Abstract
Bioremediation of contaminated soils has gained increasing interest in recent years as a low-cost and environmentally friendly technology to clean soils polluted with anthropogenic contaminants. However, some organic pollutants in soil have a low biodegradability or are not bioavailable, which hampers the use of bioremediation for their removal. This is the case of polycyclic aromatic hydrocarbons (PAHs), which normally are stable and hydrophobic chemical structures. In this review, several approaches for the decontamination of PAH-polluted soil are presented and discussed in detail. The use of compost as biostimulation- and bioaugmentation-coupled technologies are described in detail, and some parameters, such as the stability of compost, deserve special attention to obtain better results. Composting as an ex situ technology, with the use of some specific products like surfactants, is also discussed. In summary, the use of compost and composting are promising technologies (in all the approaches presented) for the bioremediation of PAH-contaminated soils.
1. Introduction
Globally, different anthropogenic activities have resulted in increasing environmental pollution, and its consequences has injured almost all components of the ecosystem [1,2,3]. Soil, as a vital component of the terrestrial ecosystem, is prone to pollution from different sources, including industrial and agricultural activities [4,5,6,7]. Wide verities of pollutants entering the soil posing a huge threat and risk to human health and natural ecosystem [8,9,10,11,12]. Polycyclic aromatic hydrocarbons (PAHs), petroleum, and related derivatives represent the main sources of soil contamination [13,14,15,16,17]. Indeed, these organic pollutant groups are listed as priorities and receive considerable attention, owing to their toxic, genotoxic, mutagenic, and potentially cancer-causing properties [18,19].
To deal with this problem, several treatment technologies are used, including chemical, physical, and biological, as well as thermal for remediation of these contaminated soils. Among the best approaches is the bioremediation technology, which is categorized as a promising approach that continues to gain more attention due to its efficiency, cost-effectiveness, and environmental-friendly byproducts [20,21,22]. The process mainly relies on the activity of a wide spectrum of microorganisms to degrade the target contaminants to lower toxic levels. Bioremediation of PAH-contaminated soil has been performed utilizing distinctive approaches [7]. In any case, composting as a remediation approach has been considered a reasonable strategy in this field, because it provides nutrients for indigenous microorganisms to degrade the target contaminants; simultaneously, applying this approach is a great opportunity for feasible and sustainable reuse of the natural biodegradable fraction of wastes. Additionally, the process is cost-effective compared with other approaches—for instance, composting costs between $50–$140 per ton, while applying slurry or biopiling treatments cost $170 per ton and $130–$260 per cubic meter, respectively [23,24,25,26,27]. Bioremediation of PAH-contaminated soil through composting could be implemented through incorporating PAH-contaminated soils to the composting process, or by adding compost to contaminated soils. Also, bioaugmentation or surfactant application might be included to achieve the final set objectives [16,25,28,29,30,31,32,33,34]. Biodegradation of PAHs intrinsically depends on microbial activity, where bacteria and fungi are considered the foremost vital variables governing the bioremediation process [35,36,37]. However, the functionality of these microorganisms is affected by different factors within the composting mixture, including biotic and abiotic factors. In this context, the environmental condition (pH, temperature, moisture,), nutrient availability, oxygen presence, and bioavailability of the contaminants are essential parameters for process control and performance [38].
This review focuses on the application of composting and compost addition for the bioremediation of soils contaminated with PAHs. In this regard, the impact of different controlling factors like temperature, PAH structure and concentration, co-substrate stability, co-substrate mixing ration, and bioaugmentation are discussed. Moreover, other issues, such as bioavailability, surfactant application, and the degradation pathways of PAHs are illustrated, in order to provide an insight into the process that is necessary for new development.
2. Soil Contamination with PAHs
Soil represents a vital component of all terrestrial ecosystems. However, it is subjected to degradation or decline in its quality as a result of different anthropogenic activities that have resulted in increasing the rate of contamination [4,5,7]. Therefore, polluted land is a worldwide concern, and can be viewed as major obstruction to sustainable development and modern environmental protection [39]. Soil contamination has been recognized as one of the major dangers to soil function in Europe by the Communication from the European Commission “Towards a Thematic Strategy for soil Protection” [40,41]. The issue has expanded with expanding public awareness and concern about the presence of chemicals in the environment, particularly due to their different unfavorable impacts on the ecosystem and human health. Polycyclic aromatic hydrocarbons (PAHs) have been recorded as pollutants of priority importance due to their properties and ubiquitous occurrence, as well as their recalcitrance [18,42,43]. Consequently, great efforts worldwide have been directed toward remediating these pollutants from the environment.
2.1. PAHs: Properties and Sources
PAHs are a group of ubiquitous organic pollutants with at least two aromatic rings (Figure 1), and are poorly soluble in water (Table 1). Due to their chemical structure, PAHs have hydrophobic properties, which refers to their ability to accumulate on the surface of solid materials like soil, sediment, sewage sludge, and solid wastes. The dangers emerging from the presence of PAHs in soil are related to the toxic nature of those pollutants [42,43]. It is noteworthy that some substances in this group have been recognized as mutagenic, carcinogenic, and teratogenic [18,19].
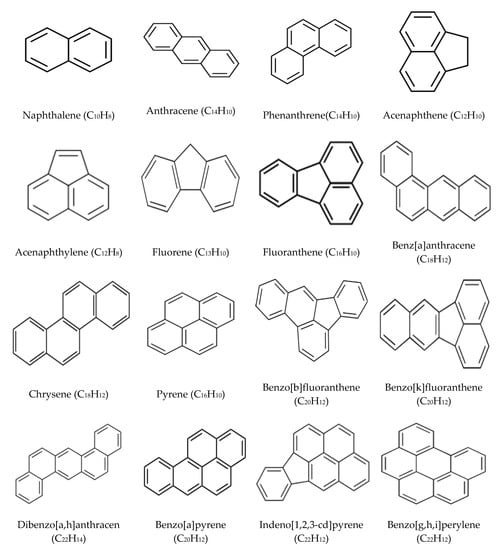
Figure 1.
Structure and chemical formula of the 16 polycyclic aromatic hydrocarbons (PAHs) listed as priority pollutants by the United States Environmental Protection Agency (USEPA).

Table 1.
Selected properties of the 16 USEPA PAHs.
Sources of PAHs are categorized into natural as well as anthropogenic sources: hydrothermal process volcanoes, forest fires, and waste burning are natural sources of PAHs. Anthropogenic sources include waste incinerators, burning of fossil fuels during heating processes, incomplete combustion of organic matter, petrochemical spills on land, wood burning, petrol and diesel oil combustion, gasification, and plastic waste incineration [13,14,15,17]. Globally, 16 to 32 PAH compounds are subjected to mandatory control, due to their harmful properties [44]. PAH persistence and hydrophobicity in environmental components are the main factors that exacerbate the pollution problem, taking into account that soils receives a considerable share of this pollution (sink), due to their complex matrix structure that facilitates the sorption of these pollutants. Soil organic matter is a decisive factor in determining the degree of PAH sorption into the soil, along with the physicochemical properties of the PAHs themselves [18,19,23,45,46]. Therefore, the remediation of soils polluted by aged PAHs has become a major issue for environmental scientists in recent years [12,13,14,47,48].
2.2. Bioremediation of PAH-Contaminated Soils
When natural biodegradation processes cannot achieve the desired goals, in this case, human intervention becomes necessary to stimulate the process above naturally occurring microbial process [49]. Accordingly, several approaches have been used to enhance bioremediation efficiency. These approaches, which could be used separately or in combination (two or more) include, but are not limited to, biostimulation (providing nutrients for increasing the microbial activity), bioaugmentation (introducing a consortium of indigenous or exogenous microorganisms), using surfactants, and co-metabolism [50,51]. Recently, various studies have been done in attempt to understand the process hierarchy and to provide solutions for different process limitations. For instance, much research has been carried out to better understand the microbial behavior and its interaction with the contaminants during the bioremediation process, whereas others have focused on introducing exogenic and genetically engineered microbes for process enhancement [52].
3. Composting Technology
Composting is defined as an aerobic process, which fundamentally requires oxygen, optimal moisture content, and porosity to stabilize the organic waste, and its common control variables are temperature, oxygen, and moisture [53]. Thus, composting bioremediation is the adaptation and application of the composting technology for wastes and contaminant treatments.
In order to achieve optimum results within a reasonable time during any composting treatment, process-controlling parameters have to be adjusted within the optimum values, and the process passes through two main stages. First is the decomposition/active stage, which is characterized by extensive microbial activity that leads to a steadily increase in the temperature, passing from the mesophilic ranges (25–45 °C) to reach the thermophilic ones (more than 45 °C). To maintain aerobic conditions for effective microbial activity during this stage, a high rate of aeration is needed. Second is the curing stage; this take place at a lower temperature, and microbial activity is relatively low, as the nutrients pool has been depleted. Material humification is an important characteristic occurring in this stage [54], which gives an interesting value to the produced compost, especially for soil bioremediation, as will be discussed later in this work.
