1. Introduction
Chemical admixtures are the essential materials and core technology for manufacturing modern concrete and key elements in the development of concrete in high-tech fields. Due to their characteristics of low dosage, high water reduction, and lowering slump loss (or improving slump retention), admixture products are a hot topic of research. Water-reducing agents are being developed to offer enhanced performance in terms of a high rate of water reduction, high slump retention ability, high early strength ability, and high durability of concrete. In engineering practices, if the function of the polycarboxylic acid water-reducing agent is only limited to the rate of water reduction and the strength of the concrete, the adaptability and rheological properties of the polycarboxylic acid water-reducing agent and the material in concrete cannot be adjusted, which likely leads to the poor physical workability of the concrete mix and eventually to problems in the stability and durability of the concrete structure, thereby not meeting the service life of the project. Therefore, it is of great significance to study functional superplasticizers based on polycarboxylic acid.
Superplasticizers are widely used to produce flowable, strong, and durable Portland cement concretes and mortars. The hydration behaviors of Portland cement in the presence of superplasticizers have been investigated by a number of researchers [
1,
2,
3]. Kreppelt et al. [
2] studied the effects of different types of polycarboxylic acid superplasticizer on the early hydration of cement using scanning electron microscopy (SEM) analysis and found that the incorporation of polycarboxylic acid superplasticizers inhibited the formation of alumina, ferric oxide, tri-sulfate (AFt), as a hydration product. Roncero et al. [
4] employed 29Si MAS NMR and X-ray diffraction (XRD) techniques and discovered that when the cement and water were mixed for 15 min, there was no characteristic peak of AFt in the XRD spectrum of the blank control slurry, but it obviously appeared when polycarboxylic acid water-reducing agent was added to the mixture. The characteristic peak of AFt indicates that the incorporation of polycarboxylic acid water-reducing agent changes the growth rate of AFt. Through differential thermal research, Puertas et al. [
5] realized that polycarboxylic acid water reducer decreased the amount of Ca(OH)
2 produced in the early hydration products. It was thought that the water-reducing agent suppressed the hydration of the silicate mineral phase and therefore delayed the formation of Ca(OH)
2; thus, calcium silicate hydrate (CSH) had no significant effect on the formation of AFt. Ferrari et al. [
6] used atomic force microscopy (AFM) to observe the morphology of cement hydration products and obtained the same results as those of Puertas; they believed that the degree of influence depends on the amount of water-reducing agent and the chemical composition of the solution. Water-reducing agents have obvious effects on micro-morphology in cement pastes [
7,
8] as confirmed by the XRD peaks at different ages [
9] and SEM analysis used to study the pore structure of cement pastes [
10,
11,
12,
13,
14,
15].
Materials based on hardened cement are generally composed of complex structural systems of hydration products, unhydrated cement mineral phases, and multiscale pores and pore solutions. Multiscale pores affect the mechanical properties of cement-based materials and are of considerable importance for the characterization of the multiscale and complex pore structures of hardened cement pastes [
16]. The pore structure reflects the microstructure of the material, and the microstructure-performance relationship is the core of modern material science [
17]. Concrete has a heterogeneous and rather complex structure; therefore, its macroscopic performance is also extremely complicated. Thus, studying and mastering the relationship between concrete pore distribution and its macroscopic performance can reflect the effect of water-reducing agents on the structure and performance of concrete. Moreover, it is known that the destruction of the pore structure is the main reason for the failure of concrete.
Therefore, this work focuses on analyzing the influence of different types of polycarboxylic acid superplasticizer on concrete at certain water/cement ratios while using the same admixture and curing conditions. The impact of water-reducing agents on compressive strength, pore structure, and cement hydration process is studied by comparing the differences in pore structure parameters and the changes in XRD peaks at different ages. SEM analysis is also employed to study the pore structure, which contributes to the macroscopic mechanical properties and illustrates the effect of different functional polycarboxylic acid superplasticizers. The effect of high-performance water-reducing agents based on polycarboxylic acid on hydration process and hardening performance of concrete is also investigated.
