One-Step and Morphology-Controlled Synthesis of Ni-Co Binary Hydroxide on Nickel Foam for High-Performance Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ni-Co Binary Hydroixde on Nickel Foam
2.2. Material Characterizations
2.3. Electrochemical Measurements
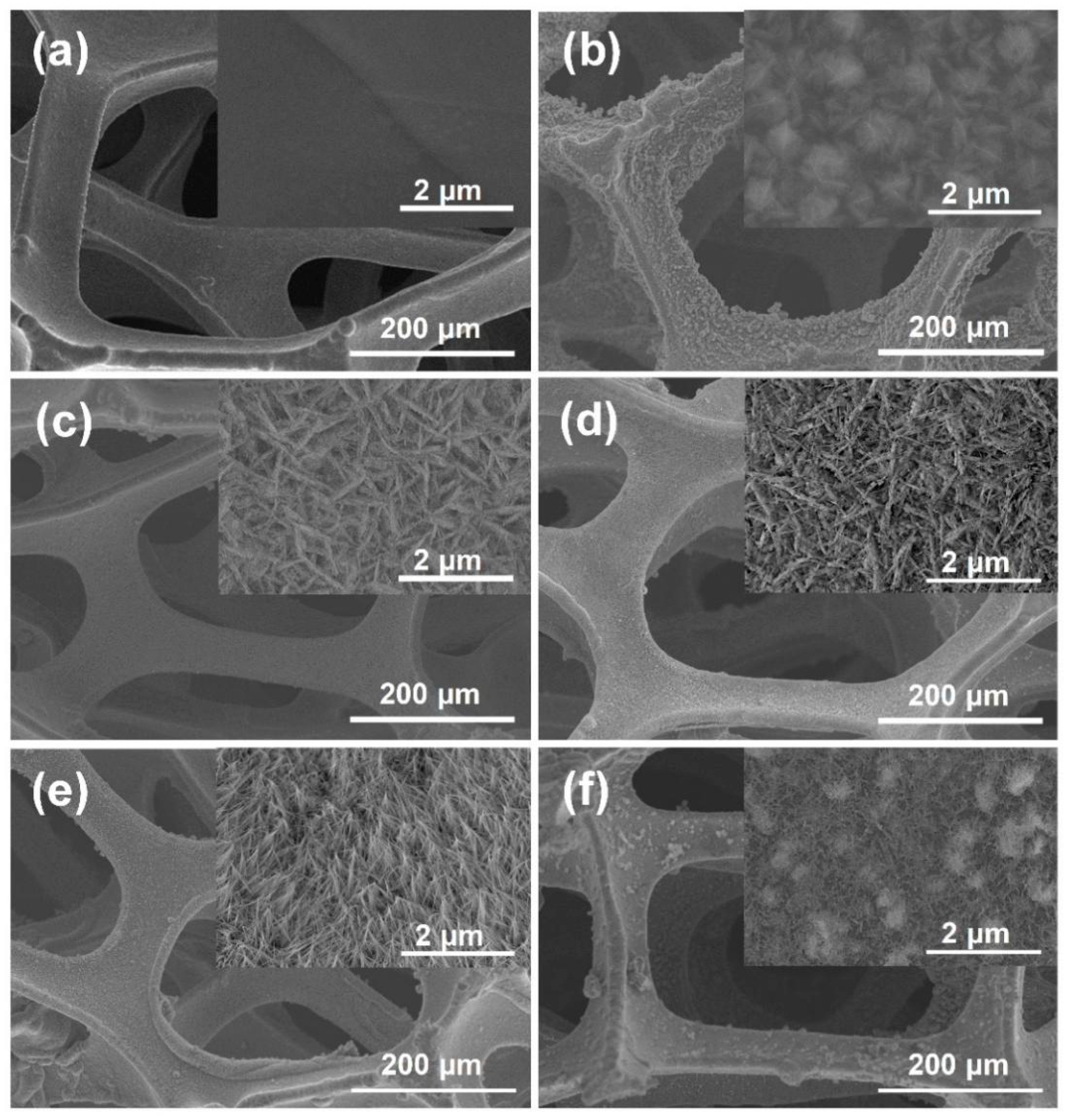
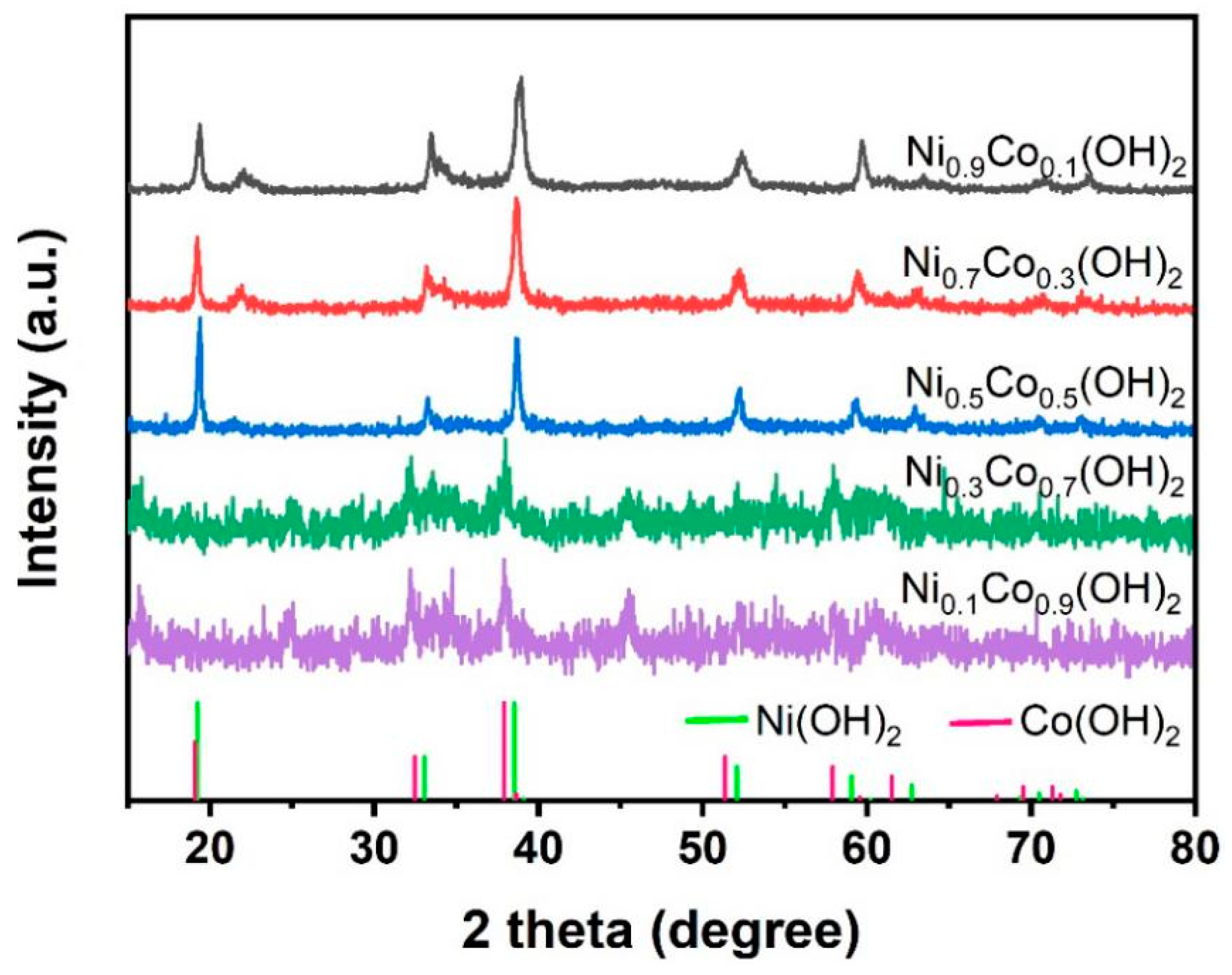
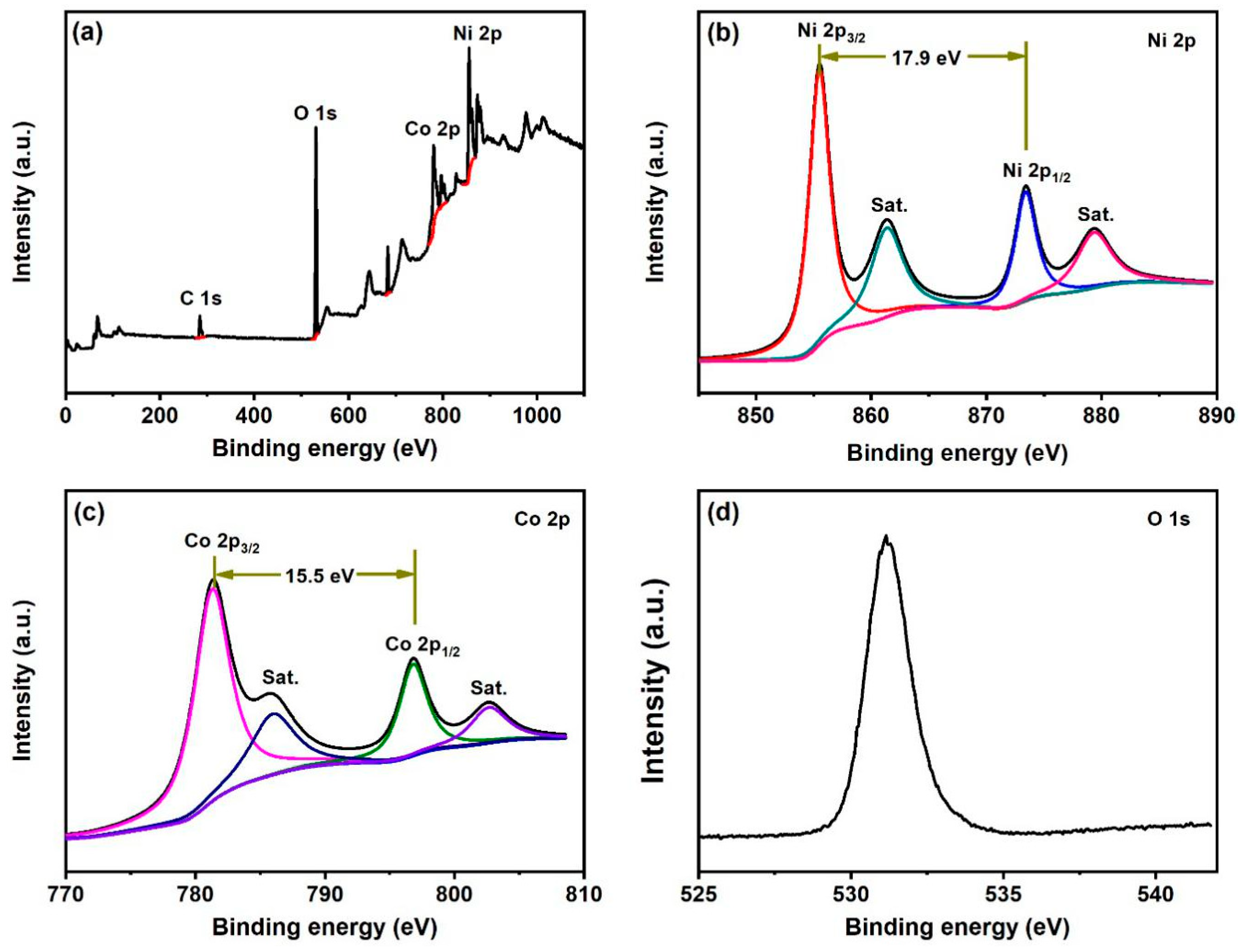
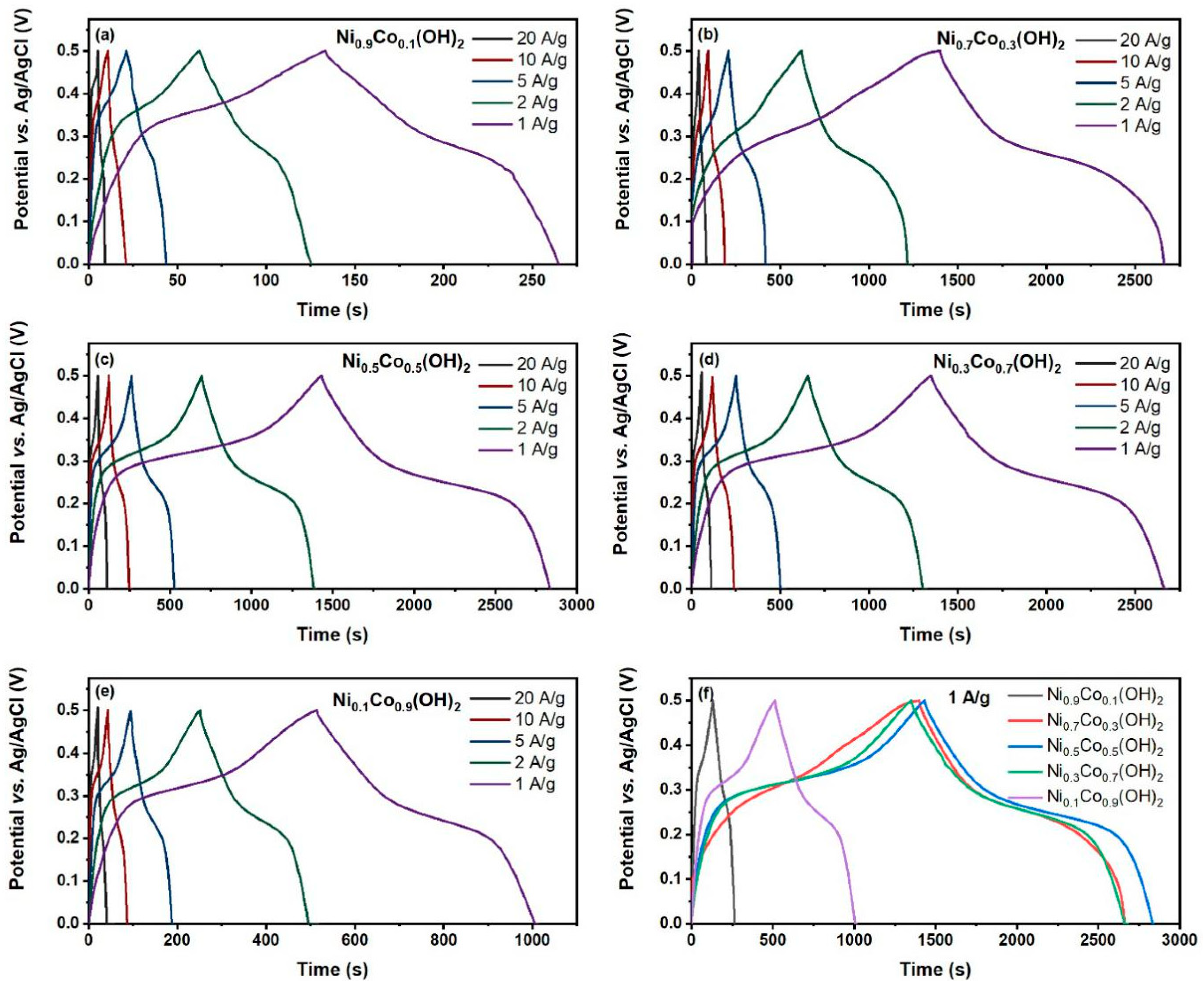
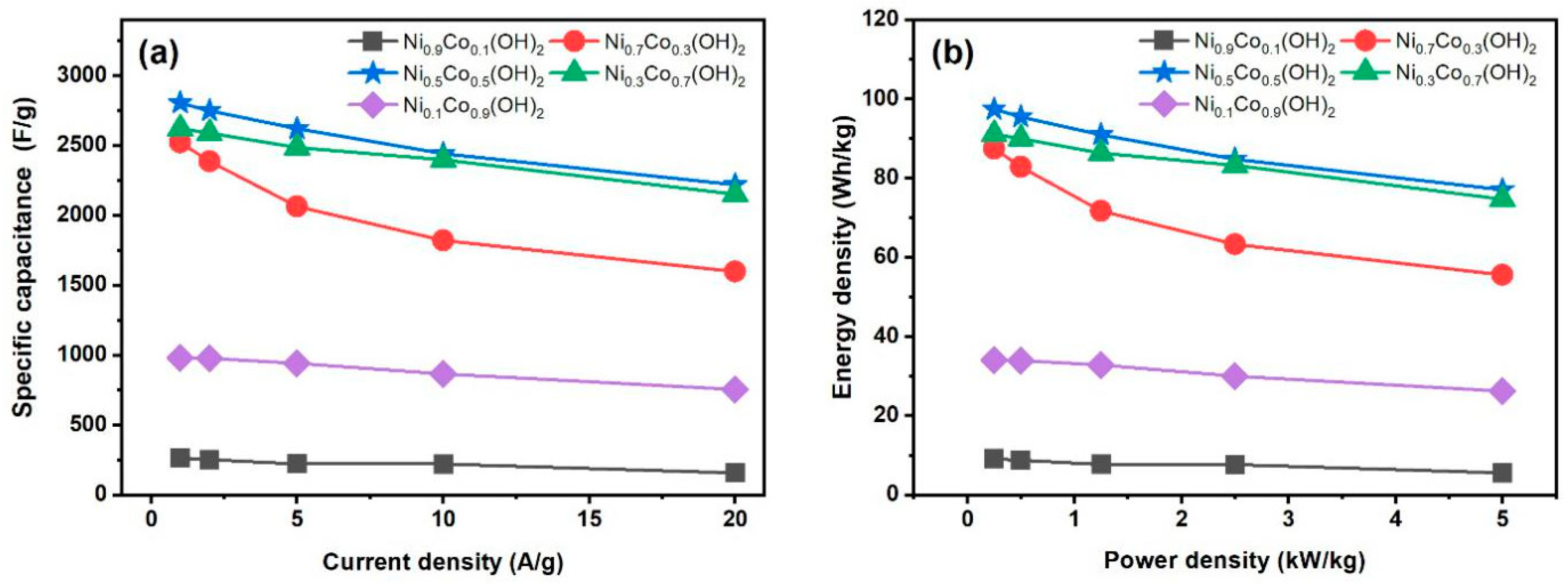
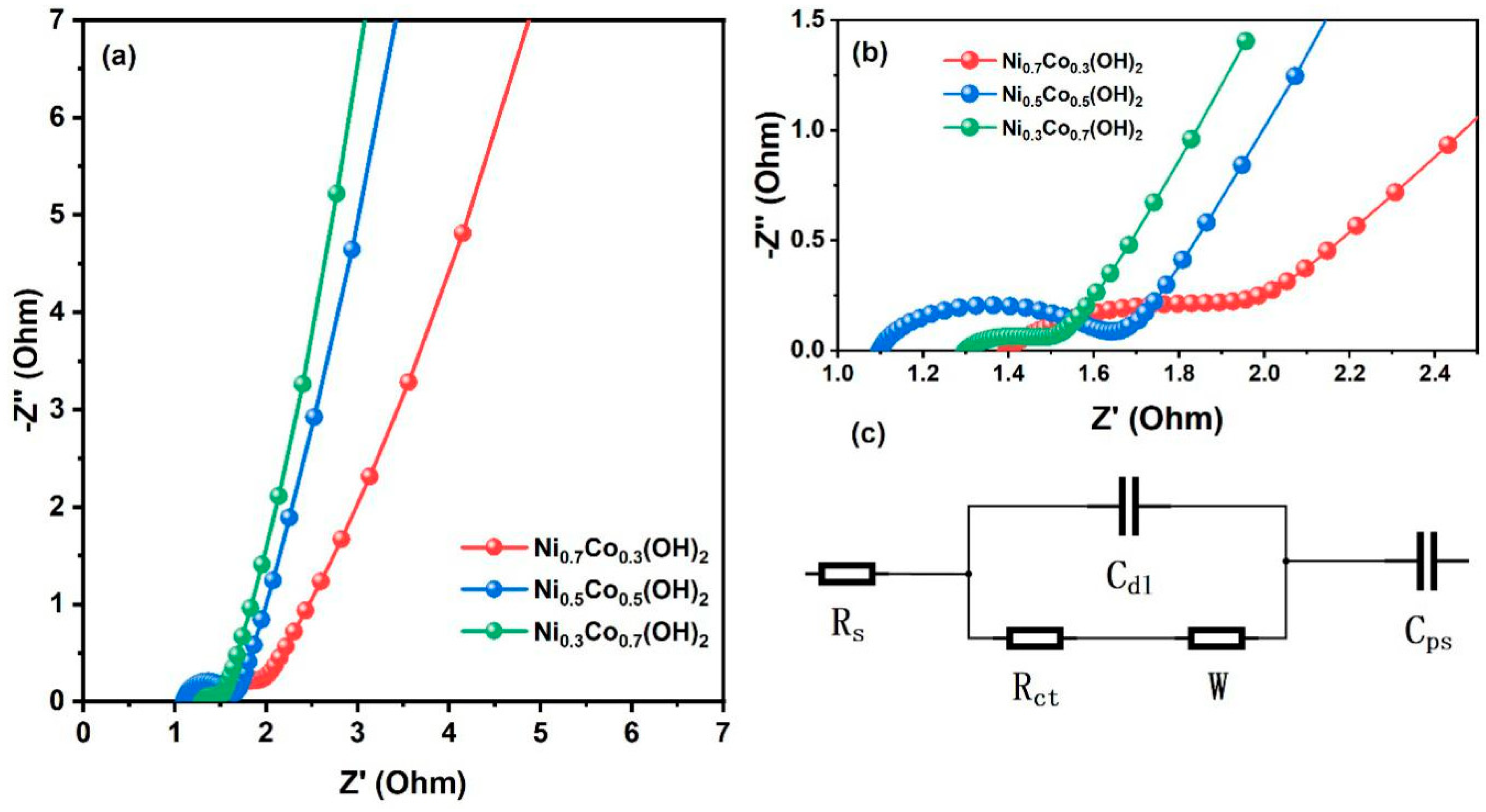
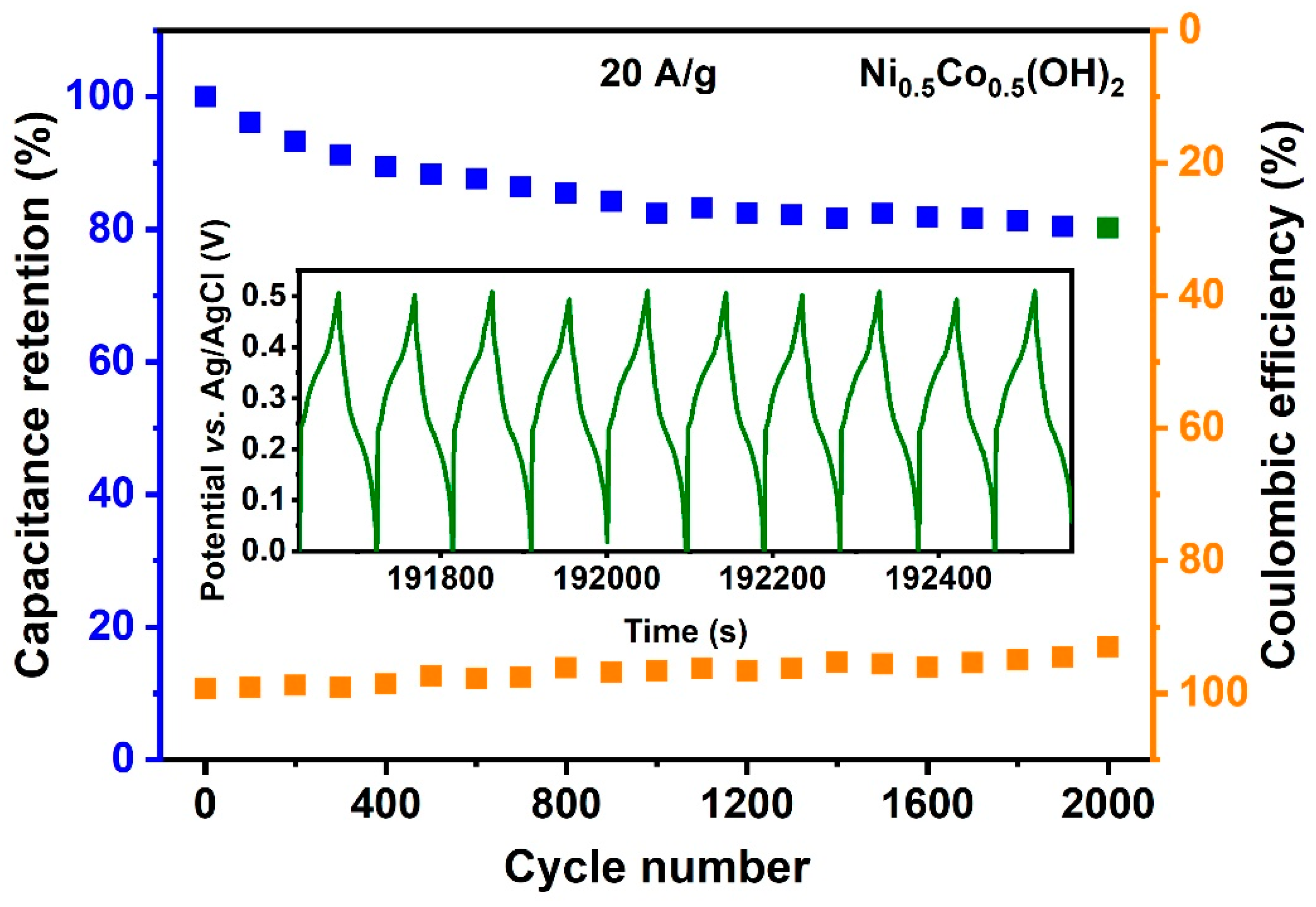
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A review of supercapacitor modeling, estimation, and applications: A control/management perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539. [Google Scholar] [CrossRef]
- Castaings, A.; Lhomme, W.; Trigui, R.; Bouscayrol, A. Comparison of energy management strategies of a battery/supercapacitors system for electric vehicle under real-time constraints. Appl. Energy 2016, 163, 190–200. [Google Scholar] [CrossRef]
- Faggioli, E.; Rena, P.; Danel, V.; Andrieu, X.; Mallant, R.; Kahlen, H. Supercapacitors for the energy management of electric vehicles. J. Power Sources 1999, 84, 261–269. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.H.; Gong, H. A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/Carbon nanotube electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Kötz, R.; Hahn, M.; Gallay, R. Temperature behavior and impedance fundamentals of supercapacitors. J. Power Sources 2006, 154, 550–555. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, S.; Zhang, Z. Three-dimensional binder-free nanoarchitectures for advanced pseudocapacitors. Adv. Mater. 2017, 29, 1700515. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes–A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, J.G.; Xiao, J.Q. Nanostructured electrodes for high-performance pseudocapacitors. Angew. Chem. Int. Ed. 2013, 52, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, J.; Yuen, M.F.; Zhang, J.; Li, Y.; Chen, X.; Zhang, W. Hierarchical composite electrodes of nickel oxide nanoflake 3D graphene for high-performance pseudocapacitors. Adv. Funct. Mater. 