4. Bioremediation of PAH-Contaminated Soil by Composting
Composting technology is categorized as ex situ technology, which has been used for the treatment of contaminated soils. During the last few decades, the process received more attention, as it has proved its high efficiency in degrading various organic contaminants like, among others, PAHs, pesticides, explosives, and chlorophenols [25,55,56,57,58,59,60]. Essentially, the process relies on the addition of compost or organic co-substrates/amendments to the contaminated soil, and while the co-substrate matures, due to the action of various microbial populations within the mixture, the target pollutants are degraded [57,61]. Thus, treatment of PAH-contaminated soil combined with composting of organic waste could be an interesting option and a sustainable method with much increasing attention. It would enable eco-friendly disposal of such waste and enhance the biodegradation rate of PAHs [7,60,62]. The biodegradation process efficiency depends fundamentally on the bioavailability of the substrates, environmental conditions (pH, moisture, temperature), the presence of oxygen, and the availability of nutrients [38]. Remarkably, the bioremediation of PAH-contaminated soils through composting has confirmed this technique’s capability to overcome most obstacles that might hinder reaching its goal, which is the removal of contaminants [10,63,64,65,66,67].
As the process is based on mixing the contaminated soil with organic co-substrates, any failure may result in producing much greater quantity of contaminated material, and this is recognized as the main concern of using such approach. This weakness, and the general scarcity of information on the toxicity, distribution, and bioavailability of such contaminants in compost-amended soils, may therefore result in the drawing up of excessively stringent soil assessment measures with remediation cost implications [68].
4.1. Effect of PAH Characteristics and Concentrations
The physical and chemical properties of PAHs have a considerable effect on their biodegradation rate. Microbial assimilation and biodegradation of these compounds basically depends on their solubility. Nevertheless, most of compounds belonging to this group are characterized as poorly soluble in water, especially with their increasing molecular weight and angularity (Table 1, Figure 1), which thus increase their hydrophobicity [69,70,71,72]. This was obvious in many studies dealing with the biodegradation of different PAHs. For instance, Han et al. [73] investigated the application of different agricultural waste on the biodegradation of aged PAHs in soil microcosms over 90 days. The initial concentration of total PAHs in the soil was 36.1 mg kg−1 dry soil, where four-ring PAHs comprised 41.7% of the total PAHs. The results demonstrated higher degradation rates of 40.7–61.2% for PAHs with low molecular weight (LMW), compared to 18.7–33.1% for those with high molecular weight (HMW) in all soil microcosms. Similarly, Lukić et al. [74] showed that LMW-PAH removal was more favorable in the mesophilic phase, with 11% and 15% residues in the soil, than in the thermophilic phase, with 29% and 31% residues. Additionally, more resistance to degradation was observed for HMW PAHs, resulting in a decrease in the total removal, which was less than 50% for both benzo[a]pyrene and benzo[k]flouranthene, in all treatments [75,76]. In this regard, even though both compounds have the same number of benzene rings (five) and molecular weights, the higher octanol–water partition coefficient (log Kow) of benzo[k]flouranthene increased its hydrophobic properties and consequently its degradation rate under the same conditions. Indeed, higher log Kow leads to a higher potential of bioaccumulation, which is the main factor responsible for the lower biodegradability of such compounds [77]. Obviously, and according to the obtained result in different studies, there is a consistent relationship between the persistence of PAHs in the environment and increasing their numbers of benzene rings, which ultimately affects their biodegradation rate.
The concentrations of PAHs also have a substantial influence on the microbial activity in such treatments, since high concentrations would lead to toxic or inhibition conditions. Meanwhile, low concentrations could be below the rate needed to stimulate microbial cultures to degrade these contaminants [78,79]. This was obvious in the study conducted by Sayara et al. [78], in which the PAH concentrations had a crucial effect. Low concentrations were found to be less than the rates that are assumed to initiate the degradation process, since microbial communities prefer the utilization of readily available nutrients, which are consumed quickly before initiating biodegradation of the target PAHs. The same results were obtained by Zappi et al. [80], where low concentrations of PAH did not degrade, even when the system was supplanted with additional carbon sources. Wu et al. [66] showed that compost addition is an effective approach for enhancing PAH removal from soils, but increasing the ratio of added compost does not necessarily help to increase removal. Nevertheless, enhanced removal by compost addition seems more effective for higher initial PAH concentrations. In this regard, Jorgensen et al. [81] demonstrated that the degradation rate of a compound is proportional to its concentration, especially for highly soluble compounds, and argued that the degradation of hydrocarbons is governed by first-order kinetics. However, this argument may be validated to some extent, as high concentration may become detrimental to microbial activity and disturb nutrient balance, especially when LMW PAHs are present [78].
4.2. Effect of Temperature
Providing optimum temperature is an intrinsic factor for the successful biodegradation of PAH. The importance of this factor stems from its influence on the metabolic activity, bioavailability, solubility, and diffusion rate of the target contaminate [82]. It is noteworthy that the solubility of PAHs increases with temperature, which ultimately increases the bioavailability of the PAH molecules. However, increasing temperature is associated with decreasing oxygen solubility, which on turn reduces the metabolic activity of aerobic microorganisms. Furthermore, and to a certain extent, the specified temperature range will determine the types of dominant microorganisms and their enzymatic activity that will undertake the degradation [73].
The successive stages during the normal composting process (mesophilic phase, thermophilic phase and curing phase) are expected to be accompanied by specific populations of bacteria, and different effects on contaminants are found with different stages of compost product. The biodegradation of PAHs occurs over a wide temperature range, and microorganisms have found to be adapted to biodegrade PAHs at extreme temperature conditions. Under mesophilic and thermophilic temperatures ranges, it has been found that the enzymatic activity of microorganisms increases, which helps in increasing the rate of hydrocarbon degradation. However, it should be underlined that a great amount of research has been directed to focus on the process behavior under mesophilic conditions, as it is believed that a wide spectrum of microbial communities could be present in active roles under these temperatures, and thus reasonable degradation rates could be achieved [78,83,84,85,86].
During in-vessel composting of pyrene-contaminated soil, composting temperature affected the prevailing of some microbial groups over others, and the predominant bacterial community changed over time. The degradation of pyrene was dominated by α-, β-, and γ-Proteobacteria, as well as Actinobacteria, at 38 °C during 14 days of composting, and then Streptomyces at 55 °C. Later, at 70 °C and after 42 days of composting, Acinetobacter and Thermobifida occupied leading position. Finally, Thermobifida and Streptomyces flourished after 60 days of composting at 38 °C [87]. Concerning the temperature effect, Lukić et al. [74] claimed that degradation rates of 89% and 59% for three-ring and four-ring PAHs, respectively, were achieved in reactors under mesophilic temperatures. In contrast, reactors displaying a thermophilic range ended with 71% and 41% removal for the same pollutants, respectively, during the bioremediation process. The addition of compost significantly promoted the removal of PAHs and alkanes up to 88% after 50 days of incubation under mesophilic temperatures (28 °C), compared to the natural biodegradation of hydrocarbons in soils without compost [67]. Additionally, the composting of PAH-contaminated soils under different conditions and different organic substrates were found to perform better under mesophilic conditions [23,25,78,88,89]. LMW-PAH concentrations, such as naphthalene, acenaphthylene, acenaphthene, fluorene, anthracene, and phenanthrene, were decreased by an average of 89% at 38 °C, which is twice that compared to the concentration reduction at 55 °C, which was an average of 45%. Simultaneously, no big difference was observed concerning HMW PAHs, including fluoranthene, pyrene, benzo[a]anthracene, and chrysene, where the removal rate was by an average of 67% at 38 °C, compared to 69% at 55 °C. Nevertheless, a high temperature was considered adverse to microbial activity, and volatilization was the leading mechanism of PAH removal [88]. Under these conditions, it is assumed that a longer incubation period under the mesophilic phase could facilitate PAH removal, due to the richest microbial diversity and possible increased microbial activity [27]. According to these studies, and others in the literature, mesophilic temperatures demonstrated their viability and were found to be more favorable for degrading LMW PAHs, with great success in many cases due to the large microbial diversity; however, these temperatures were not found to be so efficient in the degradation of recalcitrant PAHs [69,75,88,90].
On the other hand, thermophilic ranges have been documented as enhancing PAH degradation. During the composting of hydrocarbon-polluted sediments (total petroleum hydrocarbons (TPH) = 40.3 gkg−1 dw) with different organic co-substrates, Alves et al. [91] point out that fish sludge achieved higher temperatures and was able to maintain thermophilic temperature longer than other amendments, which ultimately led to greater TPH removal rates (39.5%). It was assumed that such conditions are conducive to develop fungal communities and exert a surfactant effect, thus promoting the degradation rates. Similarly, Zhu et al. [92] proposed that enhanced solubility under thermophilic conditions could explain the higher removal rate (46%) of benzo[a]pyrene in composting treatments compared to 29% under mesophilic ones. However, whether the increased solubility or microbial community changes contribute to the high-temperature impacts needs further investigation. Viamajala et al. [82] further demonstrated that the elevated temperature during the thermophilic phase of composting enhanced the solubilization rates of phenanthrene, and hence its degradation. Based on the aforementioned observations, it is clear that the impact of composting temperature is correlated to the physiochemical properties of the targeted PAH, as the corresponding degrading microorganism are specific to temperature [93]. Generally, and despite of the different observations, mesophilic temperatures and the dominant microorganisms under these conditions are believed to be more preferable for the degradation of such compounds [69,78,83,84,85,86,94].
4.3. Effect of Organic Co-Substrate Stability
Even though various organic co-substrates/amendments have demonstrated their viability in the composting of PAH-contaminated soils, composition of these materials varies significantly in the sources and stages of decomposition [25,59,78,95,96], which as a consequence influences the removal rate in different ways [59,69,78,97]. The selected organic co-substrates for the bioremediation process should contribute in improving and overcoming any deficiencies or limitations that influence the process performance and efficiency. Accordingly, selection of the most suitable organic co-substrates represents as a major challenge in such studies [59,95].