2. Experimental
2.1. Materials
Various grades of alkylene alkenyl polyoxyethylene ether (OXAB-702, MW = 3000; OXAB-608, MW = 2400; OXAB-801, MW = 4000) were kindly supplied by Liaoning Oxiranchem, Inc. (Liaoning, China). Sodium methacrylate was purchased from Taicang Xinmao Polyester Chemical Co., Ltd. (Taicang, China), and hydrogen peroxide (analytical pure, 30%) and sodium hydroxide were provided by Tianjin Bodi Chemistry (Tianjin, China). Acrylic acid (AA) was purchased from Wanhua Chemical (Yantai) Petrochemical Co., Ltd. (Yantai, China) and reducing agents (ascorbic acid) were provided by CSPC Holding Group Co., Ltd. (Shijiazhuang, China). Hydroxyethyl acrylate (HEA) was supplied by Beijing Oriental Petrochemical Co., Ltd. (Beijing, China) and mercaptopropionic acid (S) was purchased from Hebei Tongli Chemical Co., Ltd. (Hebei, China). Early-strength polycarboxylic acid superplasticizer (F, 20%) was purchased from Sika (Zurich, Switzerland), and naphthalene-based water-reducing agent (NF) was purchased from Shandong Wanshan Chemical Co., Ltd. (Shandong, China). Ordinary Portland cement PO 42.5 R was purchased from Dalian Onoda Cement Co., Ltd. (Dalian, China) and standard sand was supplied by Xiamen Esi Standard Sand Co., Ltd. (Xiamen, China). River sand with a fineness modulus of 2.7, a mud content of 1.5%, firmness of 2%, and water absorption of 0.5% was provided by Dalian Shiyutong Concrete Co., Ltd. (Dalian, China). Stone with an apparent density of 2680 kg/m3, 5–25 mm continuous grading, a mud content of 0.3%, and a water content of 0.1% was supplied by Dalian Limestone Mine (Dalian, China) and fly ash (FA, second level) was purchased from Dalian Huaneng Power Plant (Dalian, China).
2.2. Preparation of Samples
2.2.1. Synthesis of Water-Reducing Polycarboxylic Acid Superplasticizer J
To a 1 L four-neck flask, 435.6 g of deionized water, 360 g of monomer (OXAB-702), and 5.69 g of sodium methacrylic acid were added, and the flask was heated to 60 °C. Then, a mixture of 3.17 g of hydrogen peroxide and 50 g of deionized water was added to the above four-neck flask, and material A (a mix of 30.24 g of acrylic acid and 80 g of deionized water) and material B (a mix of 1.08 g of ascorbic acid and 11 g of deionized water) were reacted simultaneously. The reaction of materials A and B was completed in 3 and 3.5 h respectively while continuously stirring for 1 h. At the end of the reaction, the reaction polymer was cooled down to below 30 °C, and a mixture of 16.8 g of sodium hydroxide and 39.2 g of deionized water was added to the reaction while stirring. The pH of the reaction was adjusted at 7 to obtain superplasticizer J as a polycarboxylic acid high-performance water-reducing agent.
2.2.2. Synthesis of Sustained-Release Polycarboxylic Acid Superplasticizer H
We added 461.4 g of deionized water and 360 g of monomer (OXAB-608) to a 1 L four-neck flask and raised the temperature to 40 °C. Then, a mixture of 3.31 g of hydrogen peroxide and 50 g of deionized water was then added to the above four-neck flask, and material A (a mix of 25.92 g of acrylic acid, 27.87 g of hydroxyethyl acrylate, and 84 g of deionized water) and material B (a mix of 1.66 g of ascorbic acid, 0.80 g of mercaptopropionic acid, and 10 g of deionized water) were reacted simultaneously. The reaction of materials A and B was completed in 3 and 3.5 h respectively while continuously stirring for 1 h. At the end of the reaction, the obtained polymer was cooled down to below 30 °C, and a mixture of 14.4 g of sodium hydroxide and 33.6 g of deionized water was added while stirring. The pH of the reaction was adjusted at 7 to obtain superplasticizer H as a sustained-release polycarboxylic acid water-reducing agent.
