An Automated Data Acquisition System for Pinch Grip Assessment Based on Fugl Meyer Protocol: A Feasibility Study
Abstract
:Featured Application
Abstract
1. Introduction
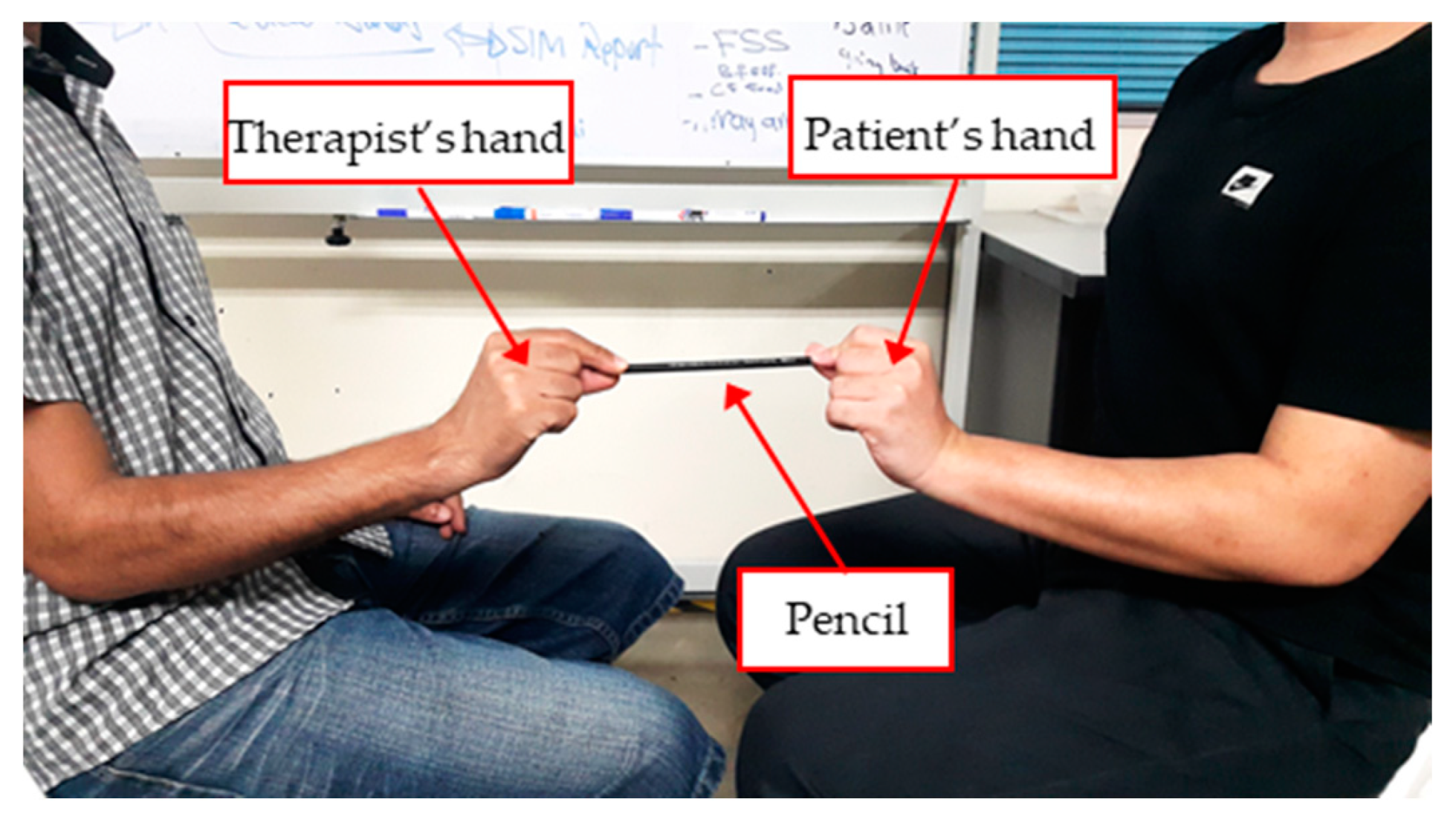
- The orientation of the pincer object is not standardized. Some therapists pull the object horizontally, while others pull it vertically.
