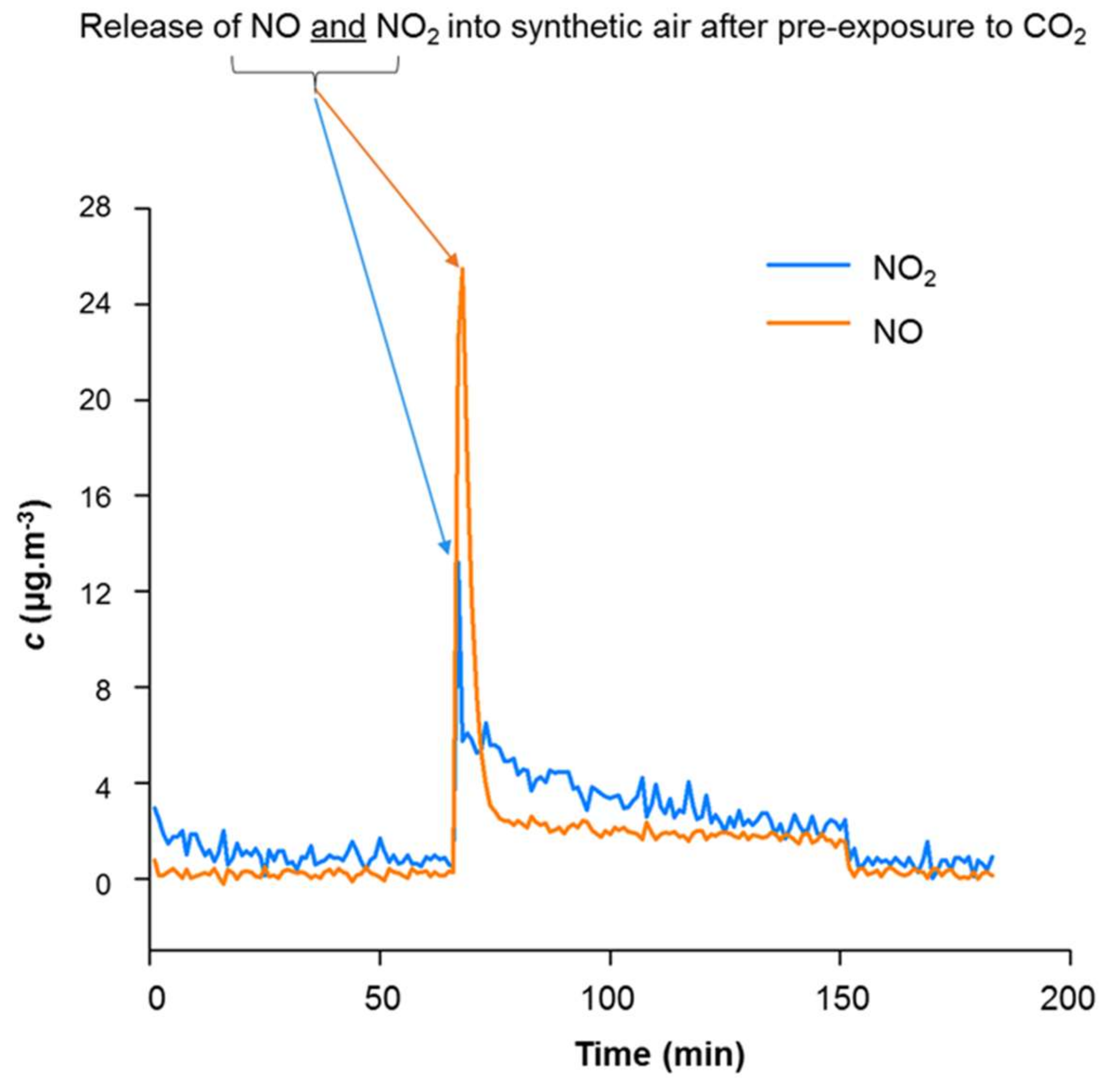
3.1.2. Proof of Principle for Coated Samples
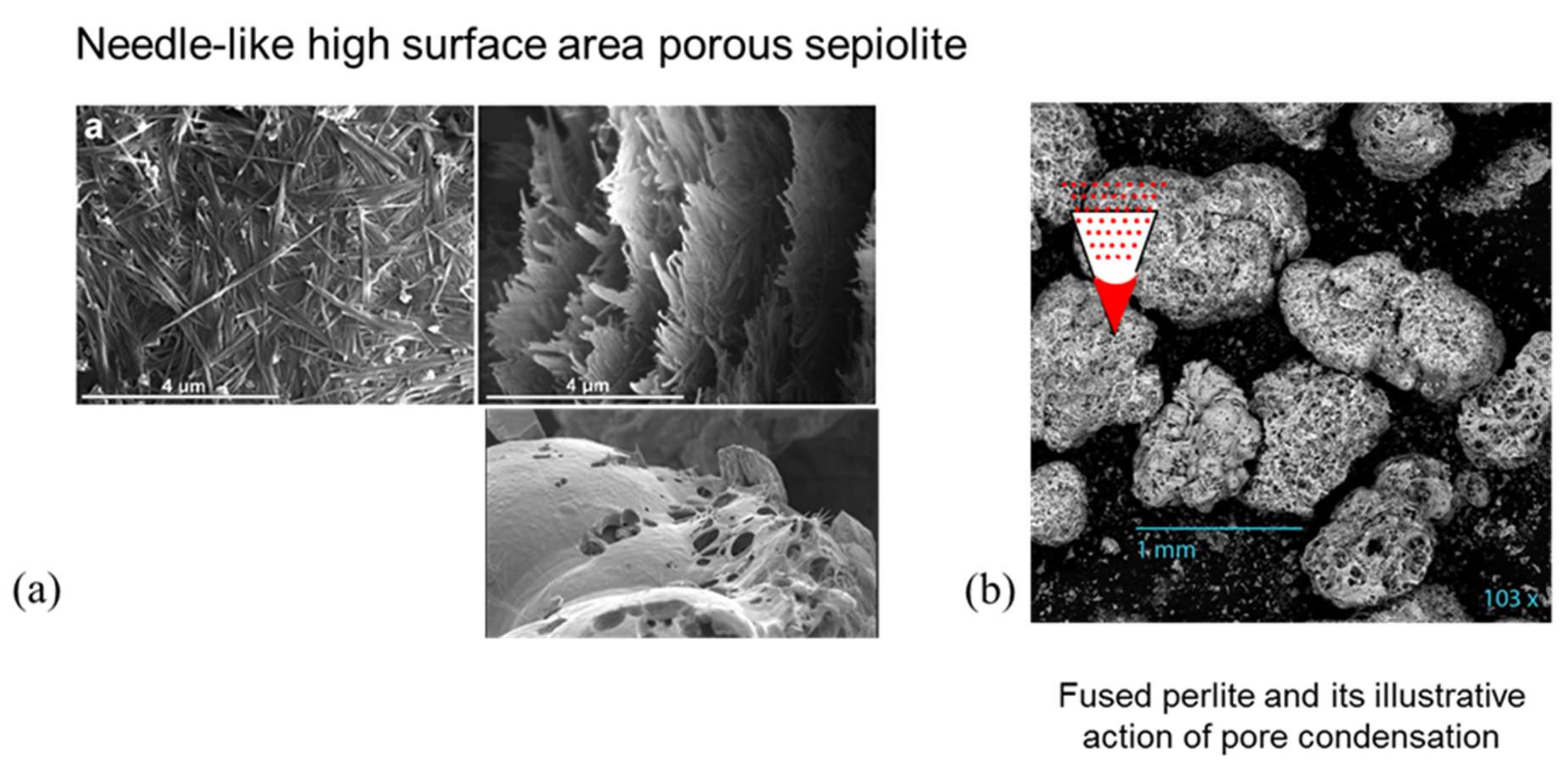
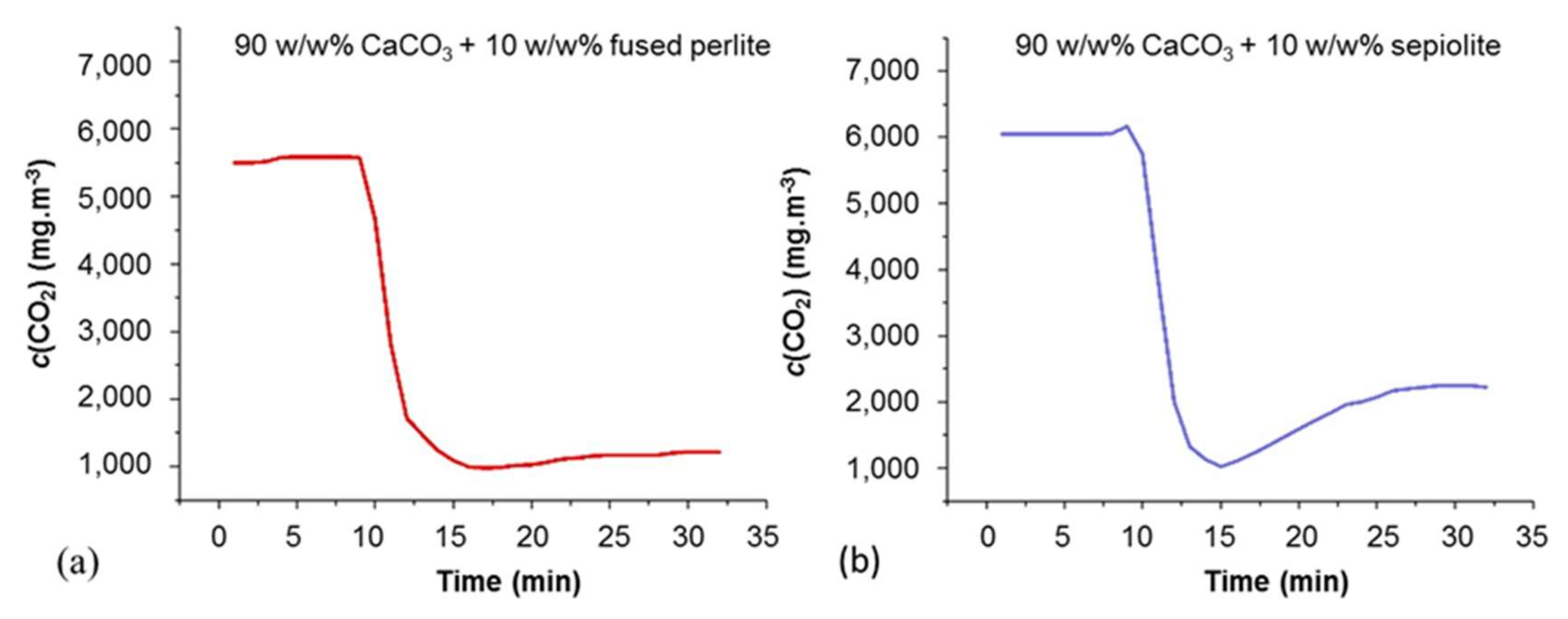
3.1.3. Pluses and Minuses of Adding Inert (Non-Reactive) Surface Area
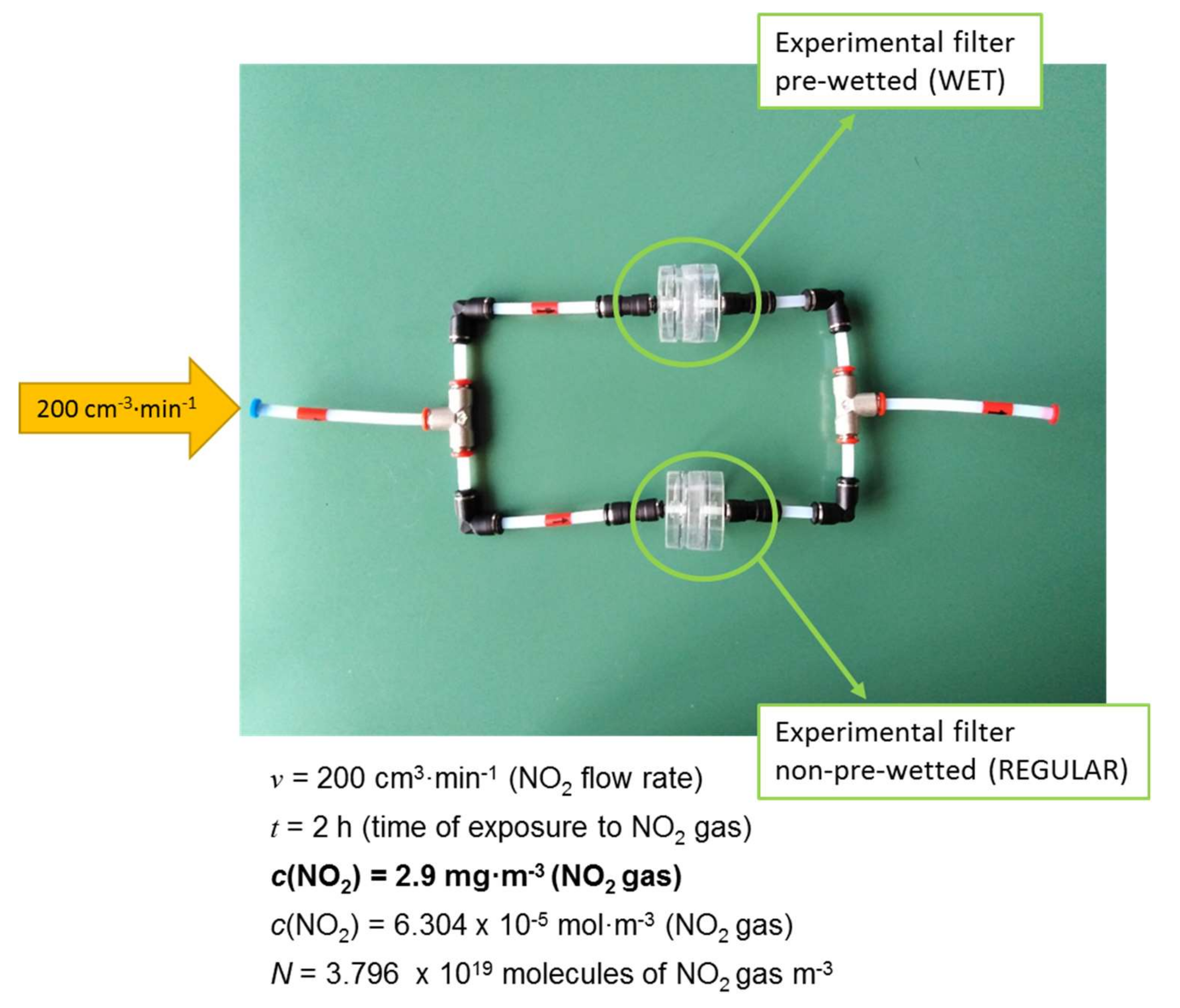
3.1.4. Effect of Moisture
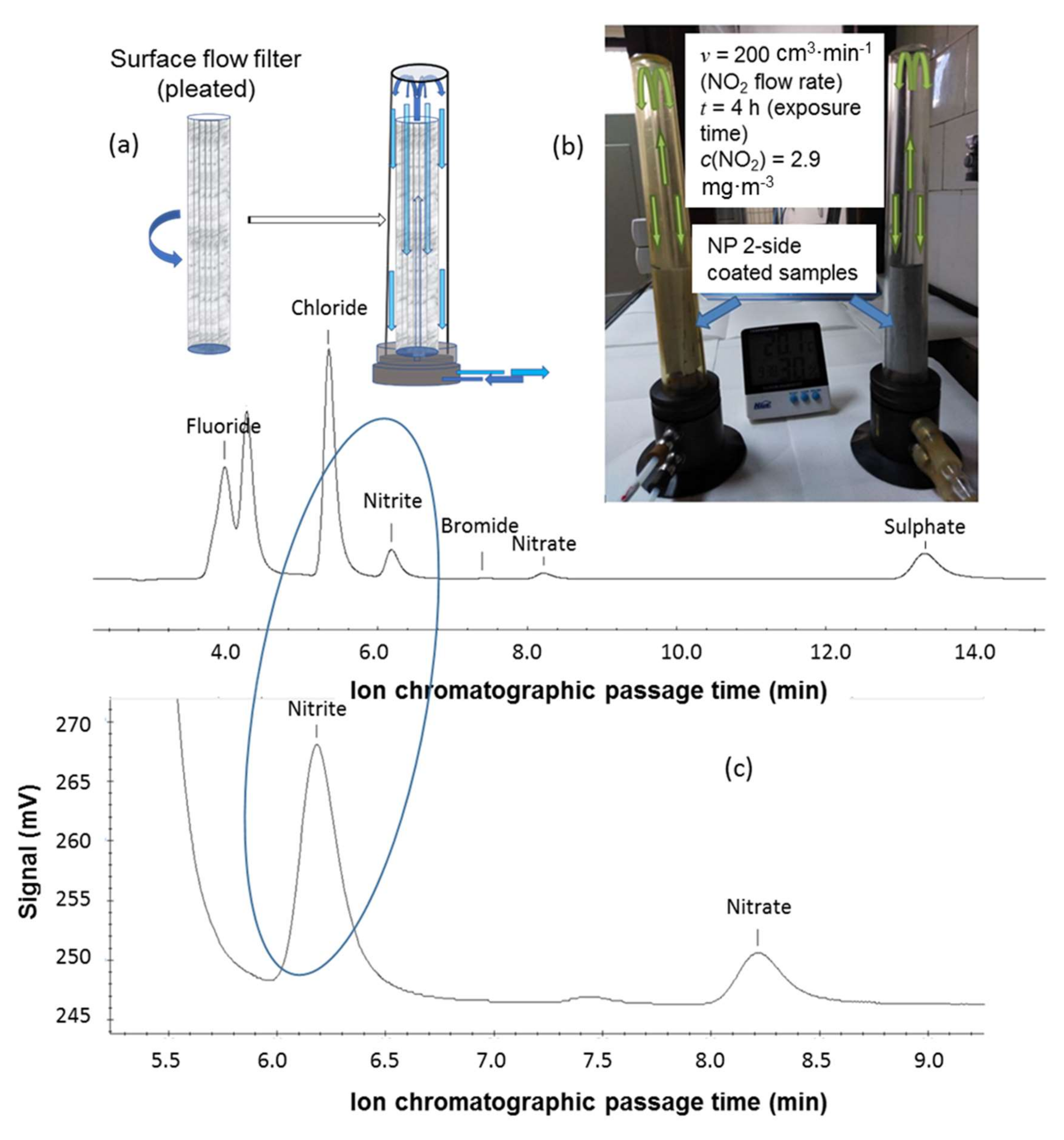
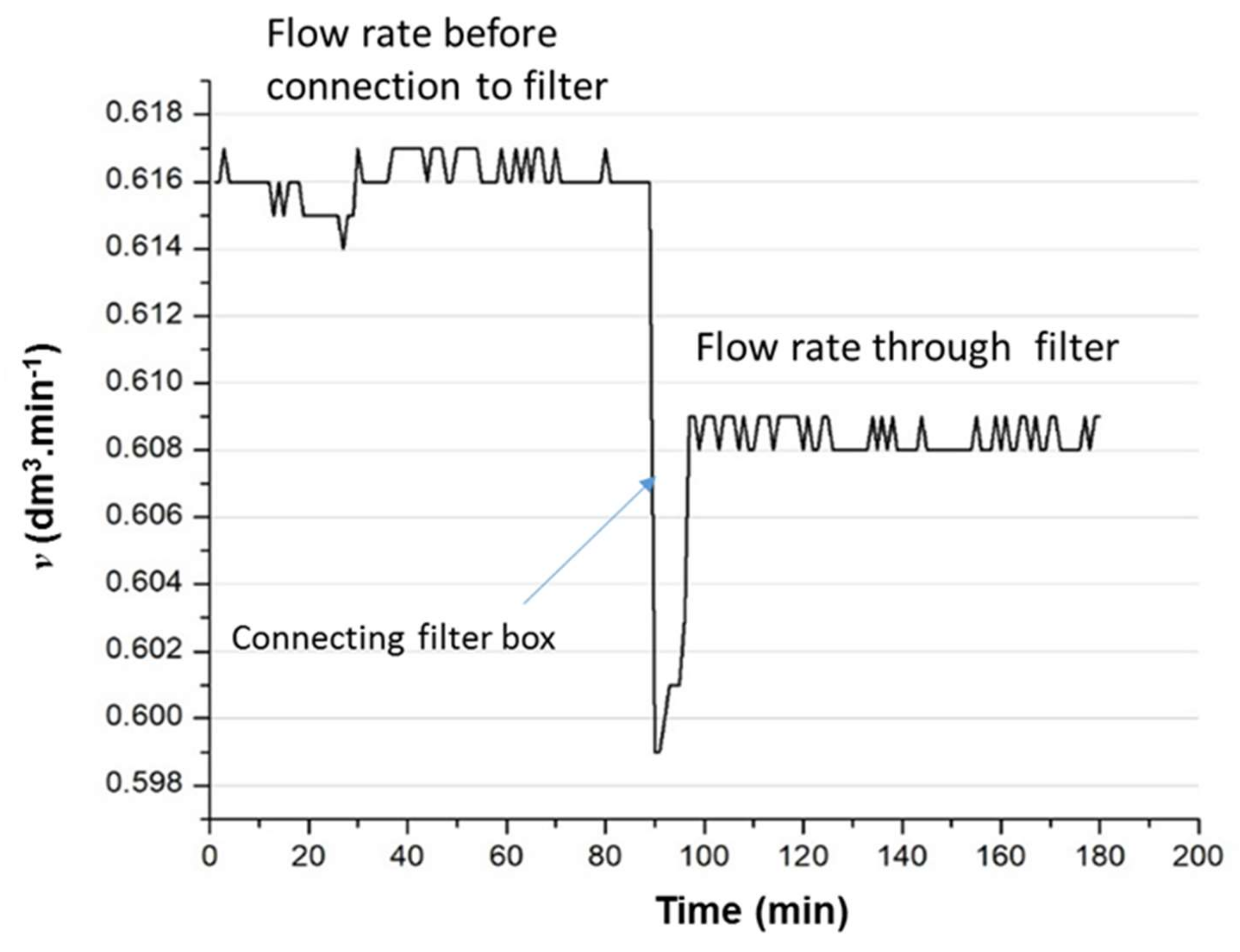
3.1.5. Surface Flow Method
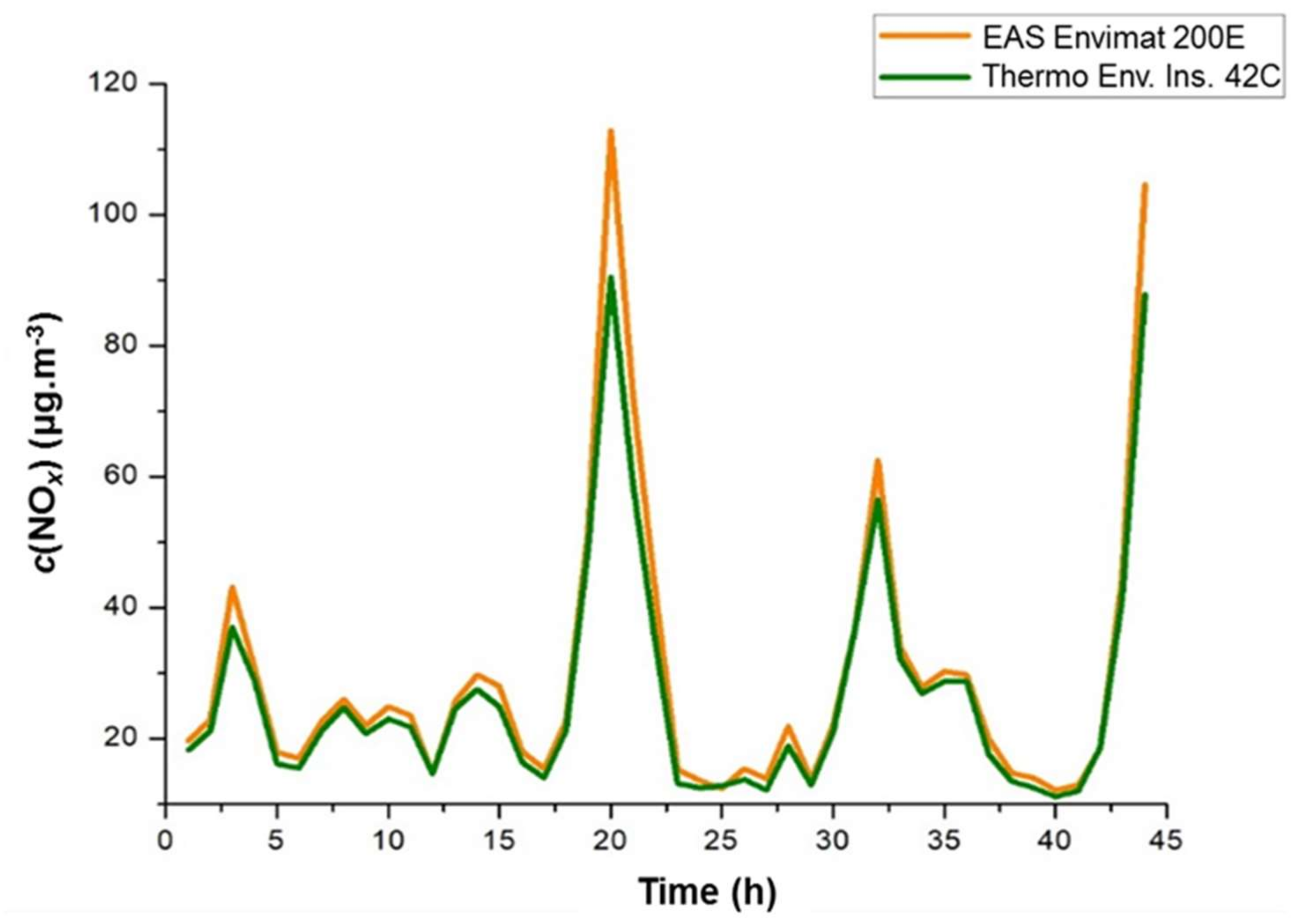
3.2. Filter–Surface Flow in Real-time Air Environment
3.3. Bacterial Activity
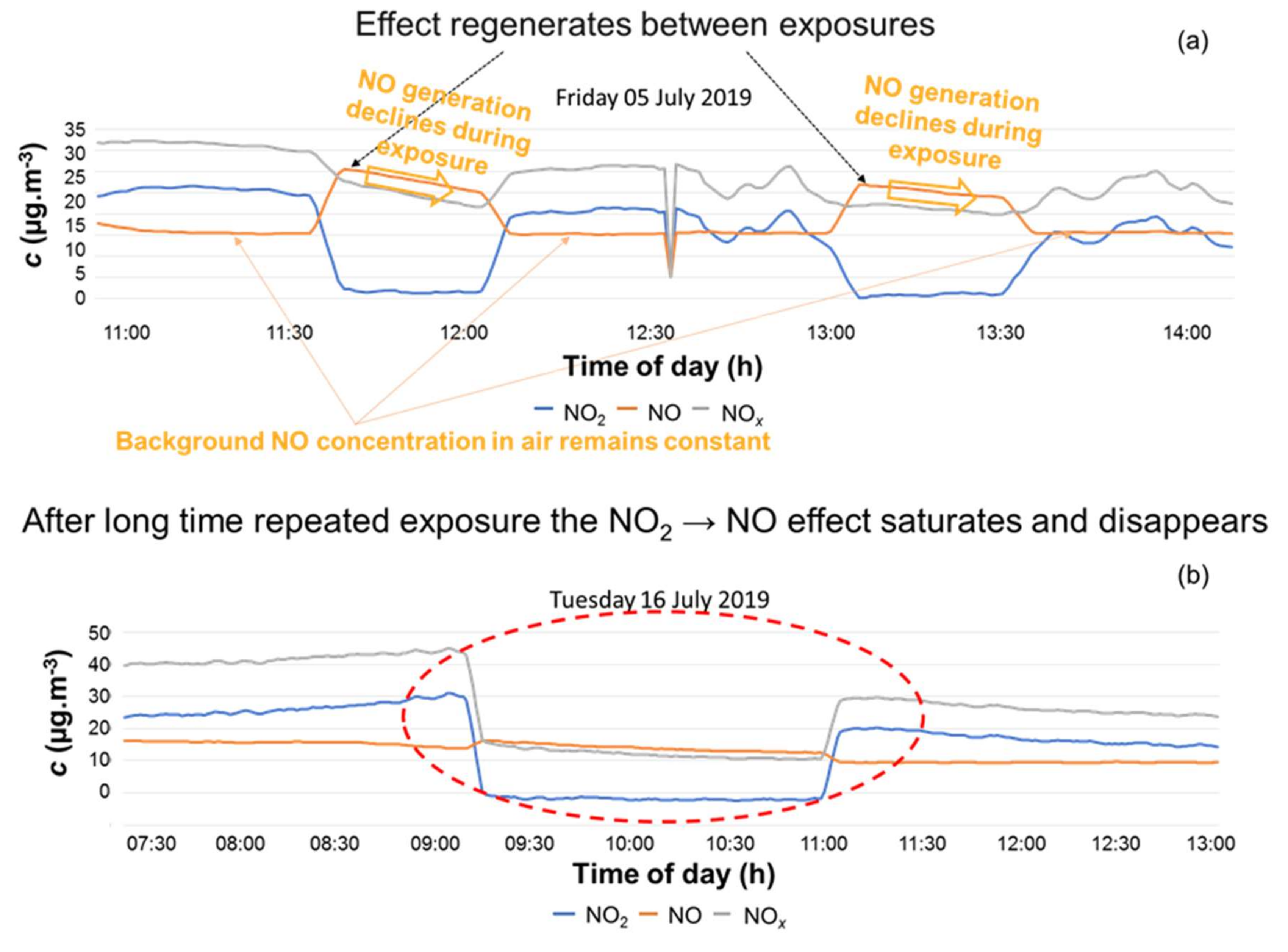
