A Practical Guide to Class IIa Medical Device Development
Abstract
1. Introduction
2. The Steps of Medical Device Development in General
2.1. Technological Steps
- At first, the intended use of the medical device must be defined. The intended use is the primary use purpose of the product, including medical indication and patient group.
- Next, the functional requirement is formulated. The requirements will form the basis for the development of the system and be used to verify and validate the design changes. These requirements need to be translated to functional requirement specifications, which will become design inputs for manufacturing.
- The design input is the base of the device planning and engineering—it means that the actual parameters regarding operation (usage, risk, standards) and physical appearance of the device are created. The parameters needed for implementation, such as the characteristics and dimensions of the product, are collected as functional requirement specification (Table 1). The design output consists of drawings, specifications and manufacturing instructions—it describes all parts of the device. These will become part of the design and development documentation of the device and therefore also part of the technical documentation.
- Prototypes are prepared according to the design output and the approved prototype can go into small quantity production. The produced devices are to be used mainly for process validation. Sterilisation and packaging plans must be finalized, and then these need to be validated as well. The design output is suggested to be patented if the medical device is novel, inventive and industrially applicable.
2.2. Quality Management and Regulatory
- Complying risks and hazards and their management according to ISO 14971.
- Classification of the device according to the applicable medical device regulations (MDR), based on the markets that are planned to be accessed. In our example, the EU is the main focus. The classification is based on the risks relating to the device—there are three main classes, Class I, Class II and Class III. This determines if the device needs to be sterile, and gives a basic idea on the magnitude of the costs and complexity of the device.
- The essential requirements (general safety and performance according to the new MDR) of the devices and all the processes that are in connection with the device are regulated by standards and it is required to be gathered according to the applicable medical device directives (MDD) and regulations (Table 2). Essential requirements are divided into two parts—Part I: general requirements and Part II: requirements for design and construction.A checklist can be obtained through the Official Journal of the European Union to identify the applicable standards. All the process validations need to be performed that are required in the applicable standards and regulations after the design and development phase is over.
- Biological evaluation needs to be performed according to ISO 10993-1 to ensure from biological and toxicological aspects, and that the use of the device is safe for both the patient and the users (Table 3).
- Accomplishing clinical evaluation. Clinical evaluation is either the analysis and demonstration of equivalence [39] using publicly available clinical data regarding the clinical, technical and biological characteristics of the medical device compared to relevant medical devices on the market, or performing a clinical investigation [40] to determine its clinical safety and effectiveness, that is stated in the intended use.
3. Demonstration of the Steps Based on the hypACT Inject Auto Device
3.1. Technological Steps
- The intended use of the medical device:The device was developed to accomplish point of care therapy for knee osteoarthritis. It is suitable to draw blood from a patient, and after a physical separation, a concentrated growth factor containing autologous serum (serum from platelet-rich fibrin, SPRF) is isolated aseptically in a closed system. In cases when the physician intends to use SPRF, this serum fraction can be isolated in an aseptic way by the device and injected to the knee of the patient immediately.
- The user needs were collected and used to create the functional requirement specifications.The user needs were found to be the following:
- Device can be used in medical facilities.
- The device can be used in autologous therapy.
- The plunger can be pulled by one or two fingers.
- The device has to withstand drawing and injecting therapeutically useful amounts of blood.
- The clotted serum fraction can be separated from the red blood cells.
- The device has to fit in a centrifuge bucket where a 50 mL conical centrifuge tube fits.
- Needles or butterfly needles can be attached to the syringe.
- The device shall not leak.
- The device shall be used to draw blood from human patients and the contents of the syringe are compatible to be used therapeutically.
- The device can be stored prior to use without any changes occurring.
- Can be centrifuged at high speed for a short period of time.
- Liquid from the clotted serum shall be pressed out.
- No infection occurs when using the device.
- Design input and outputThe user needs were converted to functional requirements (Table 1).These were translated to design inputs based on physical parameters, essential requirements and risks. Visualization of the ideas and intensive and constructive communication were conducted between the in-house or contractual developer and the initiator by the fabrication of desk models and prototypes, which resulted in the design outputs. An example to demonstrate a part of the final version of the design outputs of the device can be seen in Figure 1. To simplify the regulatory process, each part of the device is made of approved medical device grade materials.