2014, 24, 6372–6380. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; Yang, Z.; Huang, S. One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2 composites for high performance electrochemical supercapacitor. J. Power Sources 2013, 243, 555–561. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Wang, S.; Wang, Y.; Zhao, Y.; Zheng, W. Synthesis of Co(OH)2/graphene/Ni foam nano-electrodes with excellent pseudocapacitive behavior and high cycling stability for supercapacitors. Int. J. Hydrog. Energy 2012, 37, 11846–11852. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Yuan, H.; Jiao, L. Facile synthesis and high capacitive performance of 3D hierarchical Ni(OH)2 microspheres. Electrochim. Acta 2016, 196, 84–91. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, W.; Yin, Y.; Yan, Y.; Zhan, K.; Yang, J.; Zhao, B. Co(OH)2 nanoflakes grown on 3D graphene foam as a binder-free hybrid electrode for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 7884–7891. [Google Scholar] [CrossRef]
- Nguyen, T.; Boudard, M.; Carmezim, M.J.; Montemor, M.F. NixCo1-x(OH)2 nanosheets on carbon nanofoam paper as high areal capacity electrodes for hybrid supercapacitors. Energy 2017, 126, 208–216. [Google Scholar] [CrossRef]
- Xiong, G.; He, P.; Wang, D.; Zhang, Q.; Chen, T.; Fisher, T.S. Hierarchical Ni–Co Hydroxide Petals on Mechanically Robust Graphene Petal Foam for High-Energy Asymmetric Supercapacitors. Adv. Funct. Mater. 2016, 26, 5460–5470. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Bando, Y.; Sasaki, T. A general strategy to layered transition-metal hydroxide nanocones: Tuning the composition for high electrochemical performance. Adv. Mater. 2012, 24, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.D.; Charles, E.A. Some effects of cobalt hydroxide upon the electrochemical behaviour of nickel hydroxide electrodes. J. Power Sources 1989, 25, 89–97. [Google Scholar] [CrossRef]