In the bioremediation of PAH-contaminated soils, organic matter stability is of particular importance, as this parameter is directly correlated to the organic substrates’ composition and biological activity [98]. Various studies have pointed out that the fate of PAHs is dependent on the quality and nature of the amended organic matter [25,69,78,93]. Bioremediation of PAH-contaminated soils with more stable compost has proved to be more effective than with less stable or fresh organic amendments [25,78,97]. In this context, the preference of these substrates related to the presence of humic substances was found to form a considerable part of stable compost and was proportional with its degree of stability [25,78]. This humic matter was documented to enhance the organic compounds’ bioavailability [78,97]. During the composting process, organic co-substrates provided nutrients for microorganisms [99]; meanwhile, humic matter evolution is expected to facilitate the microbial accessibility to PAHs. This behavior is established as a result of decreasing humic matter binding affinity and increasing of the heterogeneity of binding sites, closer to soil humic matter, which is conducive to microbial accessibility to PAHs [97,100]. Additionally, stable compost contains low biodegradable organic matter content and a higher concentration of organic macromolecules [101], which are believed to enhance the biodegrading of the contaminant. The presence of easily degradable organic matter is assumed to reduce the process efficiency, as microbial cultures prefer to use easily degradable organic matter and thus decrease or retard utilization of the contaminant. Another important point in this item is represented by effect of the potential working surface area. In this regard, less degraded organic matter generally has coarse fractions (>5 mm), whereas most humified organic matter is generally presented in fine fraction [102]. The finest compost size fraction (<3 mm) with a higher surface area ratio provides more accessibility to microorganisms and releases more nutrients compared to coarse compost fraction [101,103].
During the bioremediation process, an increase in the content of humic matter from 0.23% to 0.70% was observed, and these changes resulted from the structural changes that occurred in the material composition. Potentiometric titrations of humic acid solution showed increases in the buffering and redox capacities of humic acids [104]. Plaza et al. [97] reported that the composting process caused a structural conversion of humic acids from an organic substrate by reducing the aliphatic fraction and increasing the polarity and aromatic polycondensation in a PAH-contaminated soil. This conversion decreased the PAH binding affinity of humic acids, and thus improved PAH-degrading microbial accessibility. Similar results were observed in other studies, which supports the application of stable organic co-substrates [24,66,73,78,105,106].
It is worth mentioning that when the same ratio of inorganic fertilizer (Nitrogen (N) .Phosphorus (P) .Potassium (K)) was compared with organic compost on the bioremediation of diesel-polluted agricultural soil over a two-month period, the results revealed that total petroleum hydrocarbon removal from polluted soil was 71.40 ± 5.60% and 93.31 ± 3.60% for N.P.K. and compost-amended options, respectively [107]. Also, after 30 weeks, the removal efficiencies of TPH in the soils were 29.3% under natural attenuation, 82.1% when nutrients (NH4NO3 and K2HPO4) were added, and 63.7% when the mixture was supplemented with 20% (w/w, dry weight basis) of aged refuse. However, a removal efficiency of 90.2% was recorded when nutrient and aged refuse were combined together. Nutrients plus aged refuse made the TPH concentration decrease to below the threshold level of commercial use required for Chinese soil quality for TPH (<3000 mg/kg) in 30 weeks. It was also found that dehydrogenase activity, bacterial counts, and degrader abundance in the soil were remarkably enhanced by the addition of aged refuse (20% w/w) [108]. All these results confirm the suitability of stable compost over other organic and inorganic substrates. Therefore, one can conclude that introducing an adequate organic co-substrate is usually more efficient in enhancing the bioremediation process, as observed in different studies. This advantage presumably corresponds to the capacity of the compost to perform simultaneously for both bioaugmentation and biostimulation.
4.4. Effect of the Mixing Ratio
The suitability of different substrates based on their physiochemical properties is recognized as an important factor in the composting of PAH-contaminated soils. Determining the appropriate quantity to be added to the mixture is also of great importance, since an inappropriate ratio may hamper or inhibit microbial activity and bioavailability [78,93]. It has been determined that even though microbial metabolism may be temporarily increased using a certain mixing ratio, the long-term inhibition of functionally important organisms may result in the failure of the bioremediation of high-molecular-weight PAHs [78]. The amount of various nutrients, and the ratio of nutrients like C, N, and P in particular, are quite conceivable as being involved in the success of the bioremediation process. Furthermore, determining the minimum quantity of the amendment that could support and maintain the desired activity with a high degradation rate is directly related to process economics [78].
As reported in the study conducted by Wang et al. [109], a microorganism’s selection of nutrients could delay the degradation of pollutants, as normally microorganisms prefer easily degradable materials over resistant ones. This study revealed that that treatments with amendment ratios of 1:1 and 2:1 had average TPH removal rates of 30.7% and 33.3%, respectively, but the amendment ratio of 3:1 had a slower net degradation rate of between 11.6% and 26.8%. An excess of readily degradable carbon might overtake the TPH and act as substrate for the metabolism of microbial degraders. Therefore, the proper amount of amendments should be taken into account in composting to balance the motivating effect on microorganisms and the competing effect with pollutants [109]. Similarly, Hickman and Reid [110] concluded that the compost additions combined with earthworms at a ratio of 1:0.5–1:1 (soil/compost, w/w) were efficient in enhancing the removal of extractable petroleum hydrocarbons and PAHs. However, when higher ratios of compost (1:2 and 1:4) were used, PAH losses were not advanced, which may indicate that the activity of earthworms were restricted by a higher addition of compost. Wu et al. [66] showed that compost addition is an effective approach for enhancing PAH removal from soils, especially for higher initial PAH concentrations, but increasing the ratio of compost added does not necessarily help to increase removal.
Experiments with different ratios of contaminated soil to green waste from 0.6:1 to 0.9:1 have demonstrated that in general, PAH removal is significantly enhanced in reactors increased with green waste until a maximum mixing ratio of 0.7:1 [89]. The same observation was found in Sayara et al. [78]. These results imply that low mixing ratios were not sufficient to stimulate the microbial growth; on the other hand, excessive amounts could eventually inhibit the targeted contaminants, as microbial communities prefer to use more available and easily degradable nutrients. Furthermore, co-composting of sediments (S) polluted by PAHs with urban green waste (GW) was performed using two mixing ratios (1:1 and 3:1; S/GW). In the first six months of treatment, the PAH concentrations in the 1:1 and 3:1 ratio scenarios was reduced by 57% and 26%, respectively. Despite the fact that only two mixing ratios were tested, the results again demonstrate that the low mixing ratio (3:1) was not sufficient to enhance the degradation process [94]. When different corn straw ratios (1%, 2%, 4%, or 6% w/w) were investigated for the remediation of aged PAHs in soils, removal rates were significantly (p < 0.05) enhanced under the 6% ratio, mainly for HMW PAHs. This indicates that the high amendment of corn straw was a potential option for the remediation of PAH-contaminated soils [111].
4.5. Bioaugmentation
When the indigenous microbial activity is not sufficient, or does not have the potential to achieve the set goals for bioremediation [112,113], it appears imperative to accelerate the process using different approaches. Among these approaches is bioaugmentation. The mechanism of this approach fundamentally depends on introducing exogenous microorganism strains that are characterized by their high capacity and diverse metabolic profiles in degrading the target contaminants [16,25,29,31,32]. However, and as concluded in many studies, the application of this approach has not always been effective in enhancing biodegradative capacity, mainly during the composting of contaminated soils [25,88,114,115]. For instance, 84% of petroleum hydrocarbon was degraded when Candida catenulate CM1 was used as an inoculant, while a removal rate of only 48% was obtained without inoculation, indicating a positive impact of bioaugmentation [29]. On the other hand, treatments using different substrates (mixing ratio = 1:1) were performed at the laboratory and field scales, and incubated with/without fungal inoculum (Phanerochaete velutina). Laboratory scale treatment showed that HMW PAHs were degraded significantly in the fungal-inoculated microcosms, such that 96% of four-ring PAHs and 39% of five- and six-ring PAHs were removed in three months, whereas 55% of four-ring PAHs and only 7% of five- and six-ring PAHs were degraded in non-inoculated ones. However, the field scale achieved similar degradation rates. Importantly, the number of gram-positive, PAH-ring, hydroxylating dioxygenase genes in the field scale experiment was found to increase 1000-fold, indicating that bacterial PAH degradation played a major role [116]. Wu et al. [117] compared bioaugmentation using Acinetobacter SZ-1 strain and biostimulation using (NH4)2SO4 and KH2PO4 in a petroleum-contaminated soil. It was found that the dissipation of total petroleum hydrocarbons (TPH) and the amounts of cultivable TPH, alkane, and PAH-degrading microorganisms were higher for biostimulation than for bioaugmentation. Similarly, Canet et al. [118] demonstrated that fungal inoculation, including four well-known PAH-degrading microorganisms (P. chrysosporium IMI 232175, Coriolus versicolor IMI 210866, Pleurotus ostreatus IMI 341687, and Wye isolate #7) in a mixture composed of non-sterile, coal-tar-contaminated soil and wheat straw, was unsuccessful in improving PAH removal. Sayara et al. [25] reported that the introduction of the white-rot fungi T. versicolor ATCC 42530 was not able to improve the decomposition of PAHs; on the contrary, organic substrates were capable of achieving significant degradation rates. Furthermore, inoculation with P. chrysosporium in a soil composting system was ineffective at enhancing the removal of benzo[a]pyrene [119].