- Review and ValidationThe development process ends with the fabrication of a prototype that was prepared according to the finalized design output, and is accepted by the legal manufacturer. This is followed by a mandatory design review and small quantity production (300–1000 units) can begin for process and manufacturing validations, which were determined by the applicable standards and regulations, e.g., as the above-mentioned biological evaluation.Besides the manufacturing related processes, that need to be validated, two processes need special attention—these are sterilisation and packaging.In the present case, the device was sterilised using ethylene-oxide, due to the glass constituent, which would become amber coloured if gamma radiation was used, thus the sterilisation method was validated according to EN 1422:2014, as listed in Table 2. It is important to note that not all medical devices need to be sterilised—this requirement needs to be evaluated according to the classification of the device.The packaging of the device consists of a tray (as seen in Figure 2, made of Poly (ethylene terephthalateco-1,4-cylclohexylenedimethylene terephthalate (PETg)) and a Tyvek lid (Tyvek 1073B), and the packaging was tested according to the applicable standards. Packaging validation needs to be performed with special care, as packaging needs to be reliable, being the first and most important barrier throughout the whole lifecycle of the device to ensure sterility from the end of manufacturing during transportation and storage until the device is used by the end user.The packaging, labelling and instructions for use need to be created according to the applicable standards and are considered as parts of the device; thus, after the medical device design is finalized, then the whole packaged device needs to pass the technology-related process validations. Therefore, the description of the device, also called as the instructions for use (IFU) needs to be part of the design and has to be placed within the packaging before the validation begins. As an example, the main part of the IFU of the present device can be seen in Figure 2.The design of the present device was patented in the EU; the identification number of the granted patent is EP3383269B1.
3.2. Demonstration of Quality Management and Regulatory Steps and Path
- Risks and hazards and their management need to be identified and the probability of occurrence needs to be reduced to as low as possible according to ISO 14971, Annex E of EN.
- Classification of the device: in our case, the device was classified according to Annex IX of 93/42/EEC (MDD):The device was categorized as a Class IIa device using rule 3 of the directive.Rule 3: “All non-invasive devices intended for modifying the biological or chemical composition of blood, other body liquids or other liquids intended for infusion into the body are in Class IIb, unless the treatment consists of filtration, centrifugation or exchanges of gas, heat, in which case they are in Class IIa”.
- The essential requirement checklist we used for our device according to MDD can be seen in Table 2.
- Biological evaluationBiological evaluation was accomplished with the use of Table 3. according to ISO 10993-1. The required tests, that are needed to assure that the device is safe are listed, are based on the nature of contact and the duration of the contact. In our case, the biological evaluation path is highlighted in green.
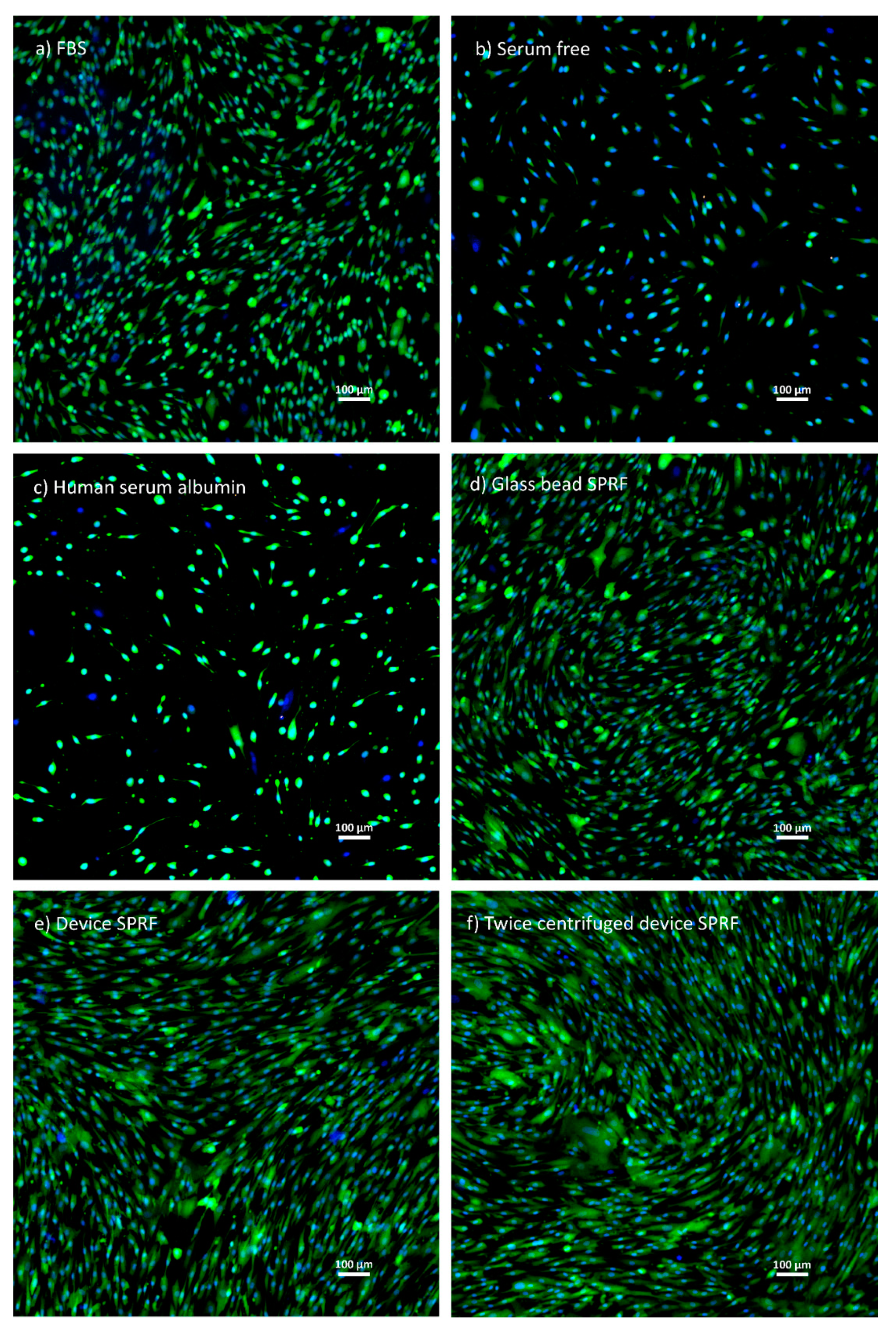
