1. Introduction
Titanium dioxide (TiO
2) is regarded as one of the most fascinating materials for environmental applications [
1,
2]. It has been intensively investigated as a photocatalyst due to its high photocatalytic activity. Despite numerous advantages, such as low fabrication cost, environmental friendliness, and high chemical stability, TiO
2 still cannot be widely applied due to its inherent drawbacks. TiO
2 has a rapid electron-hole recombination [
3] and a high bandgap energy [
4]. These drawbacks can be overcome by coupling the semiconductor with plasmonic metal nanoparticles [
5]. The plasmonic metal nanoparticles are expected to not only improve the visible light absorption, but also to minimize the electron-hole recombination reaction in TiO
2. It has been demonstrated that surfacial plasmonic resonance improved the light absorption, increased the charge separation, and enhanced the suppression of electrons and holes, and thus significantly improved hydrogen generation [
6].
There are many methods to support the adhesion of Au nanoparticles on the titania surface, such as photodeposition [
7], deposition–precipitation [
8], as well as the sol–gel method [
9,
10]. However, the improvement of the photocatalyst activity (PCA) in Au loaded titania is just several times higher than that of the original one. This may be due to the week connection between the Au nanoparticles and the TiO
2 surface. It is noted that PCA shows significantly more improvement in the one spot synthesis method than in the multistep one [
9].
In order to improve the adhesion of gold nanoparticles on its surface, TiO
2 normally follows a lengthy complicated procedure of surface activation to attach the oxygenic or nitrogenous functional groups or even both on its surface and subsequently undergoes a surface functionalization by adding the appropriate ligands (e.g., MPTMS–3-mercapto-propyl-tri-metoxy-silane). For example, Rehacek et al. [
11] used a solution of NH
4OH:H
2O
2:H
2O (5:1:1 in volume) to treat TiO
2 for 20 h at room temperature or under the UV radiation for 1 h and then illuminated with deep ultraviolet (DUV) for 10 min to improve its wettability. Higgins et al. [
12], however, incubated TiO
2 at 80 °C in a NaOH solution for 1 h to add the OH groups and then in a urea solution for 4 h to add the nitrogenous groups.
Plasma technology has been efficiently used in the fabrication of nanomaterials [
13] especially the plasma–liquid interaction method [
14]. For gold nanoparticle synthesis, Au
3+ can be reduced either by solvated electrons or by reactive species generated in the solution [
15]. The two most dominant reactions are:
and
where
stands for solvated electron.
Recently, the electrochemical reduction of HAuCl
4 acid in dense hydrosols of high-index diamond sub-micrometer sized particles was tested as a promising “green” fabrication route for “metallic nanoparticle–dielectric” clusters in their colloidal solutions. The plasma–liquid interaction approach requires only water-based solutions with the metal precursor and is completely free of any additional reducing and/or capping agents [
16]. It showed successful decoration of gold nanoparticles (AuNPs) onto the diamond surface. In the presence of the diamond particles, substantial “red” spectral shifts of the plasmonic resonance were observed for the AuNPs, being more pronounced for the decorated diamond particles (≈536 nm), than for those adsorbed AuNPs on their surfaces from the mechanical mixture of their colloids (≈530 nm). These shifts of the plasmonic resonance peak could be unambiguously related to the high-index dielectric environment of the AuNPs, provided by their attachment to the diamonds [
17].
Many studies on nonequilibrium gas phase plasmas–fluid interactions have been performed. In particular, the reactive transient species in the interfacial plasma–fluid region play a critical role in plasma-induced liquid reactivity [
18,
19]. Moreover, the reactants are activated by the plasma or the heat produced by the plasma and, in some cases, the liquid can also act as a moderator suppressing or delivering plasma processes with excess energy.
In this work we propose a novel, simple, clean, and effective surface activation of TiO2 using the plasma enhanced wet chemical surface treatment. Plasma–liquid interaction speeds up the surface treatment of TiO2 with NH3/H2O2 solution for the better attachment of the Au nanoparticles. This treatment helps minimize the need of NH3 and H2O2 as well as the treatment time, but still improves the PCA of the Au/TiO2 nanocomposites.