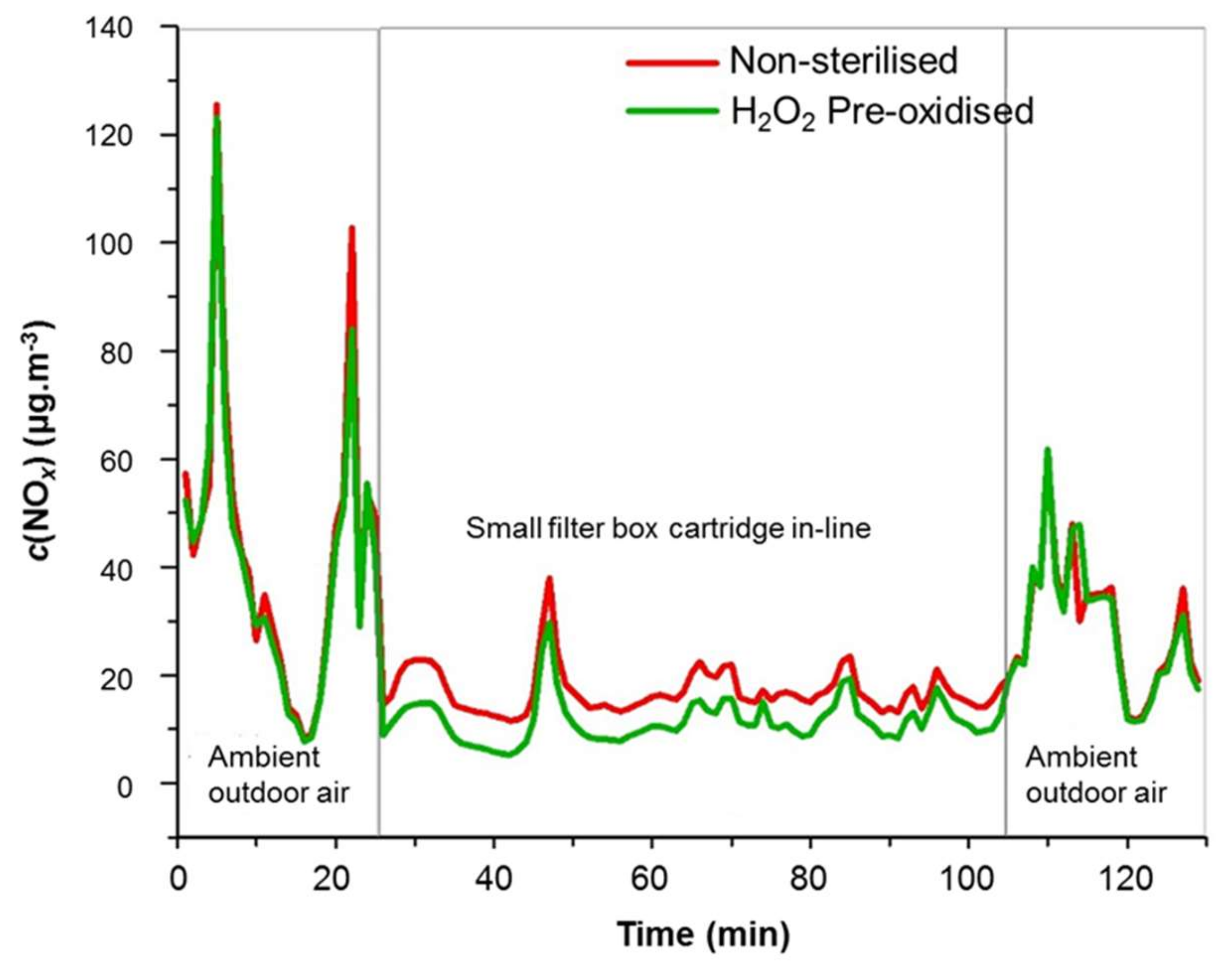
3.3.1. Sterilisation versus Pre-Oxidation
3.3.2. Cellulose Breakdown
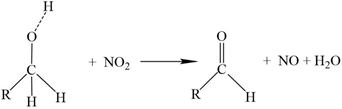
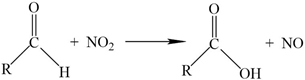

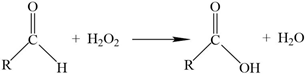
3.4. Sorption Capacity Check between Pre-Oxidised and Non-Sterilised Filters
3.5. Theoretical Filter Lifetime Capacity
= LTexperimental/{100 × 1000 dm3·min−1}/{0.607 dm3·min−1}
= LTexperimental/165,000
3.6. Capturing in-situ by-Product (CO2)
3.7. Meeting Regulatory Requirements: Heavy Element (HE) Analysis