- The posture of the patients’ shoulder, elbow, forearm, and hand may differ between each clinic resulting in different pinch force exerted at different postures.
- The amount of pulling force actuated by the therapist is subjective [16]. This opens the possibility for low intra-rater and inter-rater reliability of pinch evaluation.
2. Materials and Methods
2.1. Pinch Data Acquisition System
- Displacement sensor: LVDT sensor (1) with Low Pass Filter (9).
- Linear actuator system: including linear electric actuator (2) and servo motor driver (8).
- Customized Pinch force load cell: pincer object (4), Wheatstone bridge (5), and an amplifier (6).
- Pulling force load cell: load cell (3) and an amplifier (11).
- Data acquisition card: Arduino® Due board (Arduino LLC, Torino, Italy) (11) and Arduino® IDE 1.8.5 software (arduino.cc) [50].
- DC power supply (7).
2.2. Volunteers Recruitment and Experimental Protocol
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Appendix A



References
- Miyasaka, H.; Takeda, K.; Ohnishi, H.; Orand, A.; Sonoda, S. Effect of Sensory Loss on Improvements of Upper-Limb Paralysis Through Robot-Assisted Training: A Preliminary Case Series Study. Appl. Sci. 2019, 9, 3925. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Nataraj, R.; Marquardt, T.L.; Li, Z.-M. Directional coordination of thumb and finger forces during precision pinch. PLoS ONE 2013, 8, e79400. [Google Scholar] [CrossRef] [PubMed]
- Pessina, M.A.; Bowley, B.G.; Rosene, D.L.; Moore, T.L. A method for assessing recovery of fine motor function of the hand in a rhesus monkey model of cortical injury: An adaptation of the Fugl-Meyer Scale and Eshkol-Wachman Movement Notation. Somatosens. Mot. Res. 2019, 36, 69–77. [Google Scholar] [CrossRef]
- El-Katab, S.; Omichi, Y.; Srivareerat, M.; Davenport, A. Pinch grip strength as an alternative assessment to hand grip strength for assessing muscle strength in patients with chronic kidney disease treated by haemodialysis: A prospective audit. J. Hum. Nutr. Diet. 2016, 29, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A.; Resteghini, C.; Feys, P.; Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Valero-Cuevas, F.J.; Smaby, N.; Venkadesan, M.; Peterson, M.; Wright, T. The strength-dexterity test as a measure of dynamic pinch performance. J. Biomech. 2003, 36, 265–270. [Google Scholar] [CrossRef]
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201. [Google Scholar]
- Yozbatiran, N.; Der-Yeghiaian, L.; Cramer, S.C. A standardized approach to performing the action research arm test. Neurorehabilit. Neural Repair 2008, 22, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Johansson, G.M.; Häger, C.K. A modified standardized nine hole peg test for valid and reliable kinematic assessment of dexterity post-stroke. J. Neuroeng. Rehabil. 2019, 16, 8. [Google Scholar] [CrossRef]
- Sears, E.D.; Chung, K.C. Validity and responsiveness of the jebsen–taylor hand function test. J. Hand Surg. 2010, 35, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, P. Upper limb motor impairment after stroke. Phys. Med. Rehabil. Clin. 2015, 26, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Deakin, A.; Hill, H.; Pomeroy, V.M. Rough guide to the Fugl-Meyer Assessment: Upper limb section. Physiotherapy 2003, 89, 751–763. [Google Scholar] [CrossRef]