- Clinical evaluationIn our case, clinical evaluation was achieved by proving equivalence to a product that is already used. We used manually produced SPRF to compare to hypACT SPRF using mesenchymal stem cells with the exact methods and results which can be seen below.5.1 Maintaining Mesenchymal Stem Cell (MSC) cultureCell culture procedures were carried out in a laminar flow tissue culture hood. Bone marrow-derived mesenchymal stem cells (MSCs; ATCC, Manassas, VA, USA) were cultured in T-75 flasks in an incubator at 37 °C, 5% CO2 and 95% humidity. MSCs were maintained in stem cell medium: Dulbecco’s modified Eagle’s medium containing 4.5 g/L glucose and L-glutamine (Lonza, Basel, Switzerland) supplemented with 10% foetal bovine serum (FBS; EuroClone, Pero, Italy), 1% Penicillin-Streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and 0.75 ng/mL basic fibroblast growth factor (Sigma-Aldrich, St. Louis, MO, USA). The culture medium was refreshed three times a week.5.2 Preparation of different supplementation containing mediaAs positive control stem cell medium (FBS medium), as negative controls serum-free medium and human serum albumin (Behring, King of Prussia, Pennsylvania, USA)-containing medium (HSA medium) were used.Phlebotomy occurred under ethical approval (IRB approval number: 33106-1/2016/EKU, 12.07.2016) from healthy donors, men and women, aged 24–45 years.SPRF was isolated using the device as shown in Figure 2 (device SPRF). The device SPRF contains a small amount of red blood cells, which may be harmful to cell viability. They can be eliminated by another centrifugation at 1700 g for 8 min after isolation (twice centrifuged device SPRF).The reference SPRF was isolated manually. Fifty millilitres of blood was drawn using an ordinary syringe and the blood was poured into a centrifuge tube, containing 10 g glass beads as glass surface to promote blood clotting. It was immediately centrifuged at 1700 g for 8 min, while the fibrin matrix was formed. To squeeze out SPRF from the fibrin matrix, it was centrifuged again at 1700 g.Each cell culture media contained 10% supplementation, except for serum-free and HSA medium, where the amount of HSA was normalized to the protein content of SPRF.5.3 Comparing the effect of different serum containing media on MSC cell viabilityMSCs (4 passages) were seeded onto 24 well plates at a density of 1500 cells/well in 500 µL stem cell medium and the culture medium was changed on the first day to the different supplementation containing media. The medium was changed every two days.Cell viability was measured on the sixth day using the Cell Proliferation Kit II (XTT; Roche, Mannheim, Germany) according to the manufacturer’s instructions. Absorbance was measured by a microplate spectrophotometer (Biotek, Winooski, Vermont, USA) at 480 nm with a reference wavelength at 650 nm.The cells were visualized by Live/Dead staining. MSCs were washed with PBS and stained in PBS, containing 1 µM Calcein-AM (Invitrogen, Carlsbad, CA, USA), 4 µg/mL ethidium homodimer (Invitrogen, Carlsbad, CA, USA) and 20 µg/mL Hoechst (Invitrogen, Carlsbad, CA, USA) for 20 min, then washed again in PBS. Images were taken by an inverse fluorescent Nikon microscope.5.4 ResultsThe results of XTT measurement (Figure 3) do not show any significant differences between device SPRF, twice centrifuged device SPRF and glass bead SPRF. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was performed to compare the means of groups using Prism 7 software. The significance level was p < 0.05 and data are presented as mean ± SEM (n = 4).The results of microscopic imaging show (Figure 4) that MSCs cultured in the three kinds of SPRF media reached roughly similar density in the wells and they have the same MSC-like shape.5.5 EvaluationThe main conclusion of the clinical evaluation was that the blood separation product prepared by our device is at least as safe to use as a PRF isolation method that is already used in the practice, thus that is a way to claim clinical safety of the device. However, the optimal path to investigate clinical safety of medical devices needs to be evaluated on a case-by-case basis; nevertheless, the present path was applicable for the device. However, due to the requirements of the MDR, clinical equivalence may become more rare and actual clinical investigations will need to be done more often in the future.