2. Materials and Methods
Commercial submicron (averaged size ca. 800 nm) rutile TiO2 particles (R800) from US Research Nanomaterials, Inc. Houston, TX 77084, United States, were used in our studies. Au(III) chloride trihydrate (99.9%; HAuCl43H2O) was purchased from Sigma–Aldrich, while 30% hydrogen peroxide solution and 25–28% ammonia solution were purchased from Xilong Scientific Co., Ltd, Chaoshan Road, Shantou, Guangdong 510663, China.
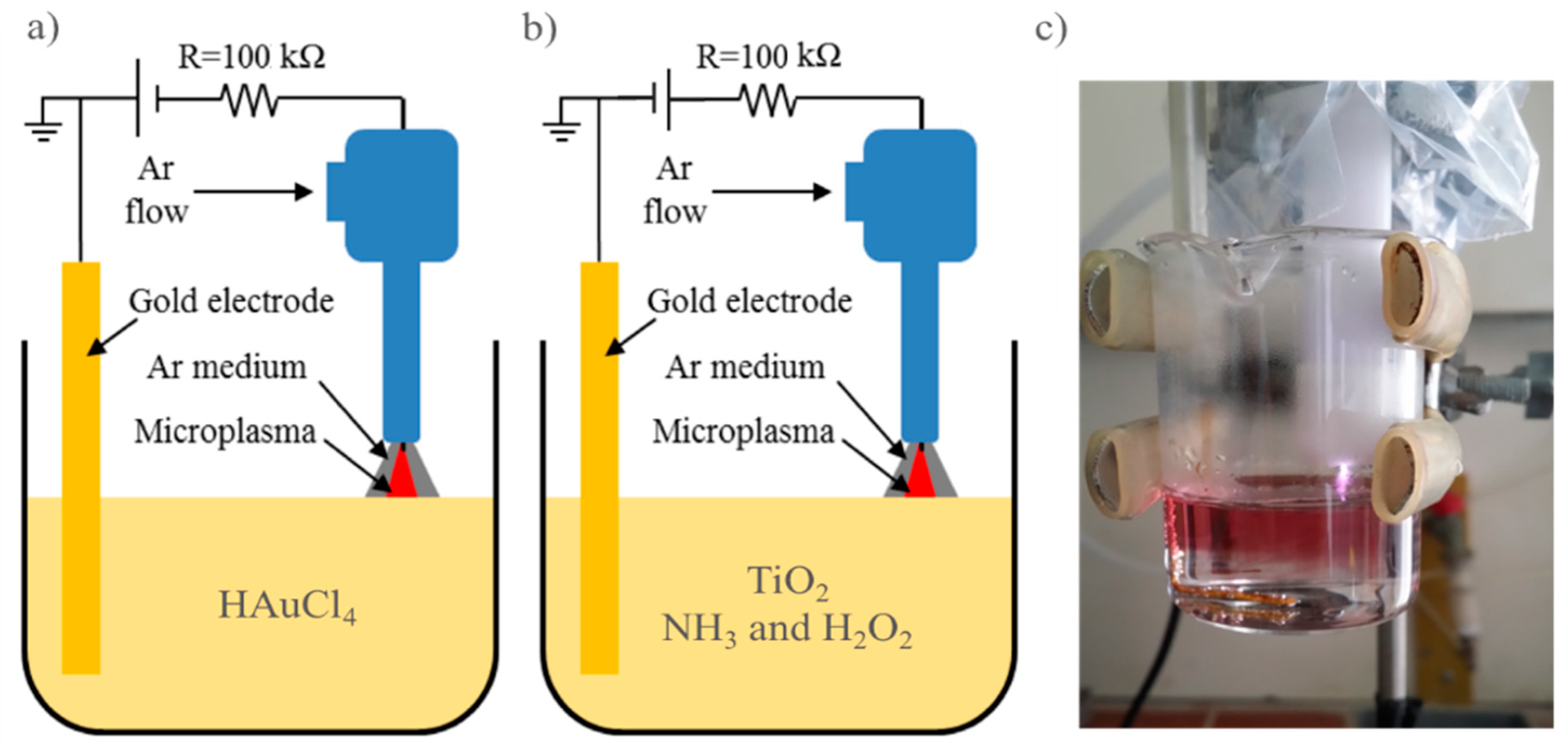
The micro-plasma system consisted of a DC high voltage source, a plasma nozzle, a gold electrode and a 50 mL glass beaker from Bomex with 20 mL treatment solution (see
Figure 1). The plasma nozzle had a Teflon housing with one end being a hollow cylinder of 15 mm in outer diameter and 10 mm in inner diameter, covering the plasma electrode, and the other end being connected to a 6 mm quick gas connector. In our experiments, the plasma electrode was a sharpened 1.6 mm diameter tungsten rod connected to the high voltage via a 100 kΩ resistor. The other polarity was grounded and connected to the gold electrode, which was submerged into the solution. The processing current was maintained constantly at 5 mA.