- Nguyen, T.; Boudard, M.; Carmezim, M.J.; Montemor, M.F. Layered Ni (OH)2-Co (OH)2 films prepared by electrodeposition as charge storage electrodes for hybrid supercapacitors. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, J.C.; Hsu, C.T.; Hu, C.C. Superior capacitive performances of binary nickel–cobalt hydroxide nanonetwork prepared by cathodic deposition. J. Power Sources 2014, 253, 205–213. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Xu, Z.; Sun, X.; Ma, Y. Ethylene glycol intercalated cobalt/nickel layered double hydroxide nanosheet assemblies with ultrahigh specific capacitance: Structural design and green synthesis for advanced electrochemical storage. ACS Appl. Mater. Interfaces 2015, 7, 19601–19610. [Google Scholar] [CrossRef]
- Hamal, E.K.; Toroker, M.C. The Effect of Fe and Co Additions on the Efficiency of NiOOH Catalyst Under Strain. ChemCatChem 2020, 12, 2801–2806. [Google Scholar] [CrossRef]
- Hwang, M.; Kang, J.; Seong, K.D.; Kim, D.K.; Jin, X.; Antink, W.H.; Lee, C.; Piao, Y. Ni-Co hydroxide nanoneedles embedded in graphene hydrogel as a binder-free electrode for high-performance asymmetric supercapacitor. Electrochim. Acta 2018, 270, 156–164. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Jang, K.; Lee, S.W.; Ahn, H. Aligned nickel-cobalt hydroxide nanorod arrays for electrochemical pseudocapacitor applications. RSC Adv. 2012, 2, 3190–3193. [Google Scholar] [CrossRef]
- Yan, T.; Li, R.; Li, Z. Nickel–cobalt layered double hydroxide ultrathin nanoflakes decorated on graphene sheets with a 3D nanonetwork structure as supercapacitive materials. Mater. Res. Bull. 2014, 51, 97–104. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Hu, Q.; Geng, B.; Zhang, X. Ultrathin porous nickel–cobalt hydroxide nanosheets for high-performance supercapacitor electrodes. RSC Adv. 2015, 5, 17007–17013. [Google Scholar] [CrossRef]