4. Conclusions and Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port 2015, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R. Knee osteoarthritis: A review of management options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef] [PubMed]
- London, N.J.; Miller, L.E.; Block, J.E. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med. Hypotheses 2011, 76, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Kardos, D.; Marschall, B.; Simon, M.; Hornyak, I.; Hinsenkamp, A.; Kuten, O.; Gyevnar, Z.; Erdelyi, G.; Bardos, T.; Paukovits, T.M.; et al. Investigation of Cytokine Changes in Osteoarthritic Knee Joint Tissues in Response to Hyperacute Serum Treatment. Cells 2019, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.; Wragg, N.M.; Wilson, S.L. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019, 376, 143–152. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal Bovine Serum (FBS): Past-Present-Future. Altex 2018, 35, 99–118. [Google Scholar] [CrossRef]
- Nacher, M.; Estil Les, E.; Garcia, A.; Nadal, B.; Pairo, M.; Garcia, C.; Secanella, L.; Novials, A.; Montanya, E. Human Serum Versus Human Serum Albumin Supplementation in Human Islet Pretransplantation Culture: In Vitro and In Vivo Assessment. Cell Transpl. 2016, 25, 343–352. [Google Scholar] [CrossRef]
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Yong, T.K.; Chan, A.; et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef]
- Hosseini, L.; Shirazi, A.; Naderi, M.M.; Shams-Esfandabadi, N.; Borjian Boroujeni, S.; Sarvari, A.; Sadeghnia, S.; Behzadi, B.; Akhondi, M.M. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed. Online 2017, 35, 343–350. [Google Scholar] [CrossRef]
- Simon, M.; Major, B.; Vacz, G.; Kuten, O.; Hornyak, I.; Hinsenkamp, A.; Kardos, D.; Bago, M.; Cseh, D.; Sarkozi, A.; et al. The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int. 2018, 2018, 4854619. [Google Scholar] [CrossRef] [PubMed]
- Vacz, G.; Major, B.; Gaal, D.; Petrik, L.; Horvathy, D.B.; Han, W.; Holczer, T.; Simon, M.; Muir, J.M.; Hornyak, I.; et al. Hyperacute serum has markedly better regenerative efficacy than platelet-rich plasma in a human bone oxygen-glucose deprivation model. Regen. Med. 2018, 13, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Kuten, O.; Simon, M.; Hornyak, I.; De Luna-Preitschopf, A.; Nehrer, S.; Lacza, Z. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part A 2018, 24, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Alser, O.H.; Goutos, I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: A literature review. Scars Burn Heal 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Maleki, A.; Dolati, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed. Pharm. 2018, 104, 652–660. [Google Scholar] [CrossRef]
