Abstract
Brownmillerite is connected to chromium leaching when present in steel slags. To prevent chromium leaching, brownmillerite in slag should be prevented. Two methods for decreasing brownmillerite content in low-alloy electric arc furnace (EAF) slag were investigated: decreasing the basicity and increasing the cooling rate. The methods were tried on both laboratory scale and in full-scale production. In the laboratory scale experiments, chromium leaching decreased as the basicity decreased until brownmillerite was no longer present, slower cooling resulted in increased chromium leaching, and faster cooling decreased chromium leaching. In full-scale production, basicity modified single batches, with a basicity below 2.2, generally leached less chromium than slag batches with higher basicity, thus verifying the correlation between basicity and chromium leaching seen in laboratory scale experiments. The cooling process in the full-scale experiments was achieved either by letting the slag cool by itself in the air or by water spraying. The water-sprayed slag, which cooled faster, had less chromium leaching than the air-cooled slag. The full-scale production experiments confirmed that both decreasing basicity below 2.2 and increasing the rate of cooling could be used to decrease chromium leaching.
1. Introduction
Slag is produced as a by-product of pyrometallurgical metal production. The steel production of Sweden almost reached 4.7 million tonnes of crude steel in 2017; at the same time, around 1.3 million tonnes of slag was produced [1]. The application areas for slag vary: fertilizer, water purification, and different construction materials are some examples. In some areas, the properties of slag make it superior to natural resources, for example the porosity of slag in asphalt reduces road noise [2,3]. By using slag, natural virgin resources can be preserved and unnecessary landfill is prevented. The properties of slag can be derived from the properties of the minerals in the slag. Different types of slag consist of different minerals, which make slag a versatile material. However, some of these properties, such as volume expansion [4], disintegration [5], and leaching of some elements, restrict how slag can be used. From the literature, it can be discerned that chromium is one of the main elements whose leaching should be restricted [6,7]. The European Council Directive (2003/3/EC) limits chromium leaching to 0.5 mg/kg for the material to be classified as inert landfill material. In Sweden, 40% of the steel is made from scrap [8]. In scrap-based production, the elements in the scrap, such as chromium, partly distribute into the slag. Slag from scrap-based steelmaking can thereby contain chromium even if no alloys were added to the furnace. The first step in producing steel by scrap-based steelmaking uses an electric arc furnace (EAF). In this paper, low-alloy EAF slag from Ovako in Hofors was used as a starting material. Ovako in Hofors produced about 400,000 tonnes of ingot steel and with that about 40,000 tonnes of EAF slag in 2017; this corresponds to almost half of the low-alloy EAF slag in Sweden, which was 100 tonnes in 2015 [9]. Ovako Hofors regards EAF slag as a product and wants to guarantee that the slag is as environmentally friendly as possible, and has therefore continuously investigated the possibility of making a better slag with less chromium leaching [10,11,12,13]. Chromium leaching has been reduced by changing the slag composition, but it can be further reduced to ensure it always maintains a level below the limit of inert material. The slag usually consists of larnite (β-Ca2SiO4), magnesiowüstite (MeO), spinel (AB2O4), and brownmillerite (Ca2(AlFe)2O5), but occasionally merwinite (Ca3MgSi2O8) has been detected. The leaching of slag has been connected to the dissolution of the minerals in the slag [14,15]. In order for a mineral to contribute to chromium leaching, two criteria must be fulfilled: the mineral has to contain chromium and dissolve or react in contact with water. The stoichiometric formula of a mineral does not have to include chromium for a mineral to contain chromium, as is the case for brownmillerite. The chromium may substitute for another atom or be an interstitial addition. Of the minerals in Ovako Hofors slag, merwinite is not the main mineral responsible for chromium leaching, as it is only present occasionally. Larnite dissolves [16] and has been reported to contain chromium [17]. However, chromium has not been detected in analyses of the investigated slag [18] and is therefore unlikely to cause the chromium leaching in this slag. The spinels are stable and do not react with water [6,19] and can also be excluded. The dissolution of magnesiowüstite depends on the FeO/MgO ratio. Magnesiowüstite, with a FeO content of 60 wt% or more, does not react with water or leach chromium [20,21]. Magnesiowüstite primarily consists of FeO, MgO, MnO, and CrOx, but also contains small amounts of additional elements like calcium [22,23]. The magnesiowüstite in the studied slag is FeO rich most of the time and should therefore, in most cases, not dissolve in water and contribute to chromium leaching. Brownmillerite has been determined to both contain chromium as well as to leach chromium [24]. As the chromium mineral leaching is known, appropriate action can be taken to prevent chromium leaching. The dissolution of brownmillerite increases with the Al/Fe ratio [25]. Unfortunately, as the low-alloy EAF slag contains aluminum, brownmillerite in the slag also contains aluminum. The only alternative to avoid leaching of chromium from brownmillerite is to avoid brownmillerite formation altogether.