- Paternostro-Sluga, T.; Grim-Stieger, M.; Posch, M.; Schuhfried, O.; Vacariu, G.; Mittermaier, C.; Bittner, C.; Fialka-Moser, V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J. Rehabil. Med. 2008, 40, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Van Beers, R.J.; Haggard, P.; Wolpert, D.M. The role of execution noise in movement variability. J. Neurophysiol. 2004, 91, 1050–1063. [Google Scholar] [CrossRef]
- Tanaka, K.; Yano, H. Errors of visual judgement in precision measurements. Ergonomics 1984, 27, 767–780. [Google Scholar] [CrossRef]
- Simbaña, E.D.O.; Baeza, P.S.-H.; Jardón, A.; Balaguer, C. Review of Automated Systems for Upper Limbs Functional Assessment in Neurorehabilitation. IEEE Access 2019, 7, 32352–32367. [Google Scholar] [CrossRef]
- de los Reyes-Guzmán, A.; Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; Monasterio-Huelin, F.; Torricelli, D.; Gil-Agudo, A. Quantitative assessment based on kinematic measures of functional impairments during upper extremity movements: A review. Clin. Biomech. 2014, 29, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Xiong, D.; Guo, L.; Wang, J. A remote quantitative Fugl-Meyer assessment framework for stroke patients based on wearable sensor networks. Comput. Methods Programs Biomed. 2016, 128, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; Waller, S.M. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Wang, J.; Guo, L.; Zhang, Q.; Li, P.; Li, Y.; Yu, X.; Huang, Y.; Wu, Z. Transfer learning based quantitative assessment model of upper limb movement ability for stroke survivors. In Proceedings of the 2017 2nd International Conference on Information Technology (INCIT), Nakhon Pathom, Thailand, 2–3 November 2017; pp. 1–4. [Google Scholar]
- Page, S.J.; Levine, P.; Hade, E. Psychometric properties and administration of the wrist/hand subscales of the Fugl-Meyer Assessment in minimally impaired upper extremity hemiparesis in stroke. Arch. Phys. Med. Rehabil. 2012, 93, 2373–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westling, G.; Johansson, R. Factors influencing the force control during precision grip. Exp. Brain Res. 1984, 53, 277–284. [Google Scholar] [CrossRef]
- Hu, W.; Wei, N.; Li, Z.-M.; Li, K. Effects of muscle fatigue on directional coordination of fingertip forces during precision grip. PLoS ONE 2018, 13, e0208740. [Google Scholar] [CrossRef] [Green Version]
- Häger-Ross, C.; Cole, K.J.; Johansson, R.S. Grip-force responses to unanticipated object loading: Load direction reveals body-and gravity-referenced intrinsic task variables. Exp. Brain Res. 1996, 110, 142–150. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Tilson, J.K.; Cen, S.Y.; Rose, D.K.; Hershberg, J.; Correa, A.; Gallichio, J.; McLeod, M.; Moore, C.; Wu, S.S. Fugl-Meyer assessment of sensorimotor function after stroke: Standardized training procedure for clinical practice and clinical trials. Stroke 2011, 42, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Frisoli, A.; Procopio, C.; Chisari, C.; Creatini, I.; Bonfiglio, L.; Bergamasco, M.; Rossi, B.; Carboncini, M.C. Positive effects of robotic exoskeleton training of upper limb reaching movements after stroke. J. Neuroeng. Rehabil. 2012, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Ellis, M.D.; Schut, I.; Dewald, J.P. Flexion synergy overshadows flexor spasticity during reaching in chronic moderate to severe hemiparetic stroke. Clin. Neurophysiol. 2017, 128, 1308–1314. [Google Scholar] [CrossRef]