- Houdek, M.T.; Wyles, C.C.; Collins, M.S.; Howe, B.M.; Terzic, A.; Behfar, A.; Sierra, R.J. Stem Cells Combined With Platelet-rich Plasma Effectively Treat Corticosteroid-induced Osteonecrosis of the Hip: A Prospective Study. Clin. Orthop. Relat. Res. 2018, 476, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. PRP Therapy. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Lang, S.; Loibl, M.; Herrmann, M. Platelet-Rich Plasma in Tissue Engineering: Hype and Hope. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2018, 59, 265–275. [Google Scholar] [CrossRef]
- Kardos, D.; Hornyak, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Vardai, R.; Kallay-Menyhard, A.; Pinke, B.; Meszaros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. [Google Scholar] [CrossRef]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef]
- McDermott, I.D. Patellar chondral defect treatment with a cell-free polyglycolic acid-hyaluronan-based implant and platelet-rich fibrin glue after previously failed microfracture. SAGE Open Med. Case Rep. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Machado, V.; Botelho, J.; Proenca, L.; Mendes, J.J.; Alves, R. Effect of A-PRF Application on Palatal Wound Healing after Free Gingival Graft Harvesting: A Prospective Randomized Study. Eur. J. Dent. 2020, 14, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 011. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, J.; Wadhwa, P.; Jiang, H.; Jang, H.-S.; Lee, E. Bone Regeneration in Peri-Implant Defect Using Autogenous Tooth Biomaterial Enriched with Platelet-Rich Fibrin in Animal Model. Appl. Sci. 2020, 10, 1939. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: Intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Meza, G.; Urrejola, D.; Saint Jean, N.; Inostroza, C.; Lopez, V.; Khoury, M.; Brizuela, C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J. Endod. 2019, 45, 144–149. [Google Scholar] [CrossRef]
- Chien, C.S.; Ho, H.O.; Liang, Y.C.; Ko, P.H.; Sheu, M.T.; Chen, C.H. Incorporation of exudates of human platelet-rich fibrin gel in biodegradable fibrin scaffolds for tissue engineering of cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 948–955. [Google Scholar] [CrossRef]
- Koh, Y.-G.; Lee, J.-A.; Lee, H.-Y.; Kim, H.-J.; Kang, K.-T. Biomechanical Evaluation of the Effect of Mesenchymal Stem Cells on Cartilage Regeneration in Knee Joint Osteoarthritis. Appl. Sci. 2019, 9, 1868. [Google Scholar] [CrossRef]
- Witek, L.; Tian, H.; Tovar, N.; Torroni, A.; Neiva, R.; Gil, L.F.; Coelho, P.G. The effect of platelet-rich fibrin exudate addition to porous poly(lactic-co-glycolic acid) scaffold in bone healing: An in vivo study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Lollobrigida, M.; Maritato, M.; Bozzuto, G.; Formisano, G.; Molinari, A.; De Biase, A. Biomimetic Implant Surface Functionalization with Liquid L-PRF Products: In Vitro Study. BioMed. Res. Int. 2018, 2018, 9031435. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.; Zhang, Z.; Yan, Z.; Lv, H.; Zhang, Y.; Wu, B. Plateletrich fibrin exudate promotes the proliferation and osteogenic differentiation of human periodontal ligament cells in vitro. Mol. Med. Rep. 2018, 18, 4477–4485. [Google Scholar] [CrossRef] [PubMed]