Heavy Element Analysis
4. Discussion
4.1. Perspectives on Real Application
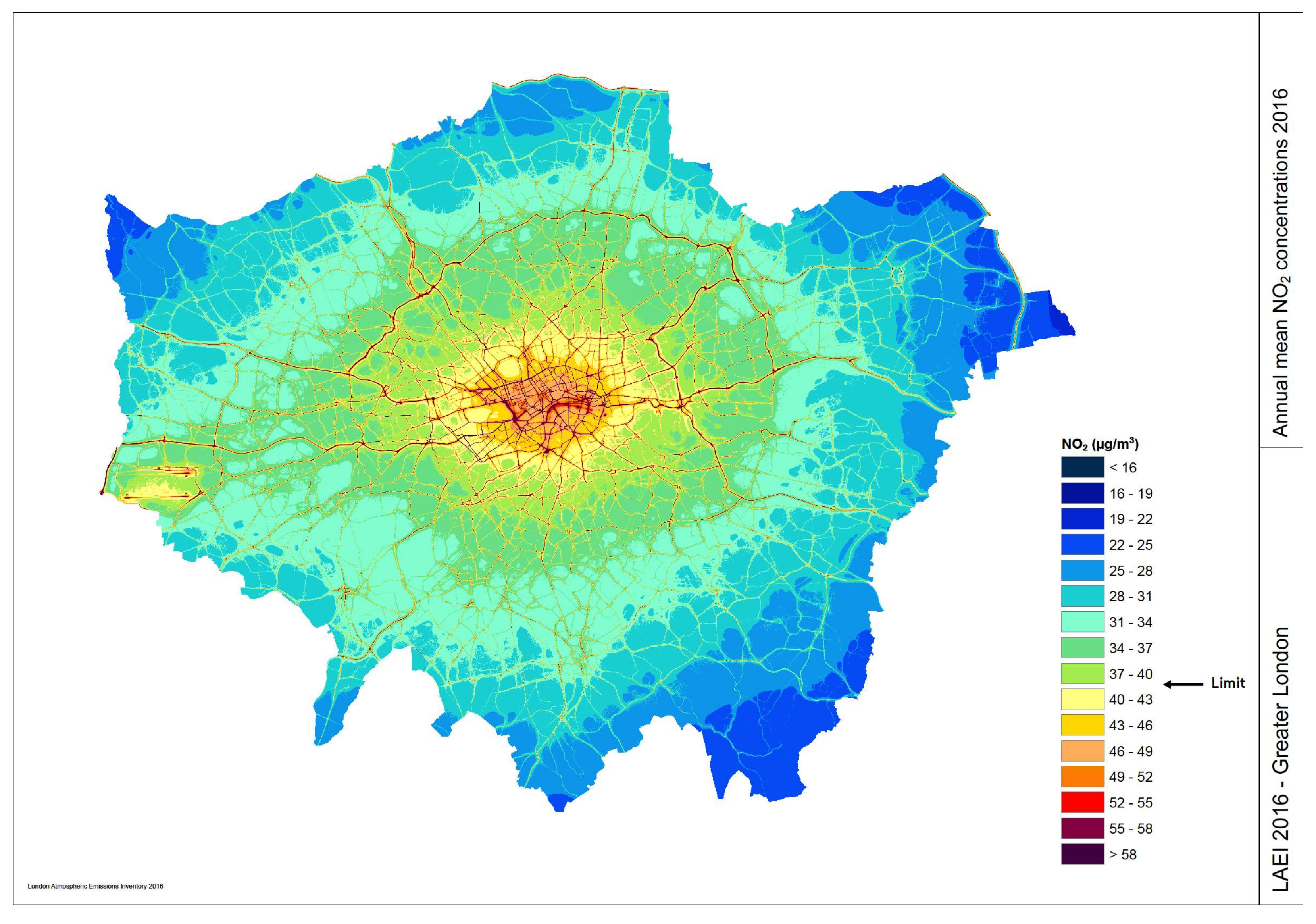
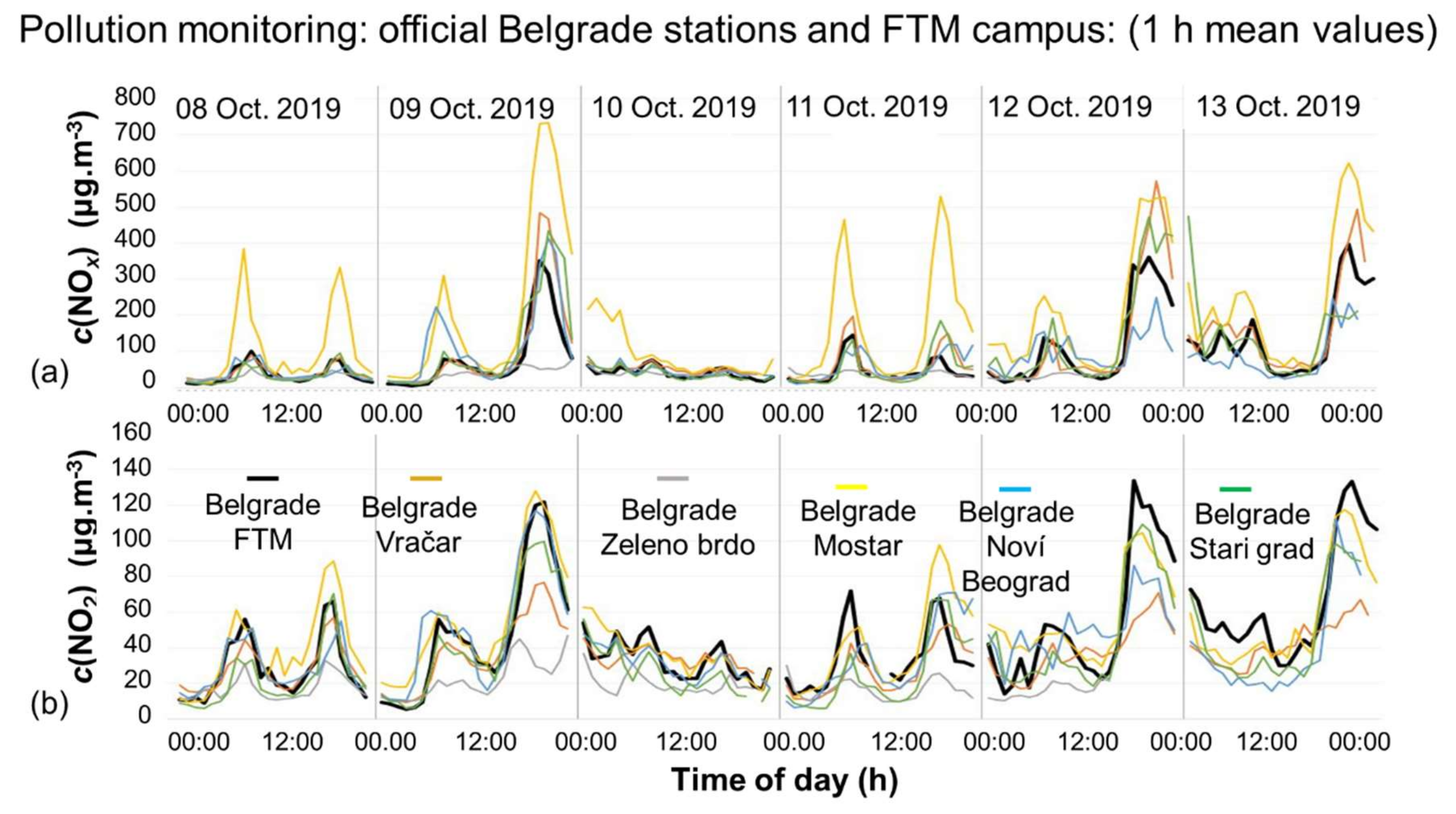
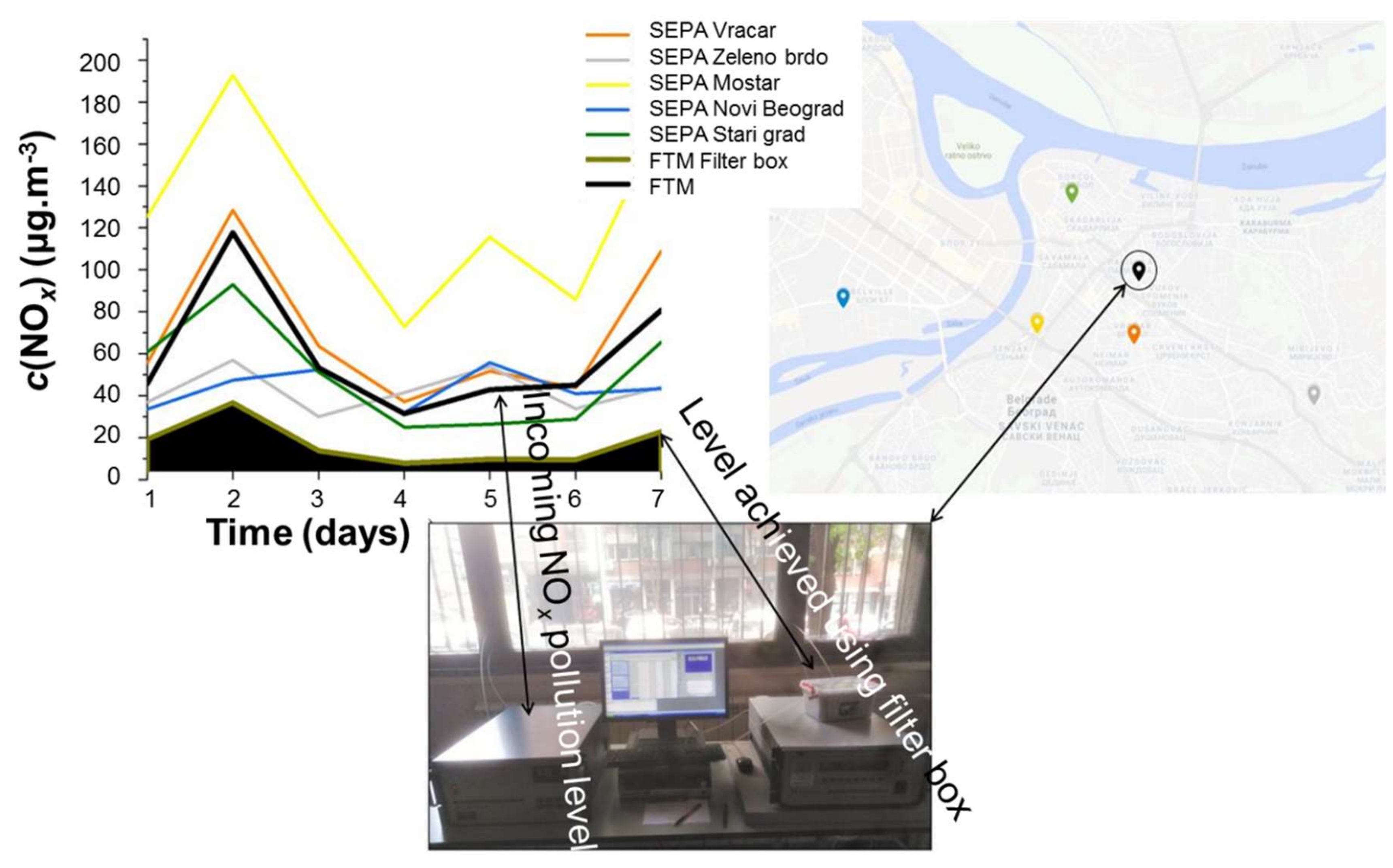
4.1.1. Belgrade City Air
4.1.2. Putting the Filter into Action
5. Conclusions
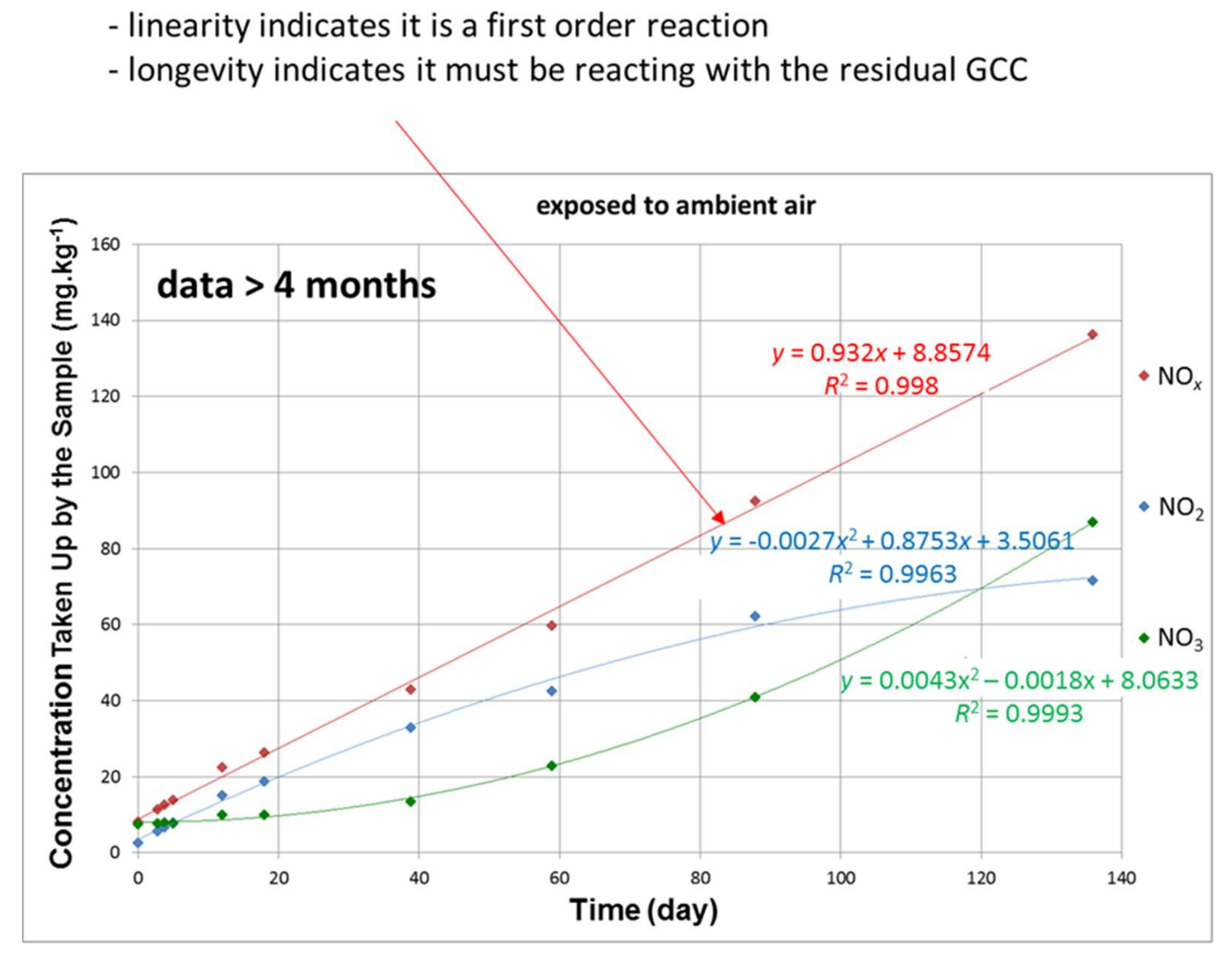
- Proof of principle was shown for a filter design for the mitigation of gaseous environmental NOx based on no longer viable, multiple, recycled cellulose fibre substrates (newsprint fibre) coated with a reactive coating comprising a fine