2.2.3. Synthesis of Early-Strength Polycarboxylic Acid Superplasticizer Z
To a 1 L four-neck flask, 457.46 g of deionized water and 360 g of monomer (OXAB-801) were added and heated to 40 °C. Then, a mixture of 3.22 g of hydrogen peroxide and 50 g of deionized water was added to the above four-neck flask, and material A (a mix of 25.92 g of acrylic acid, 12.79 g of acrylamide, and 71.29 g of deionized water) and material B (a mix of 1.61 g of ascorbic acid, 0.48 g of mercaptopropionic acid, and 10.5 g of deionized water) were titrated simultaneously. The titration of materials A and B was completed in 3 and 3.5 h respectively while continuously stirring for 1 h. At the end of the reaction, the reaction polymer was cooled down to below 30 °C, and a mixture of 14.4 g of sodium hydroxide and 33.6 g of deionized water was added to the action while stirring. The pH of the reaction was adjusted at 7 to obtain superplasticizer Z as an early-strength polycarboxylic acid water-reducing agent.
2.2.4. Properties of Functional Polycarboxylic Acid Superplasticizers
Table 1 tabulates the physical properties of the synthesized functional polycarboxylic acid high-performance water-reducing agents.
2.2.5. Concrete Composition
The composition of the various concrete samples prepared is summarized in
Table 2.
2.3. Characterization
2.3.1. Compressive Strength Analysis
Procedure for Compressive Strength Test of Cement Slurry
Small cubic samples (20 mm × 20 mm × 20 mm) were prepared to minimize the cement pastes used in the analysis of the compressive strength tests. A group of test pieces consists of six test pieces. The clinker samples with or without gypsum addition were incorporated using deionized water and a water-to-cement ratio of 0.4. In the molding process, only simple ramming was performed, and the mold did not use the vibration table because the water-to-cement ratio is a typical ratio used in general projects and sufficient to form workable pastes. When the pastes were poured into molds, every mold surface was covered with plastic film, and they were then placed in a standard curing box. After one day of curing, the mold was removed, and the cubic samples were put into deionized water at a constant temperature of 20 °C and cured for a certain age of 1, 7, and 28 days. The test specimens were taken out at the proper age and dried, and the sides of the samples were used as the upper and lower compression surface when molding to conduct the compressive strength test. The loading speed during the compressive strength test was 1.0–1.5 kN/s; when the specimen is near failure and starts to deform, the adjusting throttle of the testing machine should be stopped until the specimen is destroyed, and the ultimate damage load is recorded. The compressive strength is the average of the values obtained for a group of test pieces. When the maximum or minimum value of the compressive strength in a group of samples differs from the average value by more than 20%, the average value of the strength of the four test pieces in the middle should be considered. After testing the compressive strength of the samples, the center of the block was extracted as the sample pastes for characterization.
Concrete Compressive Strength Test Process
A concrete test piece with the dimensions 150 mm × 150 mm × 150 mm was formed. A group of test pieces consists of three test pieces, which were cured to a specified age under standard curing conditions. The test piece was taken out and dried during the test, and the sides were used as the upper and lower pressure surface when forming. The test piece was placed on the ball seat that was placed in the center of the press; the load was applied at a speed of 0.5 to 0.8 MPa/s. When the test specimen approaches deformation and begins to deform, the adjusting throttle of the test machine is stopped until the test specimen is destroyed, and the ultimate damage load is recorded.