- Straathof, P.T.; Lobo-Prat, J.; Schilder, F.; Kooren, P.N.; Paalman, M.I.; Stienen, A.H.; Koopman, B.F. Design and control of the A-Arm: An active planar arm support for adults with Duchenne muscular dystrophy. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1242–1247. [Google Scholar]
- Heyer, P.; Orihuela-Espina, F.; Castrejón, L.R.; Hernández-Franco, J.; Sucar, L.E. Sensor Abstracted Extremity Representation for Automatic Fugl-Meyer Assessment. In Applications for Future Internet; Springer: Cham, Switzerland, 2017; pp. 152–163. [Google Scholar]
- Wang, J.; Yu, L.; Wang, J.; Guo, L.; Gu, X.; Fang, Q. Automated Fugl-Meyer assessment using SVR model. In Proceedings of the 2014 IEEE International Symposium on Bioelectronics and Bioinformatics (IEEE ISBB 2014), Chung Li, Taiwan, 11–14 April 2014; pp. 1–4. [Google Scholar]
- Villán-Villán, M.A.; Pérez-Rodríguez, R.; Gómez, C.; Opisso, E.; Tormos, J.M.; Medina, J.; Gómez, E.J. A First Step for the Automation of Fugl-Meyer Assessment Scale for Stroke Subjects in Upper Limb Physical Neurorehabilitation. In Proceedings of the International Conference on Informatics, Management and Technology in Healthcare, Athens, Greece, 9–11 July 2015; pp. 45–48. [Google Scholar]
- Oña, E.D.; Jardón, A.; Monge, E.; Molina, F.; Cano, R.; Balaguer, C. Towards Automated Assessment of Upper Limbs Motor Function Based on Fugl-Meyer Test and Virtual Environment. In Proceedings of the International Conference on NeuroRehabilitation, Pisa, Italy, 16–20 October 2018; pp. 297–301. [Google Scholar]
- Eichler, N.; Hel-Or, H.; Shimshoni, I.; Itah, D.; Gross, B.; Raz, S. 3D motion capture system for assessing patient motion during Fugl-Meyer stroke rehabilitation testing. IET Comput. Vis. 2018, 12, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-S.; Cho, S.; Baek, D.; Bang, H.; Paik, N.-J. Upper extremity functional evaluation by Fugl-Meyer assessment scoring using depth-sensing camera in hemiplegic stroke patients. PLoS ONE 2016, 11, e0158640. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Yen, S.-C.; Tay, E.B.A.; Zhao, Z.; Xu, T.M.; Ling, K.K.M.; Ng, Y.-S.; Chew, E.; Cheong, A.L.K.; Huat, G.K.C. A smartphone-centric system for the range of motion assessment in stroke patients. IEEE J. Biomed. Health Inform. 2014, 18, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Olesh, E.V.; Yakovenko, S.; Gritsenko, V. Automated assessment of upper extremity movement impairment due to stroke. PLoS ONE 2014, 9, e104487. [Google Scholar] [CrossRef] [PubMed]
- Otten, P.; Kim, J.; Son, S. A framework to automate assessment of upper-limb motor function impairment: A feasibility study. Sensors 2015, 15, 20097–20114. [Google Scholar] [CrossRef] [Green Version]
- Otten, P.; Son, S.H.; Kim, J. Automating stroke patient evaluation using sensor data and SVM. In Proceedings of the 2014 IEEE 7th International Conference on Service-Oriented Computing and Applications, Matsue, Japan, 17–19 November 2014; pp. 223–229. [Google Scholar]
- Lee, S.; Lee, Y.-S.; Kim, J. Automated evaluation of upper-limb motor function impairment using Fugl-Meyer assessment. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 26, 125–134. [Google Scholar] [CrossRef]
- Lee, S.-H.; Song, M.; Kim, J. Towards clinically relevant automatic assessment of upper-limb motor function impairment. In Proceedings of the 2016 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Las Vegas, NV, USA, 24–27 February 2016; pp. 148–151. [Google Scholar]