particulate calcium carbonate combined with a binder-humectant, which is derived from the same recycled fibre source as the substrate, in the form of micro nano-fibrillated cellulose.
- A laboratory filter construct suitable for illustrating a basis for upscaling was designed by employing the filter material developed. The construct was shown to achieve successful depletion of NOx via the sorption primarily of NO2. NO2 is a pulmonary irritant considered to be highly detrimental to health, and it leads to reduced life expectancy with prolonged exposure.
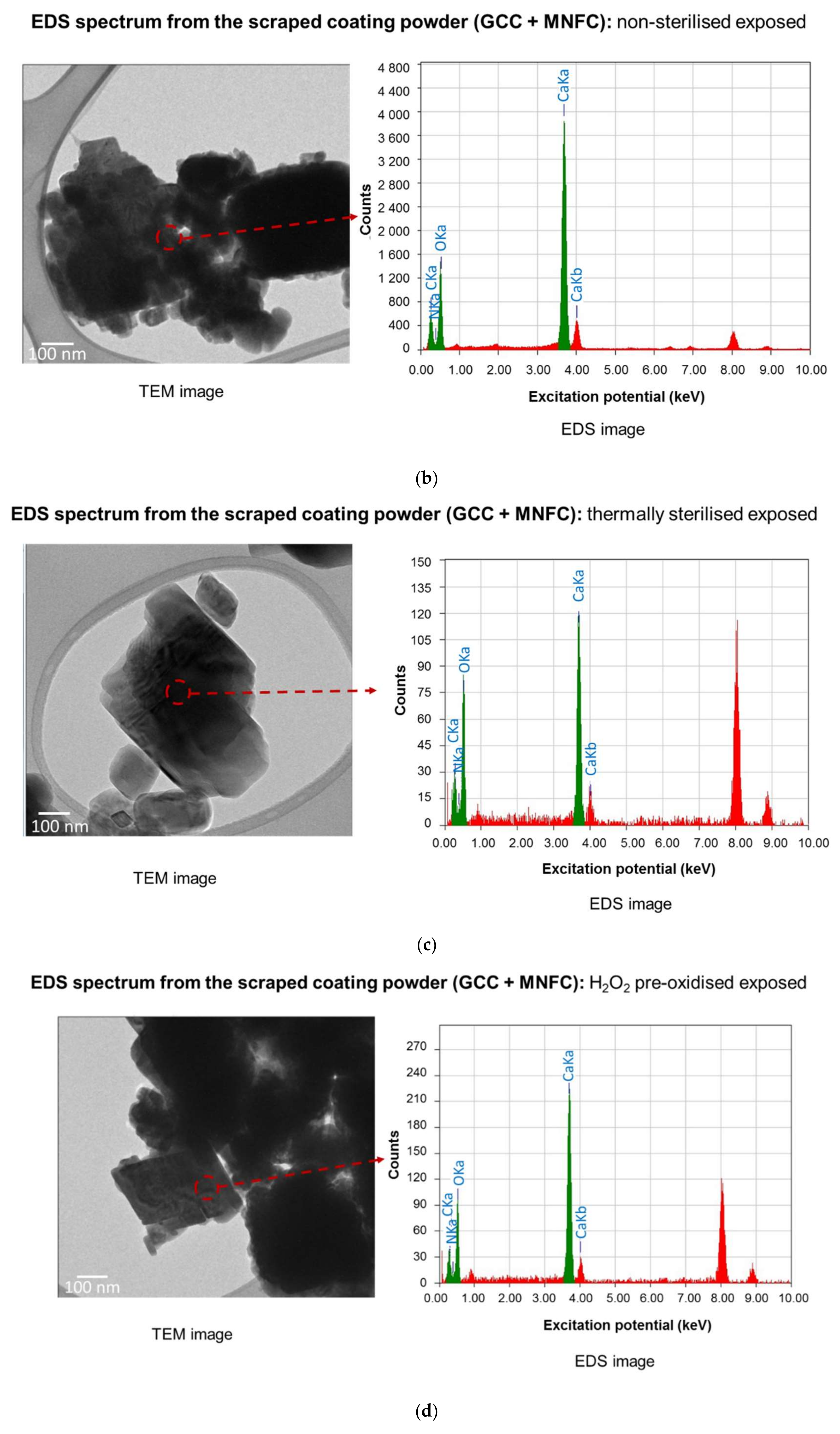
- Since EDS analysis initially failed to identify retention of N species, the additional action of de-nitrification bacteria was suspected. Microbial DNA analysis confirmed the presence of several species of de-nitrification bacteria.
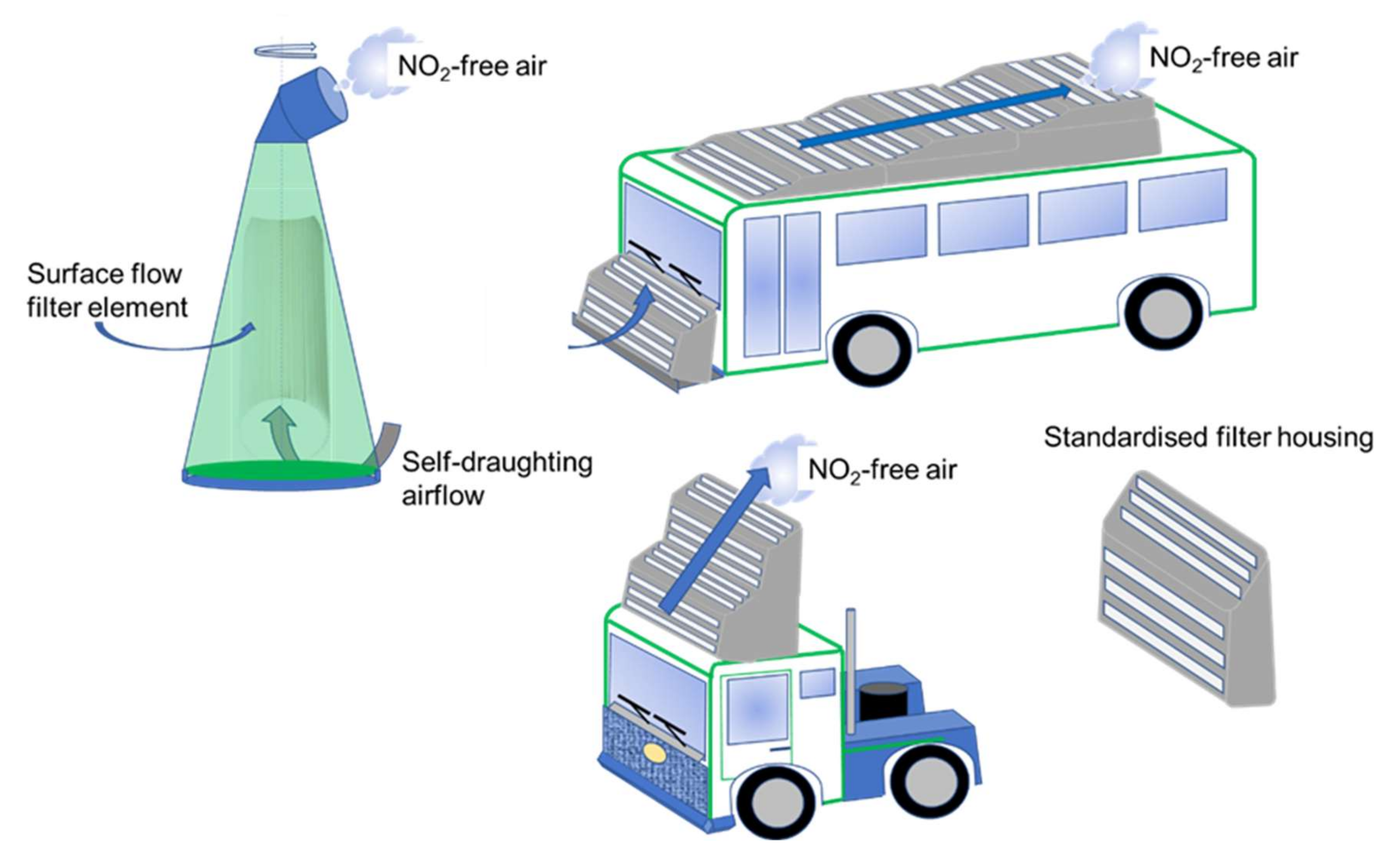
- Proposals for application include street-side self-draughting installations and/or motion-draughted filters mounted on vehicles. The most likely successful option is to consider municipal and public transport vehicles followed by haulage-trucks.