2.3.2. Procedure for Measuring Fluidity of Cement Slurry
The glass plate was placed in a horizontal position, and the glass plate and the truncated cone mold were wiped evenly with a damp cloth to make the surface wet without water stains. The truncated cone mold was then placed in the center of the glass plate and covered with a damp cloth for use. The pure slurry was quickly mixed into the truncated cone mold and scraped with a spatula; the truncated cone mold was put in the vertical direction. We then let the cement paste flow on the glass plate for 30 s and used a ruler to measure the maximum diameter of the flowing part in two directions perpendicular to each other. The average value was regarded as the cement paste fluidity.
2.3.3. XRD Analysis
The XRD analyses were conducted on different clinker and hydrated samples. To this end, the cured sample pastes were stored in water at a constant temperature of 20 °C until it was time to stop the hydration, and then the crushed hydrated pastes were immersed in ethyl alcohol. Afterward, the immersed pastes were stored in a desiccator at room temperature for 24 h over a silica gel to remove the solvent and/or water and prevent moisturization. Next, the cured samples were dried in a vacuum drying oven at 35 °C for 24 h. The clinker samples and the cured samples were all ground and sieved on a 45-lm standard sieve. A Bruker D8 Advance Davinci design X-ray diffractometer was used with CuKa1,2 radiation (k1 = 0.15406 nm, k2 = 0.15444 nm) at 40 kV and 40 mA for conducting the XRD analyses on the powder. The overall measurement time for every sample was about 31 min per individual pattern to obtain a favorable signal-to-noise ratio in the angular range of 5–80° (2θ) with a step size of 0.02. Finally, the XRD patterns were analyzed utilizing an evaluation (EVA) software package to determine the crystalline phases of the samples.
2.3.4. FTIR Spectroscopy
FTIR spectroscopy was conducted using a Thermo Fisher Nicolet iS5, which included an ATR iD5 attenuated total reflection attachment to analyze the vibration and deflection of the molecular bonds of the samples. The analysis was performed in a wavenumber range of 4000–400 cm–1 and at a spectral resolution lower than 4 cm–1 using a sample quantity of 5 ± 1 mg.