In order to synthesize gold nanoparticles (AuNPs) from HAuCl
4 solution, we use the above mentioned plasma system with the negative polarity of the plasma electrode (
Figure 1a). Several precursor concentrations were used to study the gold nanoparticle productivity of the system.
The schematic synthesis and diagnostic procedures of Au/TiO
2 composites are presented in
Figure 2. In order to prepare the treated TiO
2 samples, 20 mL TiO
2 0.1 g∙L
−1, 3% NH
3, and 3% H
2O
2 were first subjected to a positive plasma (
Figure 1b) for 5 min to modify the TiO
2 surface. After that, 0.12 mL HAuCl
4 10 mM was added together with 0.25 mL DI water to get 20 mL solution of treated TiO
2 0.1 g∙L
−1 and HAuCl
4 0.06 mM (about 0.37 mL water was vaporized under the plasma treatment). Non-treated TiO
2 samples were prepared by simply adding NH
3, H
2O
2, and HAuCl
4 solutions to get 20 mL TiO
2 0.1 g∙L
−1, 3% NH
3, 3% H
2O
2, and 0.06 mM HAuCl
4.
Both treated and non-treated TiO
2 were then irradiated with the negative plasma (configuration in
Figure 1a) for 15 min to completely reduce Au(III) to form the desired Au/TiO
2 nanocomposites. The resulting solutions were subsequently vacuum dried to eliminate all remaining H
2O
2 and NH
3. Suppose all Au precursor has been reduced to AuNPs, one can easily calculate that the gold particles loaded on the TiO
2 in these Au/TiO
2 nanocomposite samples is of about 11.8% in weight. 10 mL DI water was added to the samples to get 10 mL 0.2236 g∙L
−1 Au/TiO
2, treated and non-treated samples.
To investigate and compare the sunlight photocatalyst activity of and among the bare TiO2, the non-treated and treated Au/TiO2, using above sample preparation procedure we fabricated in darkness 2 mL solution of treated Au/TiO2 0.1118 g∙L−1 and Methylene blue (MB) 20 mg∙L−1; 2 mL solution of non-treated Au/TiO2 0.1118 g∙L−1 and MB 20 mg∙L−1; and 2 mL solution of 0.1 g∙L−1 bare TiO2, MB 20 mg∙L−1.
The decomposition of MB under the solar simulator irradiation (AM 1.5) with a power of 100 mW cm
2 (Oriel SollA) was analyzed by following the evolution of its characteristic absorbance peak at 664 nm. The time dependent concentrations of MB obtained through the experiments were fitted to pseudo-first order kinetics as follows:
where
Co (g·L
−1) and
Ct (g·L
−1) are the concentrations of MB before irradiation and at a given time
t (in minute-min), respectively;
k is the pseudo-first order rate constant (min
−1).
The UV-vis absorbance of the aqueous samples in a 1 cm wide quartz cuvette was measured with an UV-near IR spectrometer of the type V-570 (Jasco). The particle morphologies were examined in a transmission electron microscope (TEM) JEM 1010.
3. Results and Discussion
In order to investigate the activating ability of plasma on the solution, we compared the decolorization of methylene blue (MB) with three solutions; 20 mL 10% hydroperoxide, 20 mL 10% hydroperoxide treated with 5 min of plasma (plasma activated H
2O
2), and 20 mL deionized water treated with 5 min of plasma (plasma activated DI). UV-vis absorption spectroscopy was used to trace the time evolvement of 665 nm absorption peak after 1 mL solution of MB 40 mg∙L
−1 was added into these solutions (see
Figure 3). The MB concentration gradually decreased in the untreated H
2O
2. In contrast, it showed a small drop of about 2% in plasma activated DI then remained unchanged. However, in plasma activated H
2O
2, the concentration showed a stepwise evolution with a large drop of about 17% then followed by a gradual decrease with larger slop in comparison to the untreated H
2O
2.