Actually, several biotic and abiotic barriers have been documented to be behind the failure of bioaugemtation, mainly during field application of this mechanisms [51,120,121,122]. Biotic factors, including competition between indigenous and exogenous microorganisms for nutrients and the biodiversity of indigenous microorganisms, could act as a barrier to the invasion of exogenous microorganisms, in addition to antagonistic interactions and predation by protozoa and bacteriophages. Abiotic factors include all the physicochemical properties of pollutants and soils, such as pH, contaminant concentration, soil type, temperature, humidity aeration, nutrient content, and redox potential.
5. Bioavailability of PAHs
In some cases, when optimal conditions for microbial degradation are provided but low or even no degradation take place, the bioavailability of the pollutant would be the most probable reason for disabling the process from proceeding forward. Actually, the bioavailability of PAHs is directly linked to the intrinsic relationship between physicochemical and microbiological factors within the composting matrix. In particular, this factor determines the fraction of the chemical compound in the soil that can be utilized or transformed by living microorganisms [68,123,124,125,126].
Bioremediation is governed by PAH sorption onto the soil matrix in such a way that gradual sorption diminishes the possibility of desorption, and thus the PAH overstates its persistency within the soil organic matrix. This would explain the biphasic behavior of contaminants during bioremediation processes, which are associated with high removal rates in the initial phase, which is primarily limited by microbial degradation kinetics; in the second phase, though, the removal rate is low and generally limited by slow desorption. PAHs with low bioavailability are characterized with low desorption mainly in the second phase of bioremediation [127,128].
5.1. Factors Affecting PAH Bioavailability
PAHs are characterized by their high hydrophobicity, consequently increasing their affinity for being adsorbed into soil organic matter and ultimately being less available for biological uptake. Different studies [70,71,72,129,130] have highlighted that the following factors have an essential role in determining the bioavailability of PAHs. First is contamination time (ageing): the irreversible sorption of PAH is exponentially proportional with contact time, thus decreasing the bioavailability of pollutants to microorganisms and therefore the rate and extent of biodegradation. For instance, the removal efficiency of anthracene from freshly- and age-spiked agricultural soil was investigated. The results revealed that 72% of anthracene was removed in freshly-spiked soil, while only 34% was degraded in aged soil [131]. Nevertheless, it is worth mentioning that recently contaminated soil would exhibit toxicity to or even inhibit indigenous microorganisms until they adapted to the new environment [132,133]. The second factor for determining the bioavailability of PAHs is their physicochemical properties: PAHs’ water solubility is considered a crucial factor regarding their bioavailability. It is inversely proportional with PAHs’ molecular weight, which in turn reduces their accessibility to microorganisms (Section 2.1). The last factor is the physicochemical properties of the soil: organic matter, particle size, and shape have a major influence on PAHs’ bioavailability. Mineral surfaces (i.e., clays) and organic matter of the soil matrix are characterized by their high affinity to adsorb PAHs.
The addition of organic co-substrates to the composting mixture is believed to enhance the bioavailability of PAHs, which consequently increases the biodegradation rate [25,50,71]. Kobayashi et al. [106] demonstrated that water-extractable organic matter (WEOM) from cow manure compost was observed to increase the apparent solubility of phenanthrene, pyrene, and benzo[a]pyrene to 8.4, 34, and 89 times higher than their measured water values, respectively, thus promoting their solubility and biodegradation. Additionally, in a diesel-spiked soil, Wu et al. [66] showed that compost addition initially decreased PAH removal by up to 89% because of the decreased bioavailability resulting from strong sorption. However, as time increased, compost amendment enhanced PAH removal by more than two-fold compared with unamended soil, to which 30% was contributed by desorption and 70% by degradation. In coal tar- and coal ash-contaminated soils, compost addition was beneficial overall for enhancing PAH removal up to 94%, and 40% of the total loss was due to the enhanced desorption [66].
The stability of the used co-substrates plays a major role in stimulating bioavailability and biodegradation of PAHS (as discussed in Section 4.3). This type of substrates was found to have more humic matter [71]. In this context, humic matter was able to increase the microbial activities much more than those developed in humin (aged organic matter), demonstrating that humin is able to sequester organic contaminants in a stronger way [70]. An important observation is that the bioavailability of the more readily degradable or LMW PAHs was decreased due to competitive inhibition of the enzymes, which is associated with biodegradation when the enzymes present in a multiple-PAH mixture. However, the bioavailability of those usually more recalcitrant PAHs (HMW PAHs) was increased by producing inducible enzymes for catabolism [134].
5.2. Surfactant
As mentioned in the previous sections, some of PAHs are characterized by their high hydrophobicity as well as low solubility, as they have the ability to be strongly adhere to soil particles and be slowly released into the water phase [135]. Among the different alternatives to overcome the problems of low bioavailability during bioremediation of PAHs is the application of surfactants. The functionality of these additives basically depends on reducing interfacial surface tension, and thus increasing their solubility [30,33,34,136,137]. The efficiency of these surfactants is influenced by many factors, including surfactant type and concentration, PAH hydrophobicity, temperature, pH, salinity, dissolved organic matter, and microbial community. An imperative and crucial element for effective PAH remediation is the selection of the optimum ratio of mixed surfactants to avoid the inhibition of microbial activities [33,34,137]. Nowadays, various groups of surfactants are available, and each one is being used under certain conditions to be compatible with the available environment. Furthermore, biosurfactants that are produced by microorganisms are receiving more favor, as they considered more environmentally friendly [33,138]. Interestingly, Both Tween-80 and rhamnolipid were found to improve the bioremediation fluoranthene [139]; however, it should be considered that the application of surfactants may not always lead to enhanced PAH biodegradation or removal. In fact, if the surfactant is preferentially used as an easier carbon substrate than PAHs for soil microorganisms, it may actually inhibit PAH biodegradation. Selection of surfactant types is therefore crucial for the effectiveness of surfactant-aided bioremediation of PAH-contaminated soils [140].
6. PAH Biodegradation Pathway
As illustrated in the literature, a wide spectrum of microorganisms has been classified, and these microorganisms are known for their high potential in degrading PAHs. These microorganisms include, but not limited to, bacteria, fungus, actinomycetes, protozoa, and algae [69,87]. Actually, the biodegradation of PAHs has the possibility of being undertaken either under aerobic or anaerobic conditions. However, aerobic conditions are more preferable, due to their documented efficiency [141]. As a result, composting as an aerobic technique has received more attention for treatment for such types of pollution [25,78,79,123,132,141].
Fortunately, it has been documented that a wide variety of bacterial cultures have the potential to biodegrade LMW PAHs directly, using them as the sole carbon and energy source [142,143,144,145]. Otherwise, PAHs (like HMW PAHs) have to proceed through the accumulation of these compounds in the body of microorganisms, and then be decomposed through sequential steps and multiple routes (Figure 2) into a bioavailable form that could be metabolized by microorganisms [123,141,146,147]. Hydroxylation of the aromatic ring via a di- or monooxygenase enzymes or dehydrogenase is the first step in the degradation process, with the formation of a cis-dihydrodiol, which gets rearomatized to a diol intermediate by the action of a dehydrogenase. These diol intermediates may then be cleaved by intradiol or extradiol ring-cleaving dioxygenases through either an ortho-cleavage or meta-cleavage pathway, leading to intermediates such as catechols and protocatechol acid that are ultimately converted to tricarboxylic acid cycle intermediates, which could be considered as the end of the biodegradation [123,144,146,147,148,149]. Bacteria can also degrade PAHs via the cytochrome P450-mediated pathway, with the production of trans-dihydrodiols.
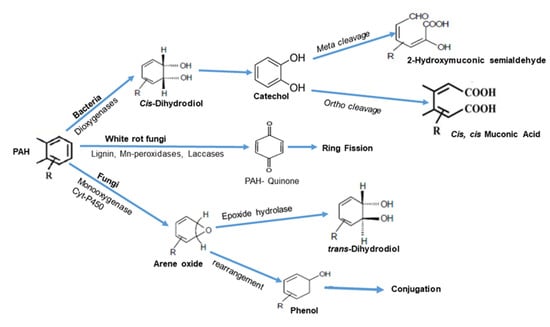
Figure 2.
Bacterial and fungal biodegradation pathways of PAHs.
It should be noted that HMW PAH degradation pathways still need more investigation, as few bacterial isolates were found to be capable of degrading them. Also, their biodegradation in some cases is complicated and passes through different routes, or even proceeds via co-metabolism, like that of benzo[a]pyrene [150,151,152].
Fungal enzymatic activity also has a key role in the biodegradation of PAHs. Lignolytic and non-lignolytic fungi have the capability of oxidizing PAHs utilizing cytochrome P-450 monooxygenase and a lignin-degrading enzyme system for oxidizing aromatic rings. Usually, an oxygen atom is incorporated into the aromatic nucleus, whereas the remaining atom is reduced to water to yield cis-transdihydrodiols. The formed arene oxide, though non-enzymatic, can undergo some rearrangement to form a phenol, which can be further conjugated with glucose, xylose, gluconeric acid, and sulfate. On the other hand, ligninolytic fungi, which are usually known as white rot fungi, have been characterized by their capability to degrade PAHs through ligninolytic and non-ligninolytic culture conditions. Ligninolytic enzymes oxidizes the PAH ring by producing hydroxyl free radicals by the donation of one electron; consequently, PAH–quinones and acids are formed instead of dihydrodiols. [123,153,154,155]. Extracellular enzymes of white rot fungi, which include laccase, LiP, and MnP, have a key role in the degradation of PAHs [153,156,157,158,159].