- Li, M.; Ma, K.Y.; Cheng, J.P.; Lv, D.; Zhang, X.B. Nickel–cobalt hydroxide nanoflakes conformal coating on carbon nanotubes as a supercapacitive material with high-rate capability. J. Power Sources 2015, 286, 438–444. [Google Scholar] [CrossRef]
- Li, H.; Musharavati, F.; Zalenezhad, E.; Chen, X.; Hui, K.N.; Hui, K.S. Electrodeposited NiCo layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors. Electrochim. Acta 2018, 261, 178–187. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Chu, W.; Wang, N. High-stable α-phase NiCo double hydroxide microspheres via microwave synthesis for supercapacitor electrode materials. Chem. Eng. J. 2017, 316, 277–287. [Google Scholar] [CrossRef]
- Tao, Y.; Ruiyi, L.; Zaijun, L.; Junkang, L.; Guangli, W.; Zhiquo, G. A free template strategy for the fabrication of nickel/cobalt double hydroxide microspheres with tunable nanostructure and morphology for high performance supercapacitors. RSC Adv. 2013, 3, 19416–19422. [Google Scholar] [CrossRef]
- He, S.; Li, Z.; Wang, J.; Wen, P.; Gao, J.; Ma, L.; Yang, Z.; Yang, S. MOF-derived NixCo1− x(OH)2 composite microspheres for high-performance supercapacitors. RSC Adv. 2016, 6, 49478–49486. [Google Scholar] [CrossRef]
- Gou, J.; Xie, S.; Liu, Y.; Liu, C. Flower-like nickel-cobalt hydroxides converted from phosphites for high rate performance hybrid supercapacitor electrode materials. Electrochim. Acta 2016, 210, 915–924. [Google Scholar] [CrossRef]
- Tao, Y.; Ruiyi, L.; Tingting, Y.; Zaijun, L. Nickel/cobalt layered double hydroxide hollow microspheres with hydrangea-like morphology for high-performance supercapacitors. Electrochim. Acta 2015, 152, 530–537. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Tao, Y.; Zaijun, L.; Ruiyi, L.; Qi, N.; Hui, K.; Yulian, N.; Junkang, L. Nickel–cobalt double hydroxides microspheres with hollow interior and hedgehog-like exterior structures for supercapacitors. J. Mater. Chem. 2012, 22, 23587–23592. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, J.P.; Li, M.; Liu, L.; Liu, F.; Zhang, X.B. Flower-like nickel–cobalt binary hydroxides with high specific capacitance: Tuning the composition and asymmetric capacitor application. J. Electroanal. Chem. 2015, 743, 38–45. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, H.; Gu, Y.; Sun, Z.; Hu, J.; Cao, L.; Lv, F.; Li, M.; Wang, W.; Wang, Z.; et al. Facile electrodeposition of 3D concentration-gradient Ni-Co hydroxide nanostructures on nickel foam as high performance electrodes for asymmetric supercapacitors. Nano Res. 2015, 8, 2744–2754. [Google Scholar] [CrossRef]
- Lu, P.; Halvorsen, E.; Ohlckers, P.; Müller, L.; Leopold, S.; Hoffmann, M.; Grigoras, K.; Ahopelto, J.; Prunnila, M.; Chen, X. Ternary composite Si/TiN/MnO2 taper nanorod array for on-chip supercapacitor. Electrochim. Acta 2017, 248, 397–408. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Sun, H.; Lu, F.; Yu, M.; Lian, J. Morphology controlled high performance supercapacitor behaviour of the Ni–Co binary hydroxide system. J. Power Sources 2013, 238, 150–156. [Google Scholar] [CrossRef]
- Gao, H.; Wang, G.; Yang, M.; Tan, L.; Yu, J. Novel tunable hierarchical Ni–Co hydroxide and oxide assembled from two-wheeled units. Nanotechnology 2011, 23, 015607. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Kim, J.; Zhang, J. Nickel and cobalt oxide composite as a possible electrode material for electrochemical supercapacitors. J. Power Sources 2012, 217, 554–561. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Wang, Y.; Zhao, Z.; Li, P.; Zhang, W.; Wang, Y.L.; Hu, J. Synthesis of MoO3/WO3 composite nanostructures for highly sensitive ethanol and acetone detection. J. Mater. Sci. 2017, 52, 1561–1572. [Google Scholar] [CrossRef]