- Kardos, D.; Simon, M.; Vacz, G.; Hinsenkamp, A.; Holczer, T.; Cseh, D.; Sarkozi, A.; Szenthe, K.; Banati, F.; Szathmary, S.; et al. The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method. Int. J. Mol. Sci. 2019, 20, 721. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M. How to optimize the preparation of leukocyte- and platelet-rich fibrin (L-PRF, Choukroun’s technique) clots and membranes: Introducing the PRF Box. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 275–278. [Google Scholar] [CrossRef]
- Su, C.Y.; Kuo, Y.P.; Tseng, Y.H.; Su, C.H.; Burnouf, T. In vitro release of growth factors from platelet-rich fibrin (PRF): A proposal to optimize the clinical applications of PRF. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 56–61. [Google Scholar] [CrossRef]
- Levy, A.; Coulthard, M.; Harvey, M.; Talbot, S.; Rich, G. Tube Anchor: Development of a small medical device from concept to market. BMJ Innov. 2015, 1, 151–156. [Google Scholar] [CrossRef]
- Wright, D.J.; Potter, J.F.; Clark, A.; Blyth, A.; Maskrey, V.; Mencarelli, G.; Wicks, S.O.; Craig, D.Q.M. Administration of aspirin tablets using a novel gel-based swallowing aid: An open-label randomised controlled cross-over trial. BMJ Innov. 2019, 5, 113–119. [Google Scholar] [CrossRef]
- Al-Halhouli, A.A.; Al-Ghussain, L.; Bouri, S.; Habash, F.; Liu, H.; Zheng, D. Clinical Evaluation of Stretchable and Wearable Inkjet-Printed Strain Gauge Sensor for Respiratory Rate Monitoring at Different Body Postures. Appl. Sci. 2020, 10, 480. [Google Scholar] [CrossRef]
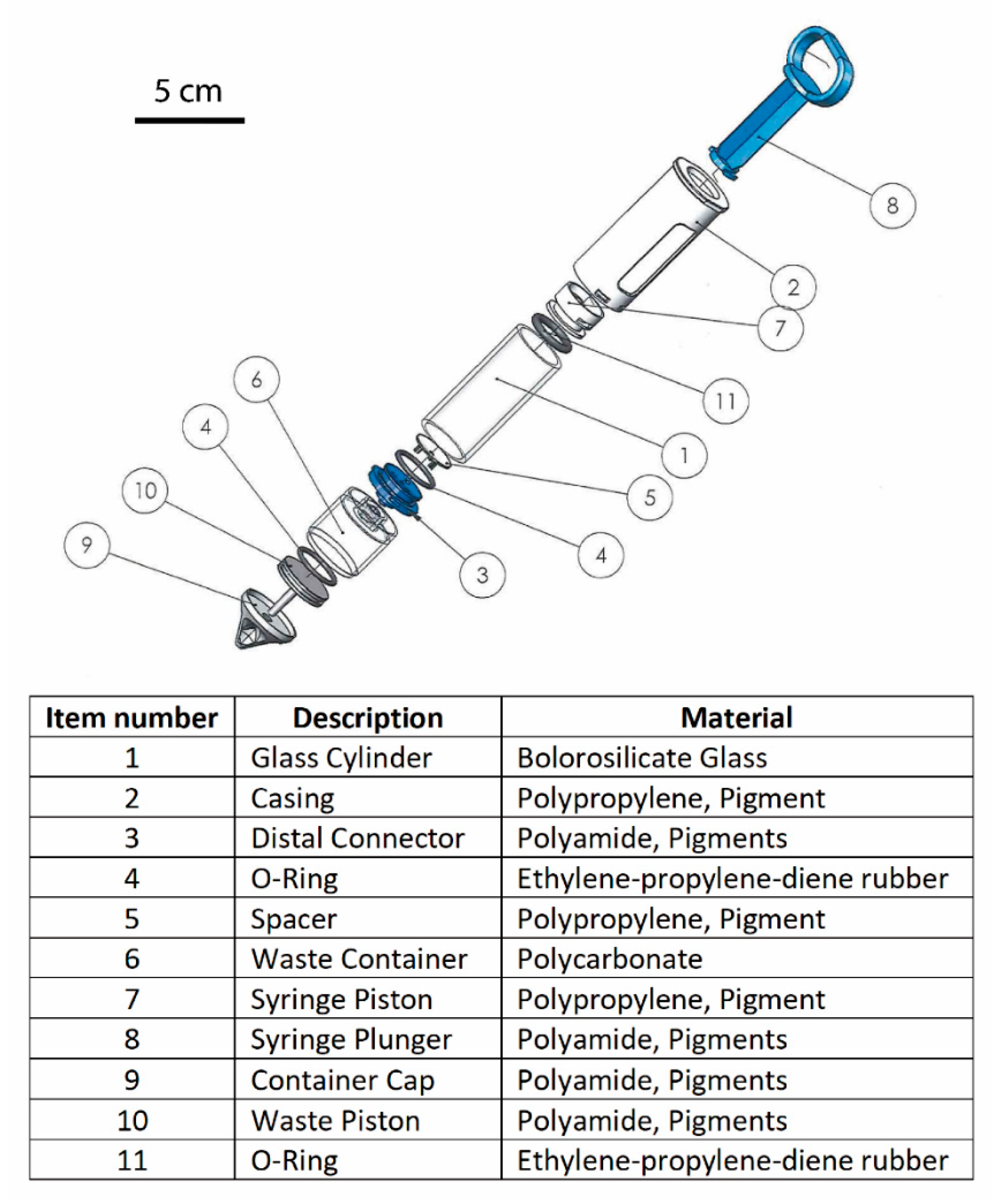
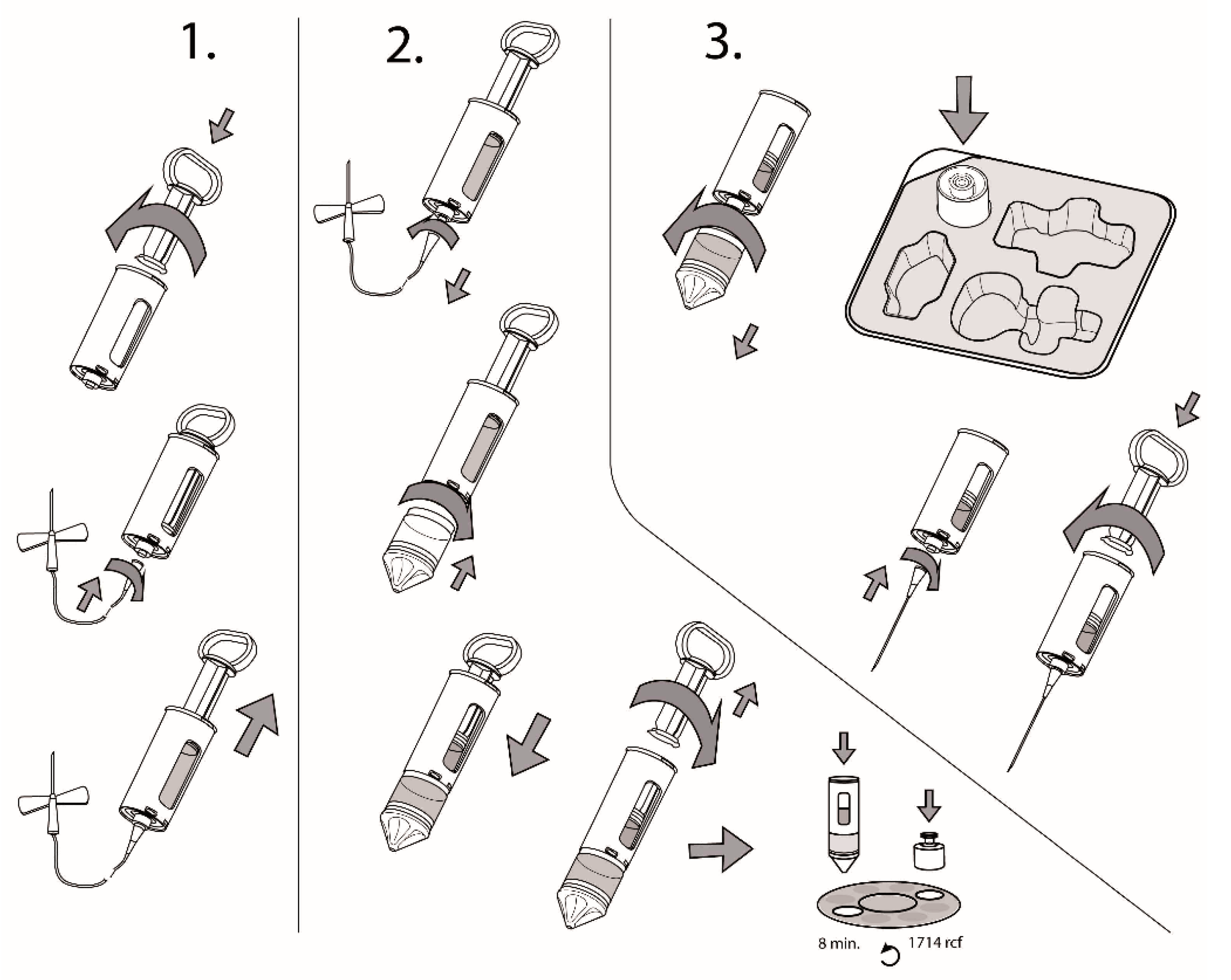

| Technical Requirements | Characteristics and Numerical Specifications (Design Input) |
|---|---|
| Use | Single use |
| Sterilisation | Ethylene oxide |
| Materials | The device must conform to ISO 10993-1 as per the essential requirement of 93/42/EEC. |
| Volume Ratio | 12 mL whole blood can be drawn The ratio of the top and bottom parts must be 40% top (serum fraction) and 60% bottom (waste). Note that 4.8 mL serum will be in the glass chamber after the centrifuging procedure |
| Mass of device | Total weight of the system with all parts, except for the plunger part, plus blood included will be 54 g |
| Dimensions of the Device | The dimensions of the device, including max diameter, length and conical bottom angle are equivalent to a 50 mL conical centrifuge tube |
| Packaging | Device packaging is to be suitable for ethylene oxide sterilization |
| Users | Nurse, Doctor |
| Medicinal Substance | None |
| External Interfaces | Swing-out rotor centrifuge for 50 mL conical centrifuge vials |
| Standard | Regarding Subject |
|---|---|
| ISO 13485:2016 | Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes |
| ISO 14971:2007 | Medical devices—Application of risk management to medical devices |
| ISO 11607-1:2019 | Packaging for terminally sterilised medical devices—Part 1: Requirements for materials, sterile barrier system and packaging systems |