- Kim, Y.M.; Son, Y.; Kim, W.; Jin, B.; Yun, M.H. Classification of children’s sitting postures using machine learning algorithms. Appl. Sci. 2018, 8, 1280. [Google Scholar] [CrossRef] [Green Version]
- Paredes-Madrid, L.; Emmi, L.; Garcia, E.; De Santos, P.G. Detailed study of amplitude nonlinearity in piezoresistive force sensors. Sensors 2011, 11, 8836–8854. [Google Scholar] [CrossRef] [Green Version]
- Parmar, S.; Khodasevych, I.; Troynikov, O. Evaluation of flexible force sensors for pressure monitoring in treatment of chronic venous disorders. Sensors 2017, 17, 1923. [Google Scholar] [CrossRef]
- Rivera, J.; Carrillo, M.; Chacón, M.; Herrera, G.; Bojorquez, G. Self-calibration and optimal response in intelligent sensors design based on artificial neural networks. Sensors 2007, 7, 1509–1529. [Google Scholar] [CrossRef] [Green Version]
- Almassri, A.; Wan Hasan, W.; Ahmad, S.; Shafie, S.; Wada, C.; Horio, K. Self-calibration algorithm for a pressure sensor with a real-time approach based on an artificial neural network. Sensors 2018, 18, 2561. [Google Scholar] [CrossRef] [Green Version]
- Likitlersuang, J.; Leineweber, M.J.; Andrysek, J. Evaluating and improving the performance of thin film force sensors within body and device interfaces. Med. Eng. Phys. 2017, 48, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ide, D.T.A. Available online: https://www.arduino.cc/en/Main/Software (accessed on 1 January 2019).
- Alsayed, A.; Kamil, R.; Ramli, H.R.; As’arry, A. Design and Calibration of Pinch Force Measurement Using Strain Gauge for Post-Stroke Patients. Int. J. Integr. Eng. 2019, 11. [Google Scholar] [CrossRef]
- Oh, J.; Kim, J.O.; Kim, Y.; Choi, H.B.; Yang, J.C.; Lee, S.; Pyatykh, M.; Kim, J.; Sim, J.Y.; Park, S. Highly Uniform and Low Hysteresis Piezoresistive Pressure Sensors Based on Chemical Grafting of Polypyrrole on Elastomer Template with Uniform Pore Size. Small 2019, 15, 1901744. [Google Scholar] [CrossRef] [PubMed]
- Matute, A.; Paredes-Madrid, L.; Gutierrez, E.; Vargas, C.A.P. Characterization of drift and hysteresis errors in force sensing resistors considering their piezocapacitive effect. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhao, M.; Jiang, J.; Song, L. Dynamic Force Transducer Calibration Based on Electrostatic Force. IEEE Access 2019, 7, 48998–49003. [Google Scholar] [CrossRef]
- Maleki-Ghahfarokhi, A.; Dianat, I.; Feizi, H.; Asghari-Jafarabadi, M. Influences of gender, hand dominance, and anthropometric characteristics on different types of pinch strength: A partial least squares (PLS) approach. Appl. Ergon. 2019, 79, 9–16. [Google Scholar] [CrossRef]
- Angst, F.; Drerup, S.; Werle, S.; Herren, D.B.; Simmen, B.R.; Goldhahn, J. Prediction of grip and key pinch strength in 978 healthy subjects. BMC Musculoskelet. Disord. 2010, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef] [Green Version]
- Blennerhassett, J.M.; Carey, L.M.; Matyas, T.A. Grip force regulation during pinch grip lifts under somatosensory guidance: Comparison between people with stroke and healthy controls. Arch. Phys. Med. Rehabil. 2006, 87, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Takamuku, S.; Gomi, H. Better grip force control by attending to the controlled object: Evidence for direct force estimation from visual motion. Sci. Rep. 2019, 9, 13114. [Google Scholar] [CrossRef] [Green Version]