- Pu, J.; Tong, Y.; Wang, S.; Sheng, E.; Wang, Z. Nickel–cobalt hydroxide nanosheets arrays on Ni foam for pseudocapacitor applications. J. Power Sources 2014, 250, 250–256. [Google Scholar] [CrossRef]
- Liu, F.; Chu, X.; Zhang, H.; Zhang, B.; Su, H.; Jin, L.; Wang, Z.; Huang, H.; Yang, W. Synthesis of self-assembly 3D porous Ni(OH)2 with high capacitance for hybrid supercapacitors. Electrochim. Acta 2018, 269, 102–110. [Google Scholar] [CrossRef]
- Xue, T.; Wang, X.; Lee, J.M. Dual-template synthesis of Co(OH)2 with mesoporous nanowire structure and its application in supercapacitor. J. Power Sources 2012, 201, 382–386. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Thorat, G.M.; Seo, J.G. Electrochemical growth of Co(OH)2 nanoflakes on Ni foam for methanol electro-oxidation. New J. Chem. 2017, 41, 9546–9553. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, B.; Cheng, J.; Li, J.; Zhang, H.; Tang, Z.; Yang, J. Hydrothermal synthesis of Ni(OH)2 nanoflakes on 3D graphene foam for high-performance supercapacitors. Electrochim. Acta 2015, 173, 399–407. [Google Scholar] [CrossRef]
- Jing, M.; Hou, H.; Banks, C.E.; Yang, Y.; Zhang, Y.; Ji, X. Alternating voltage introduced NiCo double hydroxide layered nanoflakes for an asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22741–22744. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, S.; Yan, X.; Lyu, M.; Wang, L.; Bell, J.; Wang, H. 2-Methylimidazole-derived Ni–Co layered double hydroxide nanosheets as high rate capability and high energy density storage material in hybrid supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef]
- Fan, X.; Sun, Y.; Ohlckers, P.; Chen, X. Porous Thin-Wall Hollow Co3O4 Spheres for Supercapacitors with High Rate Capability. Appl. Sci. 2019, 9, 4672. [Google Scholar] [CrossRef]
- Wu, J.; Mi, R.; Li, S.; Guo, P.; Mei, J.; Liu, H.; Lau, W.M.; Liu, L.M. Hierarchical three-dimensional NiCo2O4 nanoneedle arrays supported on Ni foam for high-performance supercapacitors. RSC Adv. 2015, 5, 25304–25311. [Google Scholar] [CrossRef]