| ISO 11607-2:2019 | Packaging for terminally sterilised medical devices—Part 2: Validation requirements for forming, sealing and assembly processes |
| EN 868-5:2018 | Packaging for terminally sterilised medical devices—Part 5: Sealable pouches and reels of porous material, and plastic film construction—Requirements and test methods |
| EN 556-1:2002 | Sterilisation of medical devices—Requirements for medical devices to be designated STERILE- Part 1: Requirements for terminally sterilised medical devices |
| EN ISO 80369-7:2016 | Conical fittings with a 6% (Luer) taper for syringes, needles and certain other medical equipment—Part 1: General requirements |
| EN ISO 80369-7:2016 | Conical fittings with a 6% (Luer) taper for syringes, needles and certain other medical equipment—Lock fittings |
| ISO 10993-1:2018 | Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process |
| ISO 10993-4:2017 | Biological evaluation of medical devices—Part 4: Selection of tests for interactions with blood |
| ISO 10993-5:2009 | Tests for in vitro cytotoxicity |
| ISO 10993-9:2009 | Framework for identification and quantification of potential degradation products |
| ISO 10993-10:2010 | Tests for irritation and skin sensitisation |
| ISO 10993-11:2017 | Tests for system toxicity |
| ISO 10993-13:2010 | Identification and quantification of degradation products for polymeric medical devices |
| ISO 1135-4:2015 | Transfusion equipment for medical use—Part 4: Transfusion Sets for Single Use |
| EN 1041:2008+A1:2013 | Information supplied by the manufacturer of Medical Devices |
| ISO 15223-1:2016 | Medical devices—Symbols to be used with medical device labels, labelling and information to be supplied—Part 1: General requirements |
| EN 1422:2014 | Sterilisers for medical purposes—Ethylene oxide sterilisers — Requirements and test methods |
| ISO 7886-1:2017 | Sterile hypodermic syringes for single use—Part 1: Syringes for manual use |
| ISO 7886-4:2018 | Sterile hypodermic syringes for single use—Part 4: Syringes with re-use prevention feature |
| ISO 11135:2014 | Sterilisation of health care products—Ethylene oxide—Part 1: Requirements for development, validation and routine control of a sterilisation process for medical devices |
| ISO 11138-2:2017 | Sterilisation of health care products—Biological indicators—Part 2: Biological indicators for ethylene oxide sterilisation processes |
| ISO 11737-1:2018 | Sterilisation of medical devices—Microbiological methods—Part 1: Determination of a population of microorganisms on products |
| ISO 11737-2:2009 | Sterilisation of medical devices—Microbiological methods—Part 2: Tests for sterility performed in the definition, validation and maintenance of a sterilisation process |
| ISO 14937:2009 | Sterilisation of health care products—General requirements for characterisation of a sterilisation agent and the development, validation and routine control of a sterilisation process for medical devices |