The concentration drops in the plasma treated solutions were attributed to the transient reactive species created by plasma which react very fast with MB. This result confirms the activating ability of plasma on reactant solutions. With the presence of H2O2 in the liquid, plasma can create significant amount of transient reactive species, making the plasma treated solution become more reactive.
In order to find out the optimal conditions for the gold nanoparticle synthesis using our plasma–liquid interaction system [
16], different precursor concentrations were used and treated with negative plasma for 15 min and the absorption spectra of the as-obtained solutions were checked. As can be seen in
Figure 4, the UV-vis absorption spectra of the as-obtained solutions show the characteristic absorption peaks of the gold nanoparticles. The position and the intensity of the absorption peaks significantly depend on the precursor concentration. When the precursor concentration increases from 0.015 mM to 0.12 mM, the absorption peak position shifts up from ca. 528 nm to ca. 558 nm, while the peak intensity first increases sharply with precursor concentration, reaches its maximum with HAuCl
4 concentration of 0.06 mM then gradually drecreases. For this investigation, therefore, we used 0.06 mM HAuCl
4 as an optimal precursor to generate AuNPs for the synthesis of Au/TiO
2 nanocomposites. At this precursor concentration, the plasma–liquid interaction creates fairly uniform spherical AuNPs with the size in the range of about 35–45 nm (see
Figure 4) and the characteristic absorption peak at about 534 nm.
Gold nanoparticles were also generated at the 0.06 mM precursor concentration with another plasma–liquid interaction configuration as reported in [
20]. The authors there have attained smaller particle sizes distributed around about 20 nm, but the characteristic absorption peak was positioned at about 534 nm like in our case. The reason for the discrepancy in the particle size may be attributed to the shape of the obtained AuNPs. As reported in [
9], AuNPs were obtained in quite diverse shapes including spherical, triangular, cubic, hexagonal, and pentagonal grains and also bars, whereas in our experiments the AuNPs were mostly in the spherical shape with rather uniform size (see
Figure 5). In their setup, the authors used a hollow electrode type so the plasma channel was surrounded by air. Meanwhile, in our case, the rod electrode was surrounded by the Ar medium. This difference in the plasma medium may be attributed to the difference in the Ar nanoparticle shapes.
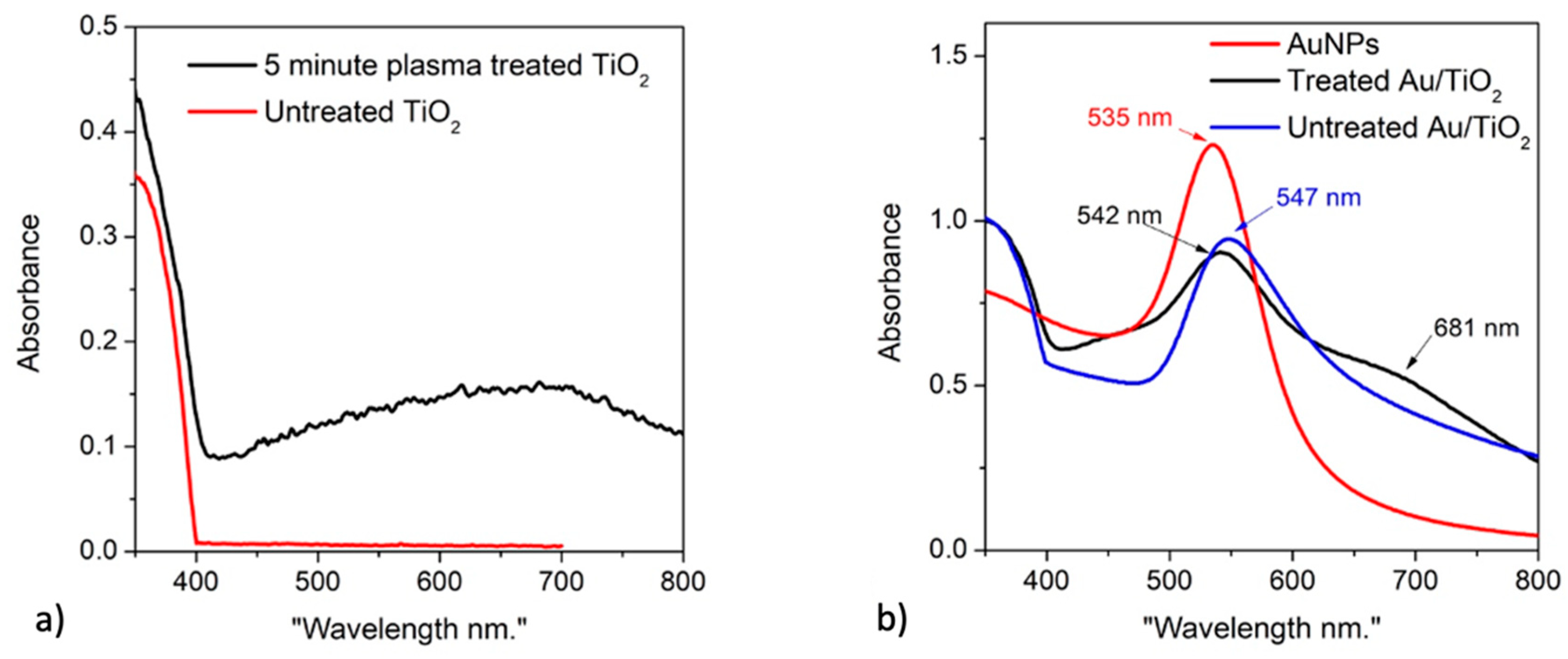
Figure 6 presents the TEM images of the nontreated Au/TiO
2 nanocomposite. One can easily see that the AuNPs are no longer homogenous and uniform in shape, but their size is distributed in the range from about 10 nm to around 55 nm. This is in line with the broader absorption band in their UV-vis absorption spectra (