7. Conclusions
The main conclusion of this review is that the use of compost and composting in several strategies significantly improves the removal of PAHs in contaminated soils. However, this strategy should be well studied and tested. For instance, future studies are required on compost stability, as it is an important parameter for considering the removal of PAHs. Composting also needs to be optimized to improve PAH removal. This could include, for instance, the use of some additives like surfactants, which can be of help for the desorption and further removal of PAHs. Furthermore, more investigations are still needed regarding the biodegradation of PAHs combined with other hydrocarbons in mixtures, biodegradation HMW PAHs, and the microbial interactions within PAH-degrading consortia. In summary, composting and compost opens a wide number of strategies to improve the bioremediation of PAH-contaminated soils. However, it is important to define this strategy and to test its efficiency before full-scale application.
Author Contributions
Writing—original draft preparation, T.S.; writing—review and editing, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Tahseen Sayara would like to thank Palestine Technical University for its administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PAHs | Polycyclic aromatic hydrocarbons |
| HMW | High molecular weight |
| LMW | Low molecular weight |
| USEPA | United States Environmental Protection Agency |
References
- Levillain, J.; Cattan, P.; Colin, F.; Voltz, M.; Cabidoche, Y.M. Analysis of environmental and farming factors of soil contamination by a persistent organic pollutant, chlordecone, in a banana production area of French West Indies. Agric. Ecosyst. Environ. 2012, 159, 123–132. [Google Scholar] [CrossRef]
- Yuan, G.L.; Qin, J.X.; Li, J.; Lang, X.X.; Wang, G.H. Persistent organic pollutants in soil near the Changwengluozha glacier of the Central Tibetan Plateau, China: Their sorption to clays and implication. Sci. Total Environ. 2014, 472, 309–315. [Google Scholar] [CrossRef]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, adsorption and rhizosphere effect: The mechanisms for Phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Kavamura, V.N.; Esposito, E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 2010, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Olson, J.; Bian, L.; Rogerson, P.A. Analysis of heavy metal sources in soil using kriging interpolation on principal components. Environ. Sci. Technol. 2014, 48, 4999–5007. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.M.; Yang, C.P.; Huang, D.L.; Zhang, J.C. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Udeigwe, T.K.; Eze, P.N.; Teboh, J.M.; Stietiya, M.H. Application, chemistry, and environmental implications of contaminant-immobilization amendments on agricultural soil and water quality. Environ. Int. 2011, 37, 258–267. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Zeng, G.M.; Chen, M.; Zeng, Z.T. Shale gas: Surface water also at risk. Nature 2013, 499, 154. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.-B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, L.; Yuan, Q.; Yan, C.; Dong, C.; Meng, C.; Sui, X.; Yao, L.; Yang, F.; Lu, Y.; et al. Airborne particulate polycyclic aromatic hydrocarbon (PAH) pollution in a background site in the North China Plain: Concentration, size distribution, toxicity and sources. Sci. Total Environ. 2015, 466–467, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Yam, R.; Leung, W. Emissions trading in Hong Kong and the Pearl River Delta region -a modeling approach to trade decisions in Hong Kong’s electricity industry. Environ. Sci. Pol. 2013, 31, 1–12. [Google Scholar] [CrossRef]
- Witter, A.; Nguyen, M.; Baidar, S.; Sak, P. Coal-tar-based sealcoated pavement: A major PAH source to urban stream sediments. Environ. Pollut. 2014, 185, 59–68. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Inoue, C. Polycyclic aromatic hydrocarbons (PAHs) biodegradation potential and diversity of microbial consortia enriched from tsunami sediments in Miyagi, Japan. J. Hazard. Mater. 2015, 283, 689–697. [Google Scholar] [CrossRef]
- Wan, J.; Zeng, G.M.; Huang, D.L.; Huang, C.; Lai, C.; Li, N.J.; Wei, Z.; Xu, P.; He, X.; Lai, M.Y.; et al. The oxidative stress of phanerochaete chrysosporium against lead toxicity. Appl. Biochem. Biotechnol. 2015, 175, 1981–1991. [Google Scholar] [CrossRef]
- Evans, M.; Davies, M.; Janzen, K.; Muir, D.; Hazewinkel, R.; Kirk, J.; de Boer, D. PAH distributions in sediments in the oil sands monitoring area and western Lake Athabasca: Concentration, composition and diagnostic ratios. Environ. Pollut. 2016, 213, 671–687. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. The levels and composition of persistent organic pollutants in alluvial agriculture soils affected by flooding. Environ. Monit. Assess. 2013, 185, 9935–9948. [Google Scholar] [CrossRef]
- Tsibart, A.; Gennadiev, A. Polycyclic aromatic hydrocarbons in soils: Sources, behavior, and indication significance. Eurasian Soil Sci. 2013, 46, 728–741. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M. Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J. Soils Sediment. 2011, 11, 62–71. [Google Scholar] [CrossRef]
- Koshlaf, E.; Shahsavari, E.; Aburto-Medina, A.; Taha, M.; Haleyur, N.; Makadia, T.H.; Morrison, P.D.; Ball, A.S. Bioremediation potential of diesel-contaminated Libyan soil. Ecotoxicol. Environ. Saf. 2016, 133, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.M.; Dadrasnia, A.; Lim, K.T.; Mahmud, A.F.; Ismail, S. Application of Biosurfactants in Environmental Biotechnology; Remediation of Oil and Heavy Metal. AIMS Bioeng. 2016, 3, 289–304. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Lopez-Real, J.; Beck, A. Degradation of polycyclic aromatic hydrocarbons (PAHs) in an aged coal tar contaminated soil under in-vessel composting conditions. Environ. Pollut. 2006, 141, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, E.; Cappa, F.; Fragoulis, G.; Trevisan, M.; Del Re, A.A.M. Bioavailability and degradation of phenanthrene in compost amended soils. Chemosphere 2007, 67, 548–556. [Google Scholar] [CrossRef]
- Sayara, T.; Borràs, E.; Caminal, G.; Sarrà, M.; Sánchez, A. Bioremediation of PAHs-contaminated soil through composting: Influence of bioaugmentation and biostimulation on contaminant biodegradation. Int. Biodeter. Biodegr. 2011, 65, 859–865. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.; Tejeda-Agredano, M.; Jimenez-Sanchez, C.; Congiu, E.; Sungthong, R.; Niqui-Arroyo, J.; Cantos, M. Is it possible to increase bioavailability but not environmental risk of PAHs in bioremediation? J. Hazard. Mater. 2013, 261, 733–745. [Google Scholar] [CrossRef]
- Lukić, B.; Panico, A.; Huguenot, D.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G. Evaluation of PAH removal efficiency in an artificial soil amended with different types of organic wastes. Euro-Mediterr. J. Environ. Integr. 2016, 1, 5. [Google Scholar] [CrossRef]
- Miller, M.; Stratton, G.; Murray, G. Effects of nutrient amendments and temperature on the biodegradation of pentachlorophenol contaminated soil. Water Air Soil Pollut. 2004, 151, 87–101. [Google Scholar] [CrossRef]
- Joo, H.S.; Ndegwa, P.M.; Shoda, M.; Phae, C.G. Bioremediation of oilcontaminated soil using Candida catenulata and food waste. Environ. Pollut. 2008, 156, 891–896. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, B.H. Effect of nonionic surfactants on biodegradation of phenanthrene by a marine bacteria of Neptunomonas naphthovorans. J. Hazard. Mater. 2009, 162, 66–73. [Google Scholar] [CrossRef]
- Gomez, S.M. Optimization of field scale biopiles for bioremediation of petroleum hydrocarbon contaminated soil at low temperature conditions by response surface methodology (RSM). Int. Biodeter. Biodegr. 2014, 89, 103–109. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Cheng, M.; Wan, J.; Hu, L.; Zhang, Y. Effect of Phanerochaete chrysosporium inoculation on bacterial community and metal stabilization in lead-contaminated agricultural waste composting. Bioresour. Technol. 2017, 243, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manag. 2017, 199, 46–61. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Zhong, H.; Wang, Z.; Liu, Z.; Cheng, M.; Liu, G.; Yang, X.; Liu, S. Effect of rhamnolipid solubilization on hexadecane bioavailability: Enhancement or reduction? J. Hazard. Mater. 2017, 322, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Singh, O.V.; Jain, R.K. Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. Trends Biotechnol. 2002, 20, 243–248. [Google Scholar] [CrossRef]
- Watanabe, K.; Futamata, H.; Harayama, S. Understanding the diversity in catabolic potential of microorganisms for the development of bioremediation strategies. Anton. Van Leeuw. 2002, 81, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zeng, G.M.; Chen, Y.N.; Zhang, J.C.; Yu, Y.; Li, H.; Liu, Z.-F.; Tang, L. Effects of inoculation with Phanerochaete chrysosporium on remediation of pentachlorophenol-contaminated soil waste by composting. Process. Biochem. 2011, 46, 1285–1291. [Google Scholar] [CrossRef]
- Gemmell, B.J.; Bacosa, H.P.; Liu, Z.; Buskey, E.J. Can gelatinous zooplankton influence the fate of crude oil in marine environments? Mar. Pollut. Bull. 2016, 113, 483–487. [Google Scholar] [CrossRef]
- Sosa, B.S.; Porta, A.; Lerner, J.E.C.; Noriega, R.B.; Massolo, L. Human health risk due to variations in PM10-PM2.5 and associated PAHs levels. Atmos. Environ. 2017, 160, 27–35. [Google Scholar] [CrossRef]
- EC (European Commission). Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions—Thematic Strategy for Soil Protection. COM, 2006; 231final. 2006. Available online: http://ec.europa.eu/environment/soil/pdf/com_2006_0231_en.pdf (accessed on 15 January 2020).