| ISO 2233:2000 | Packaging—Complete, filled transport packages and unit loads—Conditioning for testing |
| ISO 14155:2011 | Clinical investigation of medical devices for human subjects—Good clinical practice |
| Medical Device Categorization by | Endpoints of Biological Evaluation | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nature of Body Contact | Contact Duration | Physical and/or Chemical Information | Cytotoxicity | Sensitization | Irritation or Intracutaneous Reactivity | Material Mediated Pyrogenicity | Acute Systemic Toxicity | Subacute Toxicity | Subchronic Toxicity | Chronic Toxicity | Implantation Effects | Hemocompatibility | Genotoxicity | Carcinogenicity | Reproductive/Develop Mental Toxicity | Degradation | |
| Category | A-Limited(≤24 h) B-Prolonged (>24 h to 30 d) C-Permanent (>30 d) | ||||||||||||||||
| Surface medical device | Intact skin | A | X | X | X | X | |||||||||||
| B | X | X | X | X | |||||||||||||
| C | X | X | X | X | |||||||||||||
| Mucosal membrane | A | X | X | X | X | ||||||||||||
| B | X | X | X | X | X | X | X | ||||||||||
| C | X | X | X | X | X | X | X | X | X | X | |||||||
| Breached or compromised surfaces | A | X | X | X | X | X | X | ||||||||||
| B | X | X | X | X | X | X | X | X | |||||||||
| C | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Externally communicating medical device | Blood path, indirect | A | X | X | X | X | X | X | X | ||||||||
| B | X | X | X | X | X | X | X | X | |||||||||
| C | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Tissue/bone/dentin | A | X | X | X | X | X | X | ||||||||||
| B | X | X | X | X | X | X | X | X | X | ||||||||
| C | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Circulating blood | A | X | X | X | X | X | X | X | X | ||||||||
| B | X | X | X | X | X | X | X | X | X | X | |||||||
| C | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Implant medical device | Tissue/bone | A | X | X | X | X | X | X | |||||||||
| B | X | X | X | X | X | X | X | X | X | ||||||||
| C | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Blood | A | X | X | X | X | X | X | X | X | X | |||||||
| B | X | X | X | X | X | X | X | X | X | X | |||||||
| C | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinsenkamp, A.; Kardos, D.; Lacza, Z.; Hornyák, I. A Practical Guide to Class IIa Medical Device Development. Appl. Sci. 2020, 10, 3638. https://doi.org/10.3390/app10103638
Hinsenkamp A, Kardos D, Lacza Z, Hornyák I. A Practical Guide to Class IIa Medical Device Development. Applied Sciences. 2020; 10(10):3638. https://doi.org/10.3390/app10103638
Chicago/Turabian StyleHinsenkamp, Adél, Dorottya Kardos, Zsombor Lacza, and István Hornyák. 2020. "A Practical Guide to Class IIa Medical Device Development" Applied Sciences 10, no. 10: 3638. https://doi.org/10.3390/app10103638
APA StyleHinsenkamp, A., Kardos, D., Lacza, Z., & Hornyák, I. (2020). A Practical Guide to Class IIa Medical Device Development. Applied Sciences, 10(10), 3638. https://doi.org/10.3390/app10103638