- The designs developed with respect to special coating constructs in the form of discrete points of coating (pixelate coating) to enhance gaseous exchange and to support turbulent surface flow are not limited to the application illustrated in this work. The designs will be of value in enabling low energy surface-flow filter geometries to be used instead of high-energy through-flow systems.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission (EC). Directive 2010/75/EU. Directive of the European Parliament and of the Council of 24 November 2010 on Industrial Emission: Integrated Pollution Prevention and Control, the Industrial Emissions Directive (IED); European Union: Brussels, Belgium, 2010. [Google Scholar]
- Peters, H.A.R.; Toxopeus, M.E.; Jauregui-Becker, J.M.; Dirksen, M.-O. Prioritizing ‘design for recyclability’ guidelines, bridging the gap between recyclers and product developers. In Leveraging Technology for a Sustainable World; Dornfeld, D.A., Linke, B.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 203–208. [Google Scholar]
- FoodDrinkEurope. Guidelines on the Safe Use of Paper and Board Made from Recycled Fibres for Food Contact Use; FoodDrinkEurope: Brussels, Belgium, 2016. [Google Scholar]
- Schneider, A.; Cyrys, J.; Breitner, S.; Kraus, U.; Peters, A.; Diegmann, V.; Neunhäuserer, L. Quantifizierung von Umweltbedingten Krankheitslasten Aufgrund der Stickstoffdioxid-Exposition in Deutschland [Quantification of Environmental Disease Burden Due to Nitrogen Dioxide Exposure in Germany]; Abschlussbericht [Closing Report]; Umweltbundesamt: Dessau-Roßlau, Germany, 2018. [Google Scholar]
- Buchal, C.; Karl, H.-D.; Sinn, H.-W. Kohlemotoren, windmotoren und dieselmotoren: Was zeigt die CO2-bilanz? [Coal engines, wind engines and diesel engines: How do the carbon footprints compare?]. ifo Schnelld. 2019, 72, 40–54. [Google Scholar]
- Wietschel, M.; Kühnbach, M.; Rüdiger, D. Die Aktuelle Treibhausgasemissionsbilanz von Elektrofahrzeugen in Deutschland [The Current Greenhouse Gas Emission Balance of Electric Vehicles in Germany]; (No. S02/2019); Working Paper Sustainability and Innovation; Fraunhofer ISI: Karlsruhe, Germany, 2019. [Google Scholar]
- Wietschel, M.; Jung, A.-C. Electric Cars Purchased and Used in Germany Today Have a Much Better Carbon Footprint than Diesel or Gasoline-powered Cars; No. S 02/2019; Fraunhofer Institute for Systems and Innovation Research ISI: Karlsruhe, Germany, 2019. [Google Scholar]
- Ozcanli, M.; Bas, O.; Akar, M.A.; Yildizhan, S.; Serin, H. Recent studies on hydrogen usage in Wankel SI engine. Int. J. Hydrog. Energy 2018, 43, 18037–18045. [Google Scholar] [CrossRef]
- Dotan, H.; Landman, A.; Sheehan, S.W.; Malviya, K.D.; Shter, G.E.; Grave, D.A.; Arzi, Z.; Yehudai, N.; Halabi, M.; Gal, N.; et al. Decoupled hydrogen and oxygen evolution by a two-step electrochemical–chemical cycle for efficient overall water splitting. Nat. Energy 2019, 4, 786–795. [Google Scholar] [CrossRef]
- European Commission. Future Brief: What Are the Health Costs of Environmental Pollution? Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Desaigues, B.; Ami, D.; Bartczak, A.; Braun-Kohlová, M.; Chilton, S.; Czajkowski, M.; Farreras, V.; Hunt, A.; Hutchinson, M.; Jeanrenaud, C.; et al. Economic valuation of air pollution mortality: A 9-country contingent valuation survey of value of a life year (VOLY). Ecol. Indic. 2011, 11, 902–910. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Busker, L.H. Effects of wet pressing on paper quality. In TAPPI Engineering Conference, Proceedings of the Technical Association of the Pulp and Paper Industry; TAPPI Press: Atlanta, GA, USA, 1985; pp. 117–129. [Google Scholar]
- Carrillo, C.A.; Laine, J.; Rojas, O.J. Microemulsion systems for fiber deconstruction into cellulose nanofibrils. ACS Appl. Mater. Interfaces 2014, 6, 22622–22627. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Brief description of the pulp and papermaking process. In Biotechnology for Pulp and Paper Processing; Springer: New York, NY, USA, 2018; pp. 9–26. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sing, K.S.W. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76–77, 3–11. [Google Scholar] [CrossRef]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–813. [Google Scholar]
- Li, J.; Wang, Y.; Wei, X.; Wang, F.; Han, D.; Wang, Q.; Kong, L. Homogeneous isolation of nanocelluloses by controlling the shearing force and pressure in microenvironment. Carbohydr. Polym. 2014, 113, 388–393. [Google Scholar] [CrossRef]
- Ämmälä, A.; Laitinen, O.; Sirviö, J.A.; Liimatainen, H. Key role of mild sulfonation of pine sawdust in the production of lignin containing microfibrillated cellulose by ultrafine wet grinding. Ind. Crops Prod. 2019, 140, 111664. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Gane, P.A.C.; Paltakari, J. Micro and nanofibrillated cellulose as a rheology modifier additive in CMC-containing pigment-coating formulations. Ind. Eng. Chem. Res. 2013, 52, 16066–16083. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Puisto, A.; Gane, P.; Nieminen, K.; Alava, M.; Paltakari, J.; Maloney, T. The role of MFC/NFC swelling in the rheological behavior and dewatering of high consistency furnishes. Cellulose 2013, 20, 2847–2861. [Google Scholar] [CrossRef]
- Schenker, M.; Schoelkopf, J.; Mangin, P.; Gane, P. Rheological investigation of complex micro nanofibrillated cellulose (MNFC) suspensions: Discussion of flow curves and gel stability. TAPPI J. 2016, 15, 405–416. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Puisto, A.; Paltakari, J.; Alava, M.; Maloney, T. The influence of shear on the dewatering of high consistency nanofibrillated cellulose furnishes. Cellulose 2013, 20, 1853–1864. [Google Scholar] [CrossRef]
- Rantanen, J.; Dimic-Misic, K.; Kuusisto, J.; Maloney, T.C. The effect of micro and nanofibrillated cellulose water uptake on high filler content composite paper properties and furnish dewatering. Cellulose 2015, 22, 4003–4015. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials-binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef]
- Velthaus, K.-O.; Göbel, T.; Choi, J.H. Vector network analyser. WO Patent No. WO2017017579, 2017. [Google Scholar]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and conductive nanocellulose-based films for active and intelligent food packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef]
- RSN1 Info. Inside Europe: Belgrade Pollution Breaks Record. News Bulletin Serbian National 1. 2019. Available online: https://www.dw.com/en/inside-europe-belgrade-pollution-breaks-record/av-51545460 (accessed on 1 December 2019).
- SEPA. Godišnji Izveštaj o Stanju Kvaliteta Vazduha u Republici Srbiji za 2018 Godinu [Annual Report on the State of Air Quality in the Republic of Serbia for 2018]; Serbian Environmental Protection Agency: Belgrade, Serbia, 2019. Available online: http://www.sepa.gov.rs/download/izv/Vazduh2018_final.pdf (accessed on 13 November 2019).
- Serbian Monitor. Belgrade Becomes One of the Most Polluted Capital Cities. 2019. Available online: https://www.serbianmonitor.com/en/belgrade-becomes-one-of-the-most-polluted-capital-cities/ (accessed on 1 December 2019).
- Jernigan, A.J.R. Chemiluminescence NOx and GFC NDIR CO Analyzers for Low Level Source Monitoring; Thermo Environmental Instruments: Franklin, MA, USA, 2001. [Google Scholar]
- Hunter, R.J. Foundations of Colloid Science, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Fernandes, A.C.; Antunes, F.; Pires, J. Sepiolite based materials for storage and slow release of nitric oxide. New J. Chem. 2013, 37, 4052–4060. [Google Scholar] [CrossRef]
- Kocaoba, S. Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J. Mechanisms of trace metal transport in rivers. Science 1973, 180, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Alborés, A.F.; Cid, B.P.; Gómez, E.F.; López, E.F. Comparison between sequential extraction procedures and single extractions for metal partitioning in sewage sludge samples. Analyst 2000, 125, 1353–1357. [Google Scholar] [CrossRef]
- Real-time Air Quality. Global Open Data Index 2019. Available online: https://index.okfn.org/place/rs/ (accessed on 19 October 2019).
- SEPA. National network of automatic stations for air quality monitoring. Available online: http://www.amskv.sepa.gov.rs/?lng=en (accessed on 19 October 2019).