- EEA (European Environment Agency). Progress in Management of Contaminated Sites (CSI 015) 2007. Available online: http://themes.eea.europa.eu/IMS/ISpecs/ISpecification20041007131746/IAssessment1152619898983/view_content (accessed on 15 January 2020).
- Fu, P.P.; Xia, Q.; Sun, X.; Yu, H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J. Environ. Sci. Health C 2012, 30, 1–41. [Google Scholar] [CrossRef]
- Niepceron, M.; Martin-Laurent, F.; Crampon, M.; Portet-Koltalo, F.; Akpa-Vinceslas, M.; Legras, M.; Bru, D.; Bureau, F.; Bodilis, J. Gamma Proteobacteria as a potential bioindicator of a multiple contamination by polycyclic aromatic hydrocarbons (PAHs) in agricultural soils. Environ. Pollut. 2013, 180, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Simon, R.; Anklam, E.; Kleiner, J. Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Trends Anal. Chem. 2006, 25, 716–725. [Google Scholar] [CrossRef]
- Gabov, D.N.; Beznosikov, V.A.; Kondratenok, B.M.; Yakovleva, E.V. Polycyclic aromatic hydrocarbons in the soils of technogenic landscapes. Geochem. Int. 2010, 48, 569–579. [Google Scholar] [CrossRef]
- Gennadiev, A.; Tsibart, A. Pyrogenic polycyclic aromatic hydrocarbons in soils of reserved and anthropogenically modified areas: Factors and features of accumulation. Eurasian Soil Sci. 2013, 46, 28–36. [Google Scholar] [CrossRef]
- Luo, L.; Lin, S.; Huang, H.L.; Zhang, S.Z. Relationships between aging of PAHs and soil properties. Environ. Pollut. 2012, 170, 177–182. [Google Scholar] [CrossRef]
- Pereira, T.; Laiana, S.; Rocha, J.; Broto, F.; Comellas, L.; Salvadori, D.; Vargas, V. Toxicogenetic monitoring in urban cities exposed to different airborne contaminants. Ecotoxicol. Environ. Saf. 2013, 90, 174–182. [Google Scholar] [CrossRef]
- Mohan, S.V.; Prasanna, D.; Purushotham, R.B.; Sarma, P.N. Ex situ bioremediation of pyrene contaminated soil in bio-slurry phase reactor operated in periodic discontinuous batch mode: Influence of bioaugmentation. Int. Biodeter. Biodegr. 2008, 62, 162–169. [Google Scholar] [CrossRef]
- Hamdi, H.; Benzarti, S.; Manusadžianas, L.; Aoyama, I.; Jedidi, N. Bioaugmentation and biostimulation effects on PAH dissipation and soil ecotoxicity under controlled conditions. Soil Biol. Bioch. 2007, 39, 1926–1935. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Sayler, G.S.; Ripp, S. Field applications of genetically engineered microorganisms for bioremediation processes. Curr. Opin. Biotechnol. 2000, 11, 286–289. [Google Scholar] [CrossRef]
- Haug, R.T. The Practical Handbook of Compost Engineering; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Hsu, J.H.; Lo, S.L. Chemical and spectroscopic analysis of organic matter transformations during composting of pig manure. Environ. Pollut. 1999, 104, 189–196. [Google Scholar] [CrossRef]
- Lemmon, C.R.; Pylypiw, H.M. Degradation of diazanon, chlorpyrifos, isofenphos and pendimethalin in grass and compost. Bull. Environ. Contam. Toxicol. 1992, 48, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Breitung, J.; Bruns-Nagel, D.; Steinbach, K.; Kaminski, L.; Gemsa, D.; Von LoÈ w, E. Bioremediation of 2, 4, 6-trinitrotoluene-contaminated soils by two different aerated compost systems. Appl. Microbiol. Biotechnol. 1996, 44, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Reid, B.J.; Fermor, T.R. Impact of composting strategies on the treatment of soils contaminated with organic pollutants. Environ. Pollut. 2001, 112, 269–283. [Google Scholar] [CrossRef]
- Loick, N.; Hobbs, P.J.; Hale, M.D.C.; Jones, D.L. Bioremediation of Poly-Aromatic Hydrocarbon (PAH)-Contaminated Soil by Composting. Crit. Rev. Environ. Sci. Technol. 2009, 39, 271–332. [Google Scholar] [CrossRef]
- Sayara, T.; Sarrà, M.; Sánchez, A. Preliminary screening of co-substrates for bioremediation of pyrene-contaminated soil through composting. J. Hazard. Mater. 2009, 172, 1695–1698. [Google Scholar] [CrossRef]
- Gandolfi, I.; Sicolo, M.; Franzetti, A.; Fontanarosa, E.; Santagostino, A.; Bestetti, G. Influence of compost amendment on microbial community and ecotoxicity of hydrocarbon-contaminated soils. Bioresour. Technol. 2010, 101, 568–575. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Vaes, K.; Klammer, S.; De Clercq, D.; Coosemans, J.; Insam, H.; Swings, J. A survey of bacteria and fungi occurring during composting and self-heating processes. Ann. Microbiol. 2003, 53, 349–410. [Google Scholar]
- Tejada, M.; González, J.L.; Hernández, M.T.; García, C. Application of different organic amendments in a gasoline contaminated soil: Effect on soil microbial properties. Bioresour. Technol. 2008, 99, 2872–2880. [Google Scholar] [CrossRef]
- Tejada, M.; Hernandez, M.T.; Garcia, C. Soil restoration using composted plant residues: Effects on soil properties. Soil Tillage Res. 2009, 102, 109–117. [Google Scholar] [CrossRef]
- Hu, Z.H.; Liu, Y.L.; Chen, G.W.; Gui, X.Y.; Chen, T.H.; Zhan, X.M. Characterization of organic matter degradation during composting of manure straw mixtures spiked with tetracyclines. Bioresour. Technol. 2011, 102, 7329–7334. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.T.T.; Penfold, C.; Marschner, P. Differential effects of composts on properties of soils with different textures. Biol. Fert. Soils 2012, 48, 699–707. [Google Scholar] [CrossRef]
- Wu, G.; Kechavarzi, C.; Li, X.; Sui, H.; Pollard, S.J.T.; Coulon, F. Influence of mature compost amendment on total and bioavailable polycyclic aromatic hydrocarbons in contaminated soils. Chemosphere 2013, 90, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Jehmlichc, N.; Lima, K.; Morris, B.E.L.; Richnow, H.H.; Hernández, T.; von Bergen, M.; García, C. The ecological and physiological responses of the microbial community from a semiarid soil to hydrocarbon contamination and its bioremediation using compost amendment. J. Proteomics 2016, 135, 162–169. [Google Scholar] [CrossRef]
- Latawiec, A.E.; Swindell, A.L.; Simmons, P.; Reid, B.J. Bringing bioavailability into contaminated land decision making: The way forward? Crit. Rev. Environ. Sci. Technol. 2011, 41, 52–77. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Hamdi, H.; Revel, J.C. Fate of polycyclic aromatic hydrocarbons during composting of lagooning sewage sludge. Chemosphere 2005, 58, 449–458. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, S.; Zhang, N.; Zhang, D.Y.; Li, X.Q. The effect of soil organic matter on fate of polycyclic aromatic hydrocarbons in soil: A microcosm study. Environ. Pollut. 2010, 158, 1768–1774. [Google Scholar] [CrossRef]
- Tang, J.; Lu, X.; Sun, Q.; Zhu, W. Aging effect of petroleum hydrocarbons in soil under different attenuation conditions. Agric. Ecosyst. Environ. 2012, 149, 109–117. [Google Scholar] [CrossRef]
- Cébron, A.; Faure, P.; Lorgeoux, C.; Ouvrard, S.; Leyval, C. Experimental increase in availability of a PAH complex organic contamination from an aged contaminated soil: Consequences on biodegradation. Environ. Pollut. 2013, 177, 98–105. [Google Scholar] [CrossRef]
- Han, X.; Hu, H.; Shi, X.; Zhang, L.; He, J. Effects of different agricultural wastes on the dissipation of PAHs and the PAH-degrading genes in a PAH-contaminated soil. Chemosphere 2017, 172, 286–293. [Google Scholar] [CrossRef]
- Lukić, B.; Huguenot, D.; Panico, A.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G. Importance of organic amendment characteristics on bioremediation of PAH-contaminated soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 15041–15052. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, W.; Hwang, E.Y.; Park, J.S.; Choi, J.Y. Bioremediation of diesel-contaminated soil with composting. Environ. Pollut. 2002, 119, 23–31. [Google Scholar] [CrossRef]
- Piskonen, R.; Itävaara, M. Evaluation of chemical pretreatment of contaminated soil for improved PAH bioremediation. Appl. Microbiol. Biotechnol. 2004, 65, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo[a]pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Sayara, T.; Sarrà, M.; Sánchez, A. Effects of compost stability and contaminant concentration on the bioremediation of PAHs contaminated soil through composting. J. Hazard. Mater. 2010, 179, 999–1006. [Google Scholar] [CrossRef]
- Sayara, T.; Sarrà, M.; Sánchez, A. Optimization and enhancement of soil bioremediation by composting using the experimental design technique. Biodegradation 2010, 21, 345–356. [Google Scholar] [CrossRef]