In order to identify and investigate the possibility of preventing the formation of brownmillerite, extensive experimental work was conducted and verified in full-scale experiments. The aim of this article is to decrease chromium leaching from low-alloy EAF slag by decreasing the brownmillerite content, either by modifying the basicity or changing the cooling rate.
2. Methods and Material
2.1. Thermodynamic Calculations
As a first step to determine how to eliminate brownmillerite, thermodynamic calculations in the software FactSage [26] were conducted. The databases FToxid-SLAGA, FToxid-SPINA, FToxid-MeO_A, FToxid-Mel_A, and FToxid-C2AF were used. The gas phase was suppressed but all possible pure solids from FactPS and FToxid were included, with the activity of Fe set to 1 to simulate slag and molten steel in equilibrium. The thermodynamic calculations indicated that basicity B2, the CaO/SiO2 ratio, affects the formation of brownmillerite. The correlation of basicity and chromium leaching has been noted by Mombelli et al. who stated that chromium leaching increased as the basicity increased [24]. In addition, Mombelli et al. no longer detected brownmillerite in the slag samples after the basicity of the slag was decreased by quartz addition [27]. Studies by Kilau and Shah [28] and Neuhold et al. [29] also connected the basicity and chromium leaching.
Figure 1 shows the solidification of the chromium-containing minerals of a slag at basicity 2.5. The first mineral to form is magnesiowüstite, followed by spinel, and lastly brownmillerite. All the minerals start to form while there is still molten slag present. According to thermodynamic calculations, decreasing the basicity in the investigated low-alloy EAF slag compositions leads to brownmillerite forming at a lower temperature, when all of the melt has solidified. As a result of kinetics, reaching equilibrium and forming minerals takes longer in a solid than in a liquid. If the cooling rate exceeds the formation rate of brownmillerite, brownmillerite is not present in the slag. Thereby, two methods for avoiding brownmillerite are possible: decreasing the basicity to lower the temperature of formation or increasing the cooling rate. If the basicity is decreased too much, the mineral merwinite is formed in the thermodynamic calculations. Merwinite can contain chromium and dissolves in water and is therefore not a suitable substitute for brownmillerite and should be avoided to prevent chromium leaching [30]. Thermodynamic calculations of slag of different compositions were used to obtain the basicity, which would prevent brownmillerite and merwinite formation in most compositions. According to the calculations, there is a basicity span of around 2.2 to prevent both brownmillerite and merwinite; the size and location of the span differs depending on the slag composition.
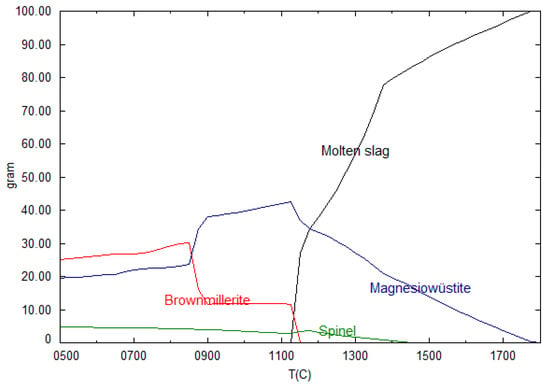
Figure 1.