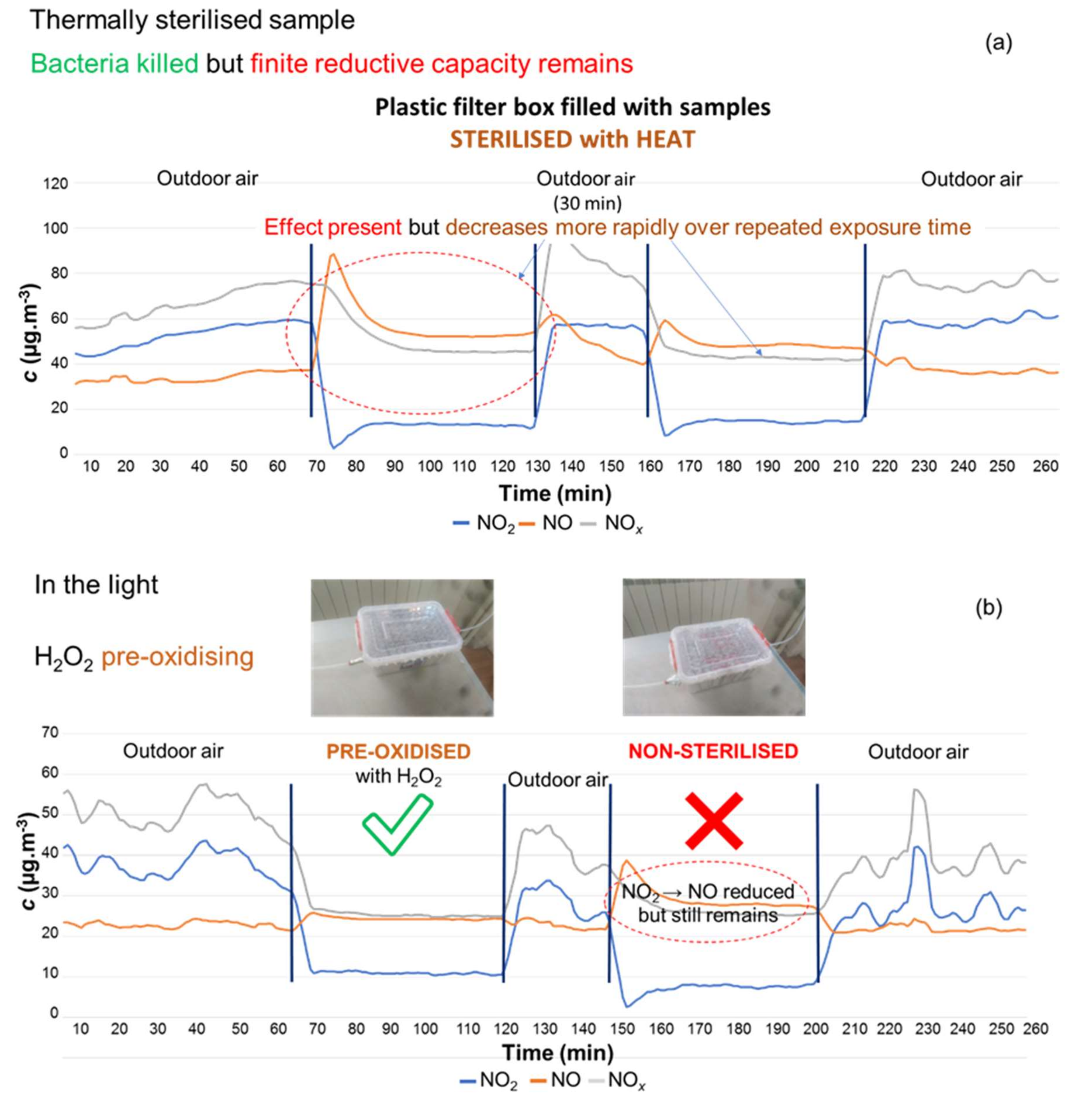
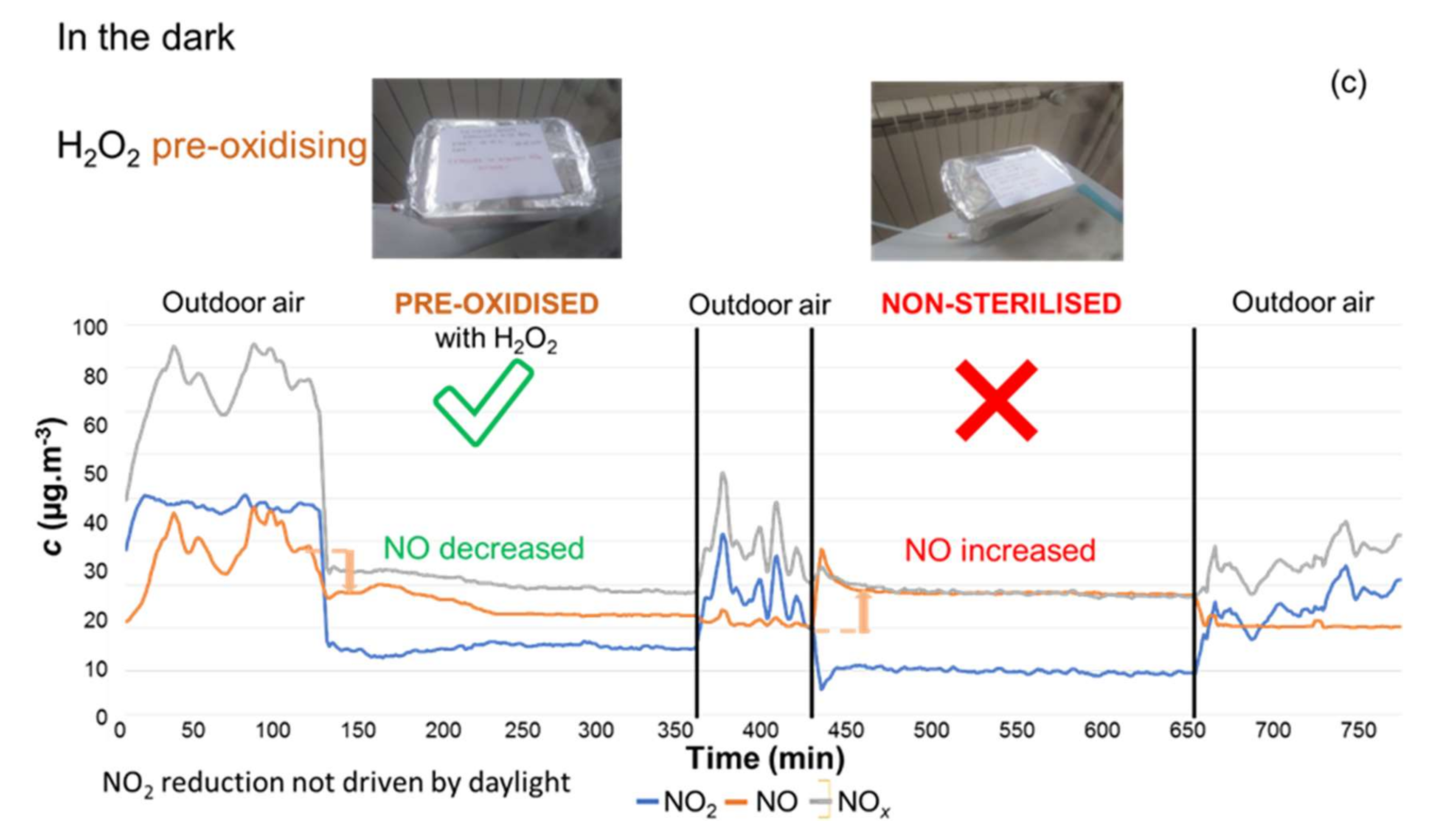
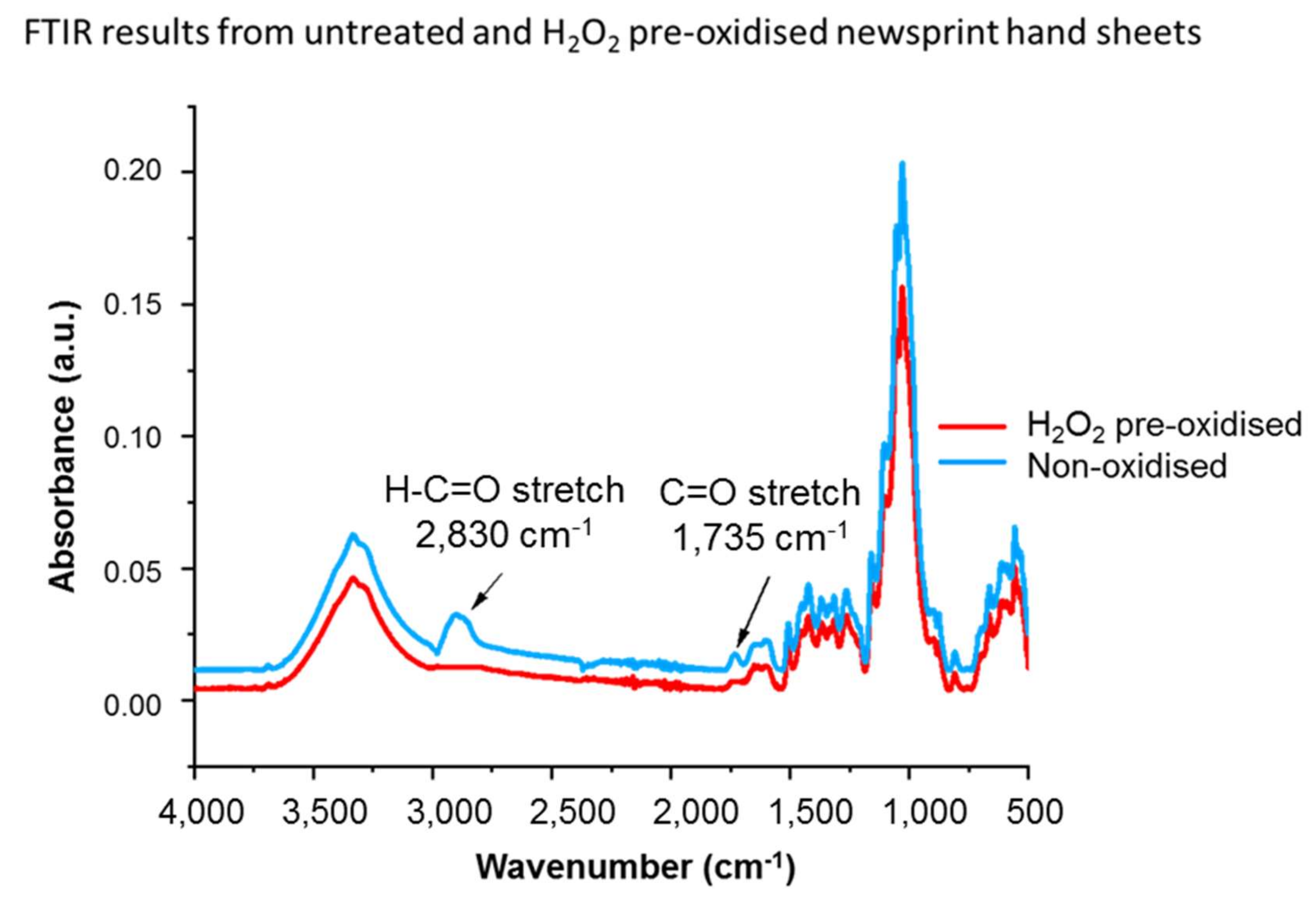
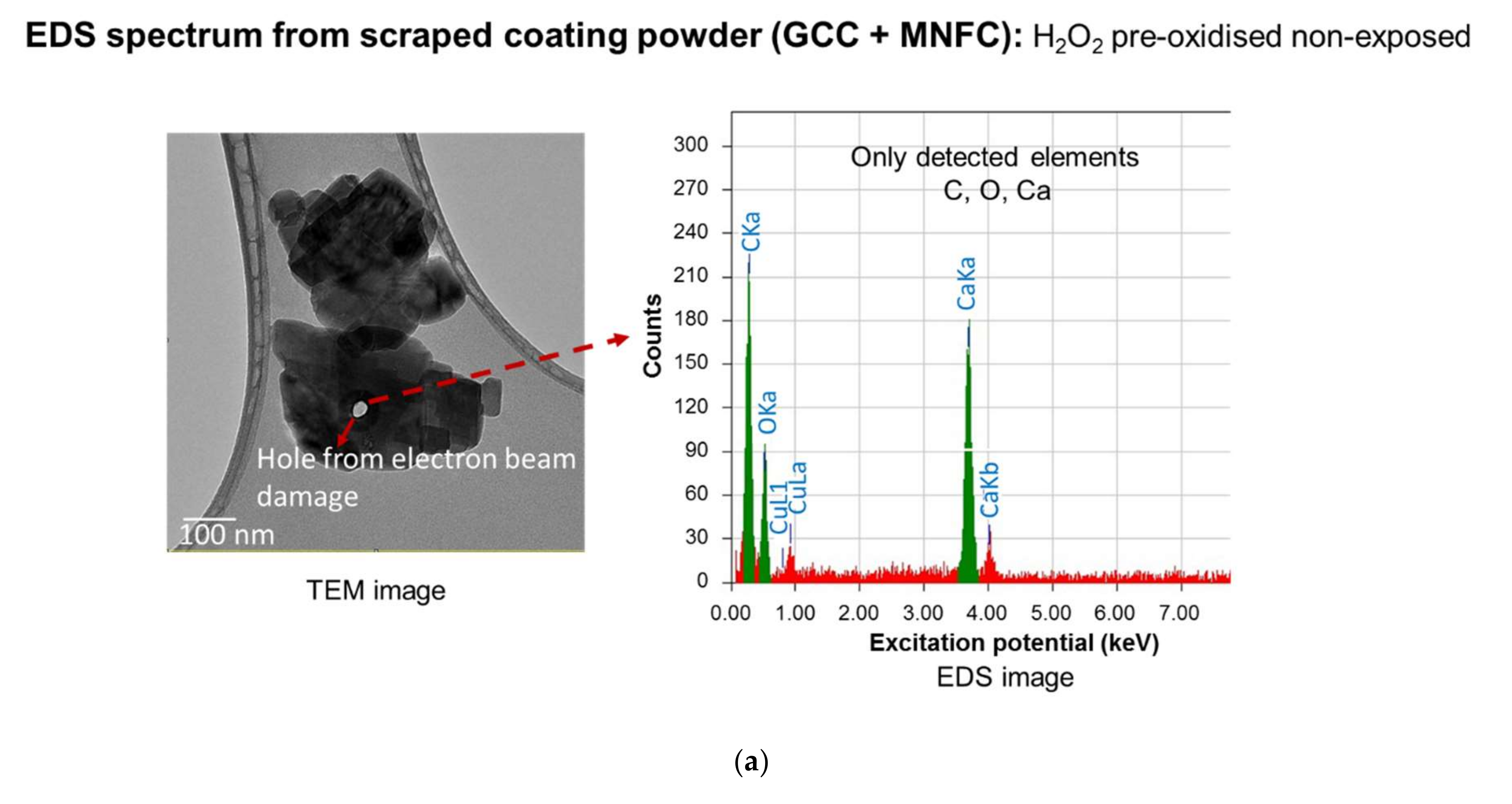

| Pollutant | Concentration (µg·m−3) | Averaging Period | Legal Requirement | Permitted Number of Exceedances per Year |
|---|---|---|---|---|
| Nitrogen dioxide (NO2) | 200 | 1 h | Limit value to be met as of 1.1.2010 | 18 |
| 40 | 1 year | Limit value to be met as of 1.1.2010 * | n/a |
| Burden of NO2 Exposure | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|---|
| Attributable fraction 1 (%) | 2.19 | 2.29 | 2.26 | 2.26 | 1.86 | 1.87 | 1.58 | 1.77 |
| Attributable deaths 2 | 7832 | 8157 | 8035 | 7960 | 6343 | 6531 | 5605 | 5966 |
| Years of life lost 3 (YLL) | 69,244 | 71,396 | 69,526 | 68,428 | 53,489 | 54,536 | 46,795 | 49,726 |
| YLL per 100,000 population | 122.78 | 126.40 | 123.04 | 120.85 | 94.10 | 95.48 | 83.13 | 87.96 |
| Sample | Area, A (m2) | Mass, m (g) | Basis Weight (g·m−2) | Thickness, t (μm) | Volume, V (cm3) | Density, D (g·cm−3) | Porosity (%) |
|---|---|---|---|---|---|---|---|
| * UK | 0.027 | 2.18 | 80 | 218 | 5.94 | 0.37 | 75.6 |
| ** NP | 0.027 | 2.18 | 80 | 183 | 4.98 | 0.44 | 70.9 |
| Volume Defined Particle Size Distribution Data Measured by Light Scattering | ||
|---|---|---|
| Particle Size 1 | Before Sonication (µm) | After Sonication (µm) |
| d10 (vol) 2 | 3.33 | 1.12 |
| d50 (vol) | 4.61 | 1.41 |
| d90 (vol) | 6.05 | 1.77 |
| Sample | Specific Surface Area, SSA (m2·g−1) | Total Pore Volume, Vtot (cm3·g−1) | Mesopore Volume, Vmeso (cm3·g−1) | Micropore Volume Vmicro (cm3·g−1) | Pore Diameter from Alpha S-Plot, dsr (nm) |
|---|---|---|---|---|---|
| CaCO3 (GCC) | 5.05 | 0.0192 | 0.0184 | 0.0023 | 20.9 |
| Nitrite and Nitrate Concentrations Determined by Ion Chromatography (mg·dm−3) | ||
|---|---|---|
| Sample | Nitrite (NO2)− | Nitrate (NO3)− |
| UK ! blank* | 0.0 | 2.0 |
| UK blank repeat * | 0.0 | 1.8 |
| NP !! blank * | 0.0 | 2.3 |
| NP blank repeat * | 0.0 | 0.8 |
| UK exposed ** | 1.5 | 3.6 |
| UK exposed repeat ** | 1.3 | 4.1 |
| NP exposed ** | 1.0 | 4.0 |
| NP exposed repeat ** | 1.1 | 7.0 |
| Sample | Specific Surface Area, SSA (m2·g−1) | Total Pore Volume, Vtot (cm3·g−1) | Mesopore Volume, Vmeso (cm3·g−1) | Micropore Volume, Vmicro (cm3·g−1) | Pore Diameter from Alpha S-Plot, dsr (nm) |
|---|---|---|---|---|---|
| CaCO3 (GCC) | 5.05 | 0.0192 | 0.0184 | 0.0023 | 20.9 |
| HAP | 98.90 | 0.5738 | 0.5743 | 0.0281 | 13.4 |
| Sample | NO2− (mg·dm−3) | NO3− (mg·dm−3) | NO2− (mg·kg−1) Dry Material | NO3− (mg·kg−1) Dry Material |
|---|---|---|---|---|
| CaCO3 exposed | 1.2 | 2.3 | 6.9 | 13.9 |
| HAP (on CaCO3) exposed | 1.6 | 2.3 | 9.7 | 13.8 |
| Sample | NO2− (mg·kg−1filter) | NO3− (mg·kg−1filter) |
|---|---|---|
| NP coated exposed REGULAR | 36.82 | 41.34 |
| NP coated exposed WET | 137.97 | 30.04 |
| NP coated non-exposed BLANK | 14.67 | 31.25 |
| Sample | Cultured Extract | ||||
|---|---|---|---|---|---|
| Total Viable Count (TVC) of Aerobic Bacteria (cfu·g−1sample) | Observed Growth | Nitrite (mg·cm−3) | Organism | Score | |
| Uncoated NP substrate non-sterilised 1 | > 105 | yes | 50 | - | - |
| Uncoated NP substrate thermally sterilised | 74 × 103 | yes | 50 | Bacillus sp. 3 | 1.839 |
| Uncoated NP substrate treated with H2O2 | > 105 | yes | 50 | Enterobacter kobei | 2.349 |
| Coated NP substrate non-sterilised | > 105 | yes | 50 | Bacillus cereus | 2.081 |
| Coated NP substrate thermally sterilised 1 | 8 × 103 | yes | 50 | - | - |