- Fan, X.; Ohlckers, P.; Chen, X. Tunable Synthesis of Hollow Co3O4 Nanoboxes and Their Application in Supercapacitors. Appl. Sci. 2020, 10, 1208. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Varanasi, C.V.; Liu, J. Improving the performance of cobalt–nickel hydroxide-based self-supporting electrodes for supercapacitors using accumulative approaches. Energy Environ. Sci. 2013, 6, 3314–3321. [Google Scholar] [CrossRef]
- Hu, Z.A.; Xie, Y.L.; Wang, Y.X.; Wu, H.Y.; Yang, Y.Y.; Zhang, Z.Y. Synthesis and electrochemical characterization of mesoporous CoxNi1−x layered double hydroxides as electrode materials for supercapacitors. Electrochim. Acta 2009, 54, 2737–2741. [Google Scholar] [CrossRef]
- Xie, L.; Hu, Z.; Lv, C.; Sun, G.; Wang, J.; Li, Y.; He, H.; Wang, J.; Li, K. CoxNi1−x double hydroxide nanoparticles with ultrahigh specific capacitances as supercapacitor electrode materials. Electrochim. Acta 2012, 78, 205–211. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Zhang, G.; Xu, M.; Lu, W.; Zhou, L.; Huang, H. Design of Hierarchical Ni-Co@Ni-Co Layered Double Hydroxide Core–Shell Structured Nanotube Array for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors. Adv. Funct. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, B.; Yu, G.; Hou, X.; Chen, D.; Shen, G. NiCo2O4 nanowire arrays supported on Ni foam for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2013, 1, 2468–2473. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-nanonet hollow structured Co3O4 for high capacity supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Yu, S.; Guo, W.; Mu, S.; Wang, H.; Zhao, Y.; Hou, L.; Fan, Y.; Gao, F. Template-free hydrothermal synthesis of nickel cobalt hydroxide nanoflowers with high performance for asymmetric supercapacitor. Electrochim. Acta 2015, 161, 279–289. [Google Scholar] [CrossRef]