- Zappi, M.E.; Rogers, B.A.; Teeter, C.L.; Gunnison, D.; Bajpai, R. Bioslurry treatment of a soil contaminated with low concentrations of total petroleum hydrocarbons. J. Hazard. Mater. 1996, 46, 1–12. [Google Scholar] [CrossRef]
- Jorgensen, K.S.; Puustinen, J.; Suortti, A.M. Bioremediation of petroleum hydrocarbon-contaminated soil by composting in biopiles. Environ. Pollut. 2000, 107, 245–254. [Google Scholar] [CrossRef]
- Viamajala, S.; Peyton, B.M.; Richards, L.A.; Petersen, J.N. Solubilization, solution equilibria, and biodegradation of PAHs under thermophilic conditions. Chemosphere 2007, 66, 1094–1106. [Google Scholar] [CrossRef]
- Bartha, R.; Bossert, I. The Treatment and Disposal of Petroleum Wastes in Petroleum Microbiology; Macmillan: New York, NY, USA, 1984; pp. 553–578. [Google Scholar]
- Cooney, J.J. The Fate of Petroleum Pollutants in Fresh Water Ecosystems. In Petroleum Microbiology; Atlas, R.M., Ed.; Macmillan: New York, NY, USA, 1984; pp. 399–434. [Google Scholar]
- Purnomo, A.S.; Koyama, F.; Mori, T.; Kondo, R. DDT degradation potential of cattle manure compost. Chemosphere 2010, 80, 619–624. [Google Scholar] [CrossRef]
- Houot, S.; Verge-Leviel, C.; Poitrenaud, M. Potential mineralization of various organic pollutants during composting. Pedosphere 2012, 22, 536–543. [Google Scholar] [CrossRef]
- Peng, J.J.; Zhang, Y.; Su, J.Q.; Qiu, Q.F.; Jia, Z.J.; Zhu, Y.G. Bacterial communities predominant in the degradation of 13C4-4,5,9,10-pyrene during composting. Bioresour. Technol. 2013, 143, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B.; Lopez-Real, J.; Beck, A.J. In-vessel composting-bioremediation of aged coal tar soil: Effect of temperature and soil/green waste amendment ratio. Environ. Int. 2005, 31, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B.; Spanova, K.; Beck, A.J.; Russell, N.J. Microbial community structure changes during bioremediation of PAHs in an aged coal-tar contaminated soil by in-vessel composting. Int. Biodeterior. Biodegrad. 2008, 61, 357–364. [Google Scholar] [CrossRef]
- Kriipsalu, M.; Marques, M.; Hogland, W.; Nammari, D.R. Fate of polycyclic aromatic hydrocarbons during composting of oil sludge. Environ. Technol. 2008, 29, 43–53. [Google Scholar] [CrossRef]
- Alves, D.; Villar, I.; Mato, S. Thermophilic composting of hydrocarbon residue with sewage sludge and fish sludge as cosubstrates: Microbial changes and TPH reduction. J. Environ. Manag. 2019, 239, 30–37. [Google Scholar] [CrossRef]
- Zhu, F.; Storey, S.; Ashaari, M.M.; Clipson, N.; Doyle, E. Benzo (a) pyrene degradation and microbial community responses in composted soil. Environ. Sci. Pollut. R. 2017, 24, 5404–5414. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Wang, J.; Deng, Y.; Liu, Y.; Peng, B. The potential impact on the biodegradation of organic pollutants from composting technology for soil remediation. Waste Manag. 2018, 72, 138–149. [Google Scholar] [CrossRef]
- Mattei, P.; Cincinelli, A.; Martellini, T.; Natalini, R.; Pascale, E.; Renella, G. Reclamation of river dredged sediments polluted by PAHs by co-composting with green waste. Sci. Total. Environ. 2016, 566–567, 567–574. [Google Scholar] [CrossRef]
- Hesnawi, R.M.; McCartney, D. Impact of compost amendments and operating temperature on diesel fuel bioremediation. Environ. Eng. Sci. 2006, 5, 37–45. [Google Scholar] [CrossRef]
- Li, J.Y.; Ye, Q.F.; Gan, J. Influence of organic amendment on fate of acetaminophen and sulfamethoxazole in soil. Environ. Pollut. 2015, 206, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Plaza, C.; Xing, B.S.; Fernández, J.M.; Senesi, N.; Polo, A. Binding of polycyclic aromatic hydrocarbons by humic acids formed during composting. Environ. Pollut. 2009, 157, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zmora-Nahum, S.; Markovitch, O.; Tarchitzky, J.; Chen, Y. Dissolved organic carbon (DOC) as a parameter of compost maturity. Soil Biol. Biochem. 2005, 37, 2109–2116. [Google Scholar] [CrossRef]
- Adam, I.K.U.; Miltner, A.; Kästner, M. Degradation of 13C-labeled pyrene in soil-compost mixtures and fertilized soil. Appl. Microbiol. Biotechnol. 2015, 99, 9813–9824. [Google Scholar] [CrossRef]
- Senesi, N.; Plaza, C. Role of humification processes in recycling organic wastes of various nature and sources as soil amendments. Clean-Soil Air Water. 2007, 35, 26–41. [Google Scholar] [CrossRef]
- He, X.S.; Xi, B.D.; Cui, D.Y.; Liu, Y.; Tan, W.B.; Pan, H.W.; Li, D. Influence of chemical and structural evolution of dissolved organic matter on electron transfer capacity during composting. J. Hazard. Mater. 2014, 268, 256–263. [Google Scholar] [CrossRef]
- Doublet, J.; Francou, C.; Pétraud, J.P.; Dignac, M.F.; Poitrenaud, M.; Houot, S. Distribution of C and N mineralization of a sludge compost within particle-size fractions. Bioresour. Technol. 2010, 101, 1254–1262. [Google Scholar] [CrossRef]
- Verma, S.L.; Marschner, P. Compost effects on microbial biomass and soil P pools as affected by particle size and soil properties. J. Soil Sci. Plant. Nut. 2013, 13, 313–328. [Google Scholar]
- Jednak, T.; Avdalović, J.; Miletić, S.; Slavković-Beškoski, L.; Stanković, D.; Milić, J.; Llic, M.; Beškoski, V.; Gojgić-Cvijović, G.; Vrvić, M.M. Transformation and synthesis of humic substances during bioremediation of petroleum hydrocarbons. Int. Biodeterior. Biodegrad. 2017, 122, 47–52. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Saiz-Jimenez, C. Effect of humic fractions and clay on biodegradation of phenanthrene by a Pseudomonas fluorescens strain isolated from soil. Appl. Environ. Microbiol. 1998, 64, 3123–3126. [Google Scholar] [CrossRef]
- Kobayashi, T.; Murai, Y.; Tatsumi, K.; Iimura, Y. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas sp. enhanced by water-extractable organic matter from manure compost. Sci. Total Environ. 2009, 407, 5805–5810. [Google Scholar] [CrossRef] [PubMed]
- Nwankwegu, A.S.; Orji, M.U.; Onwosi, C.O. Studies on organic and in-organic biostimulants in bioremediation of diesel-contaminated arable soil. Chemosphere 2016, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, X.; Zhu, Q.; Ma, J.; Hou, H.; Zhang, S. Bioremediation of petroleum-contaminated soil enhanced by aged refuse. Chemosphere 2019, 222, 98–105. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xu, Y.; Zhao, J.; Li, F.M.; Gao, D.M.; Xing, B.S. Remediation of petroleum contaminated soils through composting and rhizosphere degradation. J. Hazard. Mater. 2011, 190, 677–685. [Google Scholar] [CrossRef]
- Hickman, Z.A.; Reid, B.J. The co-application of earthworms (Dendrobaena veneta) and compost to increase hydrocarbon losses from diesel contaminated soils. Environ. Int. 2008, 34, 1016–1022. [Google Scholar] [CrossRef]
- Bao, H.; Wang, J.; Li, J.; Zhang, H.; Wu, F. Effects of corn straw on dissipation of polycyclic aromatic hydrocarbons and potential application of backpropagation artificial neural network prediction model for PAHs bioremediation. Ecotoxicol. Environ. Saf. 2019, 186, 109745. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Hausmann, T.S.; Fortman, T.J. Does bioavailability limit biodegrada- tion? A comparison of hydrocarbon biodegradation and desorption rates in aged soils. Biodegradation 2004, 15, 261–274. [Google Scholar] [CrossRef]
- Hwang, S.; Cutright, T.J. Biodegradability of aged pyrene and phenanthrene in a natural soil. Chemosphere 2002, 47, 891–899. [Google Scholar] [CrossRef]
- Kennedy, T.A.; Naeem, S.; Howe, K.M.; Knops, J.M.H.; Tilman, D.; Reich, P. Biodiversity as a barrier to ecological invasion. Nature 2002, 417, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.G.; Houot, S.; Qiao, M.; Nunan, N.; Garnier, P. Remediation of polycyclic aromatic hydrocarbon (PAH) contaminated soil through composting with fresh organic wastes. Environ. Sci. Pollut. Res. Int. 2011, 18, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Winquist, E.; Bjorklof, K.; Schultz, E.; Räsänen, M.; Salonen, K.; Anasonye, F.; Cajthaml, T.; Steffen, K.T.; Jørgensen, K.S.; Tuomela, M. Bioremediation of PAH-contaminated soil with fungi- From laboratory to field scale. Int. Biodeter. Biodegr. 2014, 86, 238–247. [Google Scholar] [CrossRef]
- Wu, M.L.; Dick, W.A.; Li, W.; Wang, X.C.; Yang, Q.; Wang, T.T.; Xu, L.M.; Zhang, M.H.; Chen, L.M. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeter. Biodegr. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Canet, R.; Birnstingl, J.G.; Malcolm, D.G.; Lopez-Real, J.M.; Beck, A.J. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by native microflora and combinations of white-rot fungi in a coal-tar contaminated soil. Bioresour. Technol. 2001, 76, 113–117. [Google Scholar] [CrossRef]