- Roberge, J.-P.; Ruotolo, W.; Duchaine, V.; Cutkosky, M. Improving industrial grippers with adhesion-controlled friction. IEEE Robot. Autom. Lett. 2018, 3, 1041–1048. [Google Scholar] [CrossRef]
- Miall, R.C.; Rosenthal, O.; Ørstavik, K.; Cole, J.D.; Sarlegna, F.R. Loss of haptic feedback impairs control of hand posture. Exp. Brain Res. 2019, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, A.; Bulens, D.C.; Lefèvre, P.; Thonnard, J.-L. Simple and reliable method to estimate the fingertip static coefficient of friction in precision grip. IEEE Trans. Haptics 2016, 9, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Hall, D. (e) in Normandy: The sociolinguistics, phonology and phonetics of the Loi de Position. J. Fr. Lang. Stud. 2019, 29, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Derler, S.; Gerhardt, L.-C.; Lenz, A.; Bertaux, E.; Hadad, M. Friction of human skin against smooth and rough glass as a function of the contact pressure. Tribol. Int. 2009, 42, 1565–1574. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Wu, G.C.; Gitis, N.V.; Maibach, H.I. Tribological testing of skin products: Gender, age, and ethnicity on the volar forearm. Ski. Res. Technol. 2003, 9, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qu, S.; Zhou, Z. Reciprocating sliding behaviour of human skin in vivo at lower number of cycles. Tribol. Lett. 2006, 23, 165. [Google Scholar] [CrossRef]
- Petersen, P.; Petrick, M.; Connor, H.; Conklin, D. Grip strength and hand dominance: Challenging the 10% rule. Am. J. Occup. Ther. 1989, 43, 444–447. [Google Scholar] [CrossRef] [Green Version]
- Li, K.W.; Yu, R. Assessment of grip force and subjective hand force exertion under handedness and postural conditions. Appl. Ergon. 2011, 42, 929–933. [Google Scholar] [CrossRef]













| Variables | Trial 1 | Trial 2 | Trial 3 | Average |
|---|---|---|---|---|
| Pinch force (N) | 14.61 | 17.81 | 15.39 | 15.93 |
| Pulling force (N) | 7.29 | 8.61 | 7.05 | 7.65 |
| Static COF | 0.498 | 0.483 | 0.458 | 0.48 |
| Variable | Mean | Standard Deviation | Range | |
|---|---|---|---|---|
| Pinch force (N) | Right hand | 12.17 | 3.02 | 5.36–18.48 |
| Left hand | 11.67 | 2.82 | 6.79–17.67 | |
| Pulling force (N) | Right hand | 6.25 | 2.19 | 2.37–10.77 |
| Left hand | 5.92 | 1.86 | 1.88–10.67 | |
| Static COF | Right hand | 0.518 | 0.146 | 0.27–0.85 |
| Left hand | 0.517 | 0.145 | 0.23–0.81 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsayed, A.; Kamil, R.; Ramli, H.; As’arry, A. An Automated Data Acquisition System for Pinch Grip Assessment Based on Fugl Meyer Protocol: A Feasibility Study. Appl. Sci. 2020, 10, 3436. https://doi.org/10.3390/app10103436
Alsayed A, Kamil R, Ramli H, As’arry A. An Automated Data Acquisition System for Pinch Grip Assessment Based on Fugl Meyer Protocol: A Feasibility Study. Applied Sciences. 2020; 10(10):3436. https://doi.org/10.3390/app10103436
Chicago/Turabian StyleAlsayed, Abdallah, Raja Kamil, Hafiz Ramli, and Azizan As’arry. 2020. "An Automated Data Acquisition System for Pinch Grip Assessment Based on Fugl Meyer Protocol: A Feasibility Study" Applied Sciences 10, no. 10: 3436. https://doi.org/10.3390/app10103436
APA StyleAlsayed, A., Kamil, R., Ramli, H., & As’arry, A. (2020). An Automated Data Acquisition System for Pinch Grip Assessment Based on Fugl Meyer Protocol: A Feasibility Study. Applied Sciences, 10(10), 3436. https://doi.org/10.3390/app10103436