| Coated NP substrate treated with H2O2 | > 105 | yes | 50 | Enterobacter kobei | 2.320 |
| GCC non-sterilised 2 | 4 × 103 | - | - | - | - |
| MNFC non-sterilised | > 105 | yes | 50 | Bacillus licheniformis | 2.057 |
| Laboratory bacteria-free control | - | no | 0 | - | - |
| Element | Excitation Potential (keV) | Mass Proportion (%) | Atom Occurrence (%) |
|---|---|---|---|
| Scraped coating powder (GCC + MNFC): non-sterilised exposed | |||
| C | 0.27 | 23.23 | 40.41 |
| N | 0.39 | 2.95 | 4.4 |
| O | 0.52 | 21.28 | 27.79 |
| Ca | 3.69 | 52.55 | 27.4 |
| Scraped coating powder (GCC + MNFC): thermally sterilised exposed | |||
| C | 0.27 | 24.94 | 38.13 |
| N | 0.39 | 7.47 | 9.8 |
| O | 0.52 | 30.59 | 35.12 |
| Ca | 3.69 | 36.99 | 16.95 |
| Scraped coating powder (GCC + MNFC): H2O2 pre-oxidised exposed | |||
| C | 0.27 | 23.14 | 38.07 |
| N | 0.39 | 9.21 | 12.99 |
| O | 0.52 | 20.99 | 25.93 |
| Ca | 3.69 | 46.67 | 23.01 |
| Samples | Mean ± SD | Median (Min–Max) | Skewness | Kurtosis |
|---|---|---|---|---|
| Filter box with H2O2 pre-oxidised samples | 11.6 ± 4.1 | 10.7 (5.3–29.8) | 1.710 | 5.249 |
| Filter box with non-sterilised samples displaying NO2 → NO conversion | 17.1 ± 4.2 | 16.2 (11.5–38.0) | 2.079 | 7.148 |
| Ambient outdoor NOx * | 36.3 ± 22.6 | 32.6 (8.1–125.5) | 1.733 | 4.021 |
| Water Soluble Fraction, c (mg·kg−1) | ||||||
| Pb | Cd | Hg | As | Sb | Cu | |
| Filter exposed | 0.42 | < DL | < DL | 0.60 | < DL | < DL |
| Blank non-exposed | 0.42 | < DL | < DL | 0.66 | < DL | < DL |
| Acid Soluble Fraction, c (mg·kg−1) | ||||||
| Pb | Cd | Hg | As | Sb | Cu | |
| Filter exposed | 0.84 | < DL | < DL | 0.48 | < DL | < DL |
| Blank non-exposed | 0.36 | < DL | < DL | 0.75 | < DL | < DL |
| Total HE Content, c (mg·kg−1) | ||||||
| Pb | Cd | Hg | As | Sb | Cu | |
| Filter exposed | 42.00 | < DL | < DL | 0.51 | < DL | 72.00 |
| Blank non-exposed | 54.00 | < DL | < DL | 0.66 | < DL | 96.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gane, P.; Dimić-Mišić, K.; Barać, N.; Imani, M.; Janaćković, D.; Uskoković, P.; Barceló, E. Unveiling a Recycling-Sourced Mineral-Biocellulose Fibre Composite for Use in Combustion-Generated NOx Mitigation Forming Plant Nutrient: Meeting Sustainability Development Goals in the Circular Economy. Appl. Sci. 2020, 10, 3927. https://doi.org/10.3390/app10113927
Gane P, Dimić-Mišić K, Barać N, Imani M, Janaćković D, Uskoković P, Barceló E. Unveiling a Recycling-Sourced Mineral-Biocellulose Fibre Composite for Use in Combustion-Generated NOx Mitigation Forming Plant Nutrient: Meeting Sustainability Development Goals in the Circular Economy. Applied Sciences. 2020; 10(11):3927. https://doi.org/10.3390/app10113927
Chicago/Turabian StyleGane, Patrick, Katarina Dimić-Mišić, Nemanja Barać, Monireh Imani, Djordje Janaćković, Petar Uskoković, and Ernest Barceló. 2020. "Unveiling a Recycling-Sourced Mineral-Biocellulose Fibre Composite for Use in Combustion-Generated NOx Mitigation Forming Plant Nutrient: Meeting Sustainability Development Goals in the Circular Economy" Applied Sciences 10, no. 11: 3927. https://doi.org/10.3390/app10113927
APA StyleGane, P., Dimić-Mišić, K., Barać, N., Imani, M., Janaćković, D., Uskoković, P., & Barceló, E. (2020). Unveiling a Recycling-Sourced Mineral-Biocellulose Fibre Composite for Use in Combustion-Generated NOx Mitigation Forming Plant Nutrient: Meeting Sustainability Development Goals in the Circular Economy. Applied Sciences, 10(11), 3927. https://doi.org/10.3390/app10113927