- McFarland, M.J.; Qiu, X.J. Removal of benzo(a)pyrene in soil composting systems amended with the white rot fungus Phanerochaete chrysosporium. J. Hazard. Mater. 1995, 42, 61–70. [Google Scholar] [CrossRef]
- Lebeau, T. Bioaugmentation for in Situ Soil Remediation: How to Ensure the Success of Such a Process. In Bio- Augmentation, Biostimulation and Biocontrol, Soil Biology; Singh, A., Parmar, N., Kuhad, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 28, pp. 129–186. [Google Scholar]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zafra, G.; Cortés-Espinosa, D.V. Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: A mini review. Environ. Sci. Pollut. Res. 2015, 22, 19426–19433. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.G.; Cerniglia, C.E.; Pritchard, P.H. Bioremediation of Environments Contaminated by Polycyclic Aromatic Hydrocarbons. In Bioremediation: Principles and Applications; Crawford, R.L., Crawford, D.L., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 1215–1294. [Google Scholar]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Riding, M.J.; McAllister, L.E.; Sopena-Vazquez, F.; Bending, G.D. Impact of black carbon on the bioaccessibility of organic contaminants in soil. J. Hazard. Mater. 2013, 261, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.D.; Paton, G.; Semple, K.T. Behaviour and assessment of bioavailability of organic contaminants in soil: Relevance for risk assessment and remediation. Soil Use Manag. 2006, 21, 475–486. [Google Scholar] [CrossRef]
- Loeher, R.C.; Mc Millen, S.J.; Webster, M.T. Predictions of biotreatability and actual results: Soils with petroleum hydrocarbons. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2001, 5, 78–87. [Google Scholar] [CrossRef]
- Trinidade, P.V.O.; Sobral, A.C.L.; Rizzo, S.G.F.; Leite Soriano, A.U. Bioremediation of weathered and recently oil-contaminated soils from Brazil: A compression study. Chemosphere 2005, 58, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Allard, A.S.; Remberger, M.; Neilson, A.H. The negative impact of aging on the loss of PAH components in a creosote-contaminated soil. Int. Biodeter. Biodegr. 2000, 46, 43–49. [Google Scholar] [CrossRef]
- Maletic, S.; Dalmacija, B.; Roncevic, S.; Agbaba, J.; Ugarcina, P.S. Impact of hydrocarbon type, concentration and weathering on its biodegradability in soil. J. Environ. Sci. Heal. A 2011, 46, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Balbuena, L.; Romero-Tepal, E.M.; Luna-Guido, M.L.; Marsch, R.; Dendooven, L. Removal of anthracene from recently contaminated and aged soils. Water Air Soil Pollut. 2013, 224, 1420. [Google Scholar] [CrossRef]
- Margesin, R.; Labbe, D.; Schninner, F.; Greer, C.W.; Whyte, L.G. Characterization of Hydrocarbon-Degrading Microbial Populations in Contaminated and Pristine Contaminated Soils. Appl. Environ. Microbiol. 2003, 69, 3085–3092. [Google Scholar] [CrossRef]
- Pawar, R.M. The Effect of Soil pH on Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHS). J. Bioremed. Biodeg. 2015, 6, 291. [Google Scholar] [CrossRef]
- Couling, N.R.; Towell, M.G.; Semple, K.T. Biodegradation of PAHs in soil: Influence of chemical structure, concentration and multiple amendment. Environ. Pollut. 2010, 158, 3411–3420. [Google Scholar] [CrossRef]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; Van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Lai, K.M.; Wong, J.W.C. Effects of pig manure compost and nonionicsurfactant Tween 80 on phenanthrene and pyrene removal from soil vegetated with Agropyron elongatum. Chemosphere 2008, 73, 791–797. [Google Scholar] [CrossRef]
- Adrion, A.C.; Nakamura, J.; Shea, D.; Aitken, M.D. Screening nonionic surfactants for enhanced biodegradation of polycyclic aromatic hydrocarbons remaining in soil after conventional biological treatment. Environ. Sci. Technol. 2016, 50, 3838–3845. [Google Scholar] [CrossRef]
- Shivlata, L.; Satyanarayana, T. Thermophilic and alkaliphilic Actinobacteria: Biology and potential applications. Front. Microbiol. 2015, 6, 1014. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, C.; Zhao, Y. Effects of surfactants on the fractionation, vermiaccumulation, and removal of fluoranthene by earthworms in soil. Chemosphere 2020, 250, 126332. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.C.; Gan, J. Influence of rhamnolipid biosurfactant and Brij-35 synthetic surfactant on 14C-Pyrene mineralization in soil. Environ. Pollut. 2018, 243, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Ambrosoli, R.; Petruzzelli, L.; Minati, J.L.; Marsan, F.A. Anaerobic PAH degradation in soil by a mixed bacterial consortium under denitrifying conditions. Chemosphere 2005, 60, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.H.; Xiong, A.S.; Xue, Y.; Fu, X.Y.; Gao, F.; Zhao, W.; Tian, Y.-S.; Yao, Q.-H. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol. Rev. 2008, 32, 927–955. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Chakraborty, J.; Dutta, T.K. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2011, 37, 64–90. [Google Scholar] [CrossRef]
- Ghosal, D.; Dutta, A.; Chakraborty, J.; Basu, S.; Dutta, T.K. Characterization of the metabolic pathway involved in assimilation of acenaphthene in Acinetobacter sp. strain AGAT-W. Res. Microbiol. 2013, 164, 155–163. [Google Scholar] [CrossRef]
- Hammel, K.E. Mechanisms for polycyclic aromatic hydrocarbon degradation by ligninolytic fungi. Environ. Health Perspect. 1995, 103, 41. [Google Scholar]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 1992, 3, 351–368. [Google Scholar] [CrossRef]
- Eaton, R.W.; Chapman, P.J. Bacterial metabolism of naphthalene: Construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J. Bacteriol. 1992, 174, 7542–7554. [Google Scholar] [CrossRef]
- van Herwijnen, R.; Wattiau, P.; Bastiaens, L.; Daal, L.; Jonker, L.; Springael, D.; Govers, H.A.J.; Parsons, J.R. Elucidation of the metabolic pathway of fluorene and cometabolic pathways of phenanthrene, fluoranthene, anthracene and dibenzothiophene by Sphingomonas sp. LB126. Res. Microbiol. 2003, 154, 199–206. [Google Scholar] [CrossRef]
- Kweon, O.; Kim, S.-J.; Jones, R.C.; Freeman, J.P.; Adjei, M.D.; Edmondson, R.D.; Cerniglia, C.E. A polyomic approach to elucidate the fluoranthene-degradative pathway in Mycobacterium vanbaalenii PYR-1. J. Bacteriol. 2007, 189, 4635–4647. [Google Scholar] [CrossRef]
- Kim, S.J.; Kweon, O.; Jones, R.C.; Freeman, J.P.; Edmondson, R.D.; Cerniglia, C.E. Complete and integrated pyrene degradation pathway in Mycobacterium vanbaalenii PYR-1 based on systems biology. J. Bacteriol. 2007, 189, 464–472. [Google Scholar] [CrossRef]
- Sutherland, J.B.; Rafii, F.; khan, A.A.; Cerniglia, C.E. Mechanisms of Polycyclic Aromatic Hydrocarbon Degradation. In Microbial Transformation and Degradation of Toxic Organic Chemicals; Young, L.Y., Cerniglia, C.E., Eds.; Wiley-Liss: New York, NY, USA, 1995. [Google Scholar]
- Bezalel, L.; Hadar, Y.; Cerniglia, C.E. Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 1997, 63, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, C.E.; Sutherland, J.B. Degradation of polycyclic aromatic hydrocarbons by fungi. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., McGenity, T.J., van der Meer, J.R., de Lorenzo, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2080–2110. [Google Scholar]
- Jerina, D.M. The 1982 Bernard, B. brodie Award Lecture. Metabolism of Aromatic hydrocarbons by the cytochrome P-450 system and epoxide hydrolase. Drug Metab. Dispos. 1983, 11, 1–4. [Google Scholar]
- Chang, B.V.; Chang, W.; Yuan, S.Y. Anaerobic degradation of polycyclic aromatic hydrocarbons in sludge. Adv. Environ. Res. 2003, 7, 623–628. [Google Scholar] [CrossRef]
- Tortella, G.R.; Diez, M.C.; Duran, N. Fungal diversity and use in decomposition of environmental pollutants. Crit. Rev. Microbiol. 2005, 31, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Karaçay, H.A.; Shahi, A.; Gökçe, S.; Ince, B.; Ince, O. Aerobic and anaerobic fungal metabolism and Omics insights for increasing polycyclic aromatic hydrocarbons biodegradation. Fungal Biol. Rev. 2017, 31, 61–72. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).