Thermodynamic calculations of the crystallization of different chromium-containing minerals as the low-alloy electric arc furnace (EAF) slag solidifies.
2.2. Experimental Overview
On the basis of the thermodynamic calculations, two methods for eliminating brownmillerite were identified and tested: modifying the basicity and varying the cooling rate. Both methods were tried on laboratory scale, and then verified in full-scale production. Table 1 shows a synopsis of the experiments conducted with the two methods.

Table 1.
Synopsis of all the trials presented in this article.
During previous investigations of this slag, it was revealed that the leaching of chromium could change over time [31]. It was also noted that autoclave treatment could age slag quickly and replicate the leaching of aged slag [31]. Therefore, the slag samples were autoclave-treated to achieve the chromium leaching of aged slag. Crushed slag was introduced together with 20 wt% added deionized water in the autoclave. The autoclave was run for 24 h at 160 °C. Afterwards, the slag was removed from the autoclave and dried at 100 °C. The leaching was performed accordingly to EN 12457-2.
2.3. EN 12457-2 Leaching Test
The EN 12457-2 leaching test uses slag crushed to 95% within 0–4 mm is leached in water at room temperature for 24 h at an L/S ratio of 10. During the leaching, the containers are rotating. After the leaching test, the leachate was sieved through a 0.45 µm filter before analysis.
2.4. Mineralogical Characterization
The mineralogical composition of the laboratory slag samples was analyzed by X-ray diffraction (XRD) using an Empyrean Series 2 PANalytical XRD unit (Malvern Panalytical, Almelo, The Netherlands) equipped with copper Kα radiation of 45 kV and 40 mA. Evaluation of data was performed using the software HighScore Plus (version 4.7, PANalytical B.V., Almelo, The Netherlands). Some of the samples were also molded in epoxy, polished, and carbon coated before being analyzed in a Gemini Zeiss Merlin SEM scanning electron microscope equipped with an Oxford Xmax 50 mm2 EDS (Zeiss, Oberkochen, Germany). The beam current was 1.0 nA, the voltage was 20 keV, and the working distance was set to 8.5 mm.
2.5. Laboratory-Scale Experiments
2.5.1. Controlling Basicity
Slag, aged outdoors, at a basicity of 2.7, was remelted with the addition of SiO2 to decrease the basicity. The slag was stored in fractions and did only require splitting before the experiments. The composition of the starting slag is shown in Table 2. The SiO2 was added to achieve a basicity of 2.3, 2.0, and 1.5, as seen in Table 1. A slag sample without any additions was included to determine if remelting did affect the leaching. The samples were placed in an MgO crucible in an inert atmosphere and heated at 6 °C/min to 1600 °C where the temperature was maintained for an hour. Then, the furnace with the slag samples was cooled to room temperature within four hours. The slag was crushed to less than 4 mm before autoclave treatment and the following EN 12457-2 leaching test.

Table 2.
Composition in wt% of the slag used in the basicity experiments.
2.5.2. Controlling Cooling
Four different cooling methods were tested on two batches of slag of different basicities, 2.1 and 2.8, to see how the cooling rate impacts chromium leaching. The reference slag samples were specifically chosen for their representation of different basicities and chromium leaching. The composition of the slag samples is shown in Table 3. Ideally, only the basicity would differ between the two slags but there was also significantly more Al2O3 in the slag at basicity 2.8 and more chromium in the slag at basicity 2.1. The sample with basicity 2.1 was at the targeted basicity and leached below 0.04 mg/kg. The sample at basicity 2.8 was chosen for the unusually high leaching of 4.5 mg/kg. Before the remelting experiments, the slag was crushed to less than 4 mm.

Table 3.
Composition in wt% of the two different slags used in the lab