Figure 7). It is likely that these AuNPs are formed in the solution before being attached to TiO
2 surface because they show similar size distribution as those generated from the pure HAuCl
4. All generated AuNPs are seen immobilized on the TiO
2 surface with clear and sharp boundaries indicating a rather weak connection between them. This also supports a small red shift of about 15 nm compared to the situation with the pristine AuNPs. The influence of TiO
2 on the reduction mechanism of Auric ions may be twofold: catalyst effect under UV radiation from plasma which enhances the particle formation, resulting in bigger particles and growth termination by capturing the growing particles onto the titania surface.
Unlike the non-treated Au/TiO
2, there are two types of AuNPs existing in the treated Au/TiO
2, one with a similar size distribution as in the non-treated Au/TiO
2 composites and the other with very small sizes ranging from 4 nm to 9 nm (
Figure 8). Both of these types exhibit noticeably close contacts to the TiO
2 surface with seemingly undetectable boundaries. The small particles even seem to be submerged into the TiO
2 matrix (see
Figure 8). The UV-vis spectra (
Figure 7) of this sample show a good agreement with the TEM results. The spectra of the treated Au/TiO
2 show two absorption peaks which can be attributed to the two classes of AuNP particle size (there is no AuNP aggregation). The absorption peaks are strongly red shifted from about 523 nm to 538 nm (for smaller AuNPs) and from 534 nm to around 680 nm (for larger AuNPs). Using the refractive index difference of 1.279 (rutile TiO
2 has refractive index of 2.609) applied for the situation presented in
Figure 5 in [
21], one can easily estimate the red shift for particles with the original plasmonic resonance of 534 nm in water (
n = 1.33) to be about 130 nm which is in good agreement with the red shift of the larger sized AuNPs in the treated Au/TiO
2. This result again confirms a strong contact between the treated TiO
2 and the AuNPs in treated Au/TiO
2 nanocomposites.
As expected from the above analysis, the MB sunlight degradation rate of the non-treated Au/TiO
2 was improved in comparison to that of bared TiO
2 (
Figure 9). Even weakly attached AuNPs already increased the degradation rate by a factor of 4 from 0.01 min
−1 with TiO
2 to 0.04 min
−1, with non-treated Au/TiO
2 supporting the electron–hole separation and absorption spectra broadening. This effect is maximized in the treated Au/TiO
2 as the degradation rate reaches the extremely high value of 0.13 min
−1. This value is far better than the MB sunlight degradation rate in the order of only 0.022 min
−1 obtained with the AuNPs doped TiO
2 prepared by the chemical method, as reported by S. Padikkaparambil et al. [
22].