- Lu, P.; Ohlckers, P.; Müller, L.; Leopold, S.; Hoffmann, M.; Grigoras, K.; Ahopelto, J.; Prunnila, M.; Chen, X. Nano fabricated silicon nanorod array with titanium nitride coating for on-chip supercapacitors. Electrochem. Commun. 2016, 70, 51–55. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Q.; Zhang, H.; Liu, J.; Li, Z.; Jing, X.; Yuan, Y.; Liu, L.; Wang, J. Nickel-cobalt layered double hydroxide nanowires on three dimensional graphene nickel foam for high performance asymmetric supercapacitors. Electrochim. Acta 2016, 215, 492–499. [Google Scholar] [CrossRef]
- Le, K.; Wang, Z.; Wang, F.; Wang, Q.; Shao, Q.; Murugadoss, V.; Wu, S.; Liu, W.; Liu, J.; Gao, Q.; et al. Sandwich-like NiCo layered double hydroxide/reduced graphene oxide nanocomposite cathodes for high energy density asymmetric supercapacitors. Dalton Trans. 2019, 48, 5193–5202. [Google Scholar] [CrossRef]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 2015, 268, 251–259. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Ohlckers, P.; Chen, X. One-Step and Morphology-Controlled Synthesis of Ni-Co Binary Hydroxide on Nickel Foam for High-Performance Supercapacitors. Appl. Sci. 2020, 10, 3814. https://doi.org/10.3390/app10113814
Fan X, Ohlckers P, Chen X. One-Step and Morphology-Controlled Synthesis of Ni-Co Binary Hydroxide on Nickel Foam for High-Performance Supercapacitors. Applied Sciences. 2020; 10(11):3814. https://doi.org/10.3390/app10113814
Chicago/Turabian StyleFan, Xiao, Per Ohlckers, and Xuyuan Chen. 2020. "One-Step and Morphology-Controlled Synthesis of Ni-Co Binary Hydroxide on Nickel Foam for High-Performance Supercapacitors" Applied Sciences 10, no. 11: 3814. https://doi.org/10.3390/app10113814
APA StyleFan, X., Ohlckers, P., & Chen, X. (2020). One-Step and Morphology-Controlled Synthesis of Ni-Co Binary Hydroxide on Nickel Foam for High-Performance Supercapacitors. Applied Sciences, 10(11), 3814. https://doi.org/10.3390/app10113814