2.3.5. Mercury Intrusion Porosimetry
Mercury intrusion porosimetry (MIP) was conducted using an AUTOPORE IV 9500 (a maximum pressure of 33,000 psi) series made by Micromeritics Instrument Corp. (Atlanta, GA, USA) to characterize the pore size distribution of the samples. To this end, some pieces of block samples of about 1.5–2.5 g were used for the analysis, and the samples were dried in a vacuum drying oven at 35 °C for 24 h to remove the water from the porous structure of the samples.
2.3.6. SEM
SEM images were employed to analyze the microstructure of the samples, to find cracks in the interfacial transition zone (ITZ), and to study the porosity of different samples.
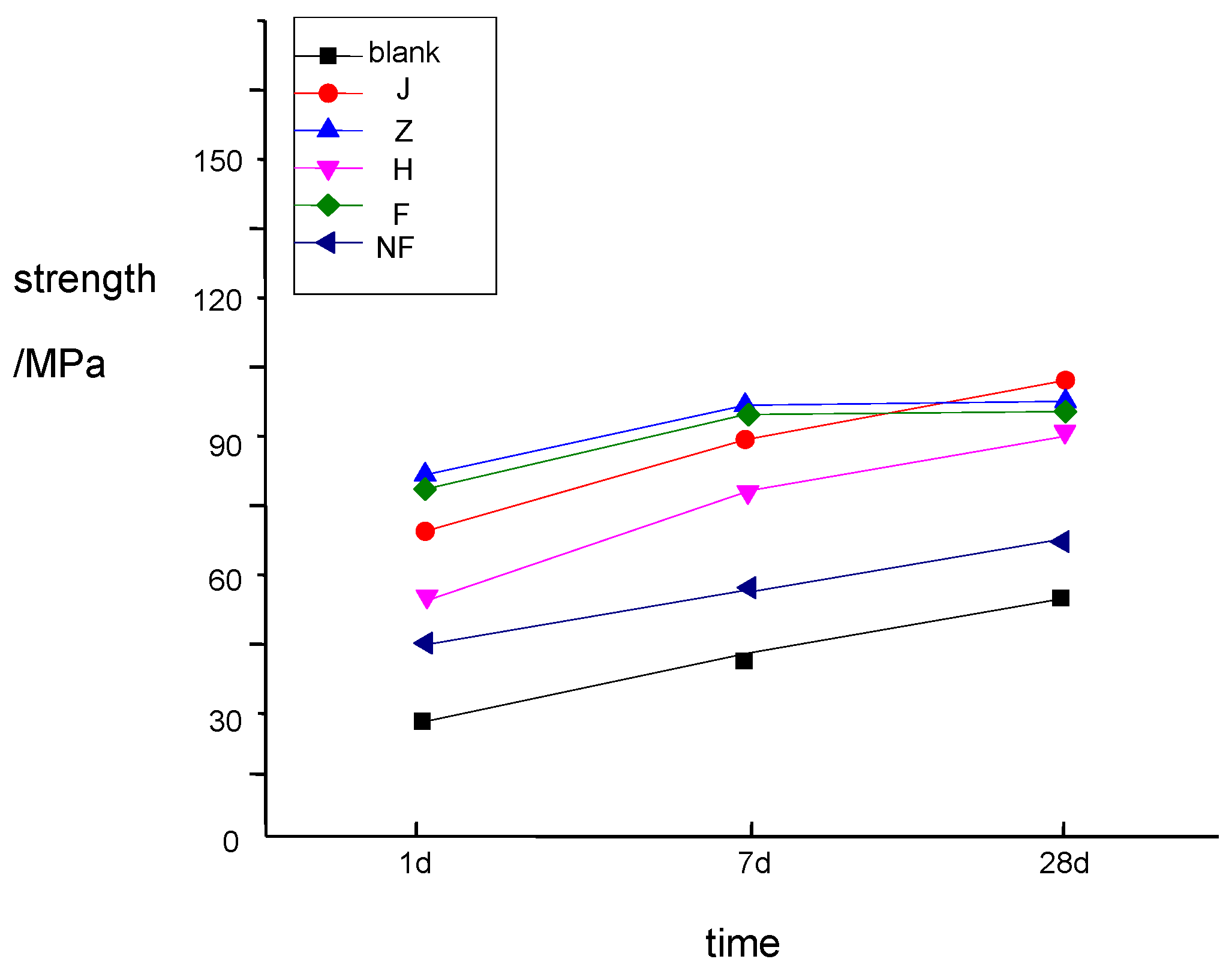
4. Conclusions
Functional polycarboxylic acid superplasticizers, named Z, J, and H, do not participate in the cement hydration reaction but rather slow down the rate of the initial reaction of the cement. Superplasticizer Z has a strong steric hindrance effect, due to the ultra-long side chains in its structure, which can increase the contact area between cement particles and water, accelerate cement hydration, promote the generation of CH and AFt, and be closely combined with CSH. It can be seen from the SEM results that the Z water-reducing agent can form a dense structure of cement stone; thus, it presents higher early-strength characteristics to cement. On the other hand, superplasticizer H with shorter side chains in its structure and its ester functional groups can slow down the cement hydration rate, thereby helping achieve long-term slump retention performance. However, it can be seen from the SEM results that compared with the Z water reducer and the J water reducer, the H water reducer makes the overall cement stone structure less dense. Superplasticizer J can ensure the homogeneity and high fluidity of concrete while satisfying a high rate of water reduction and improving the pore structure and strength of concrete. Compared with water-reducing agent NF, functional polycarboxylic acid superplasticizers (i.e., Z, J, and H) mainly rely on functional groups such as hydroxyl, carboxyl, sulfonic acid, and polyoxyethylene, in their molecular structure to more easily inhibit the precipitation of the initial phase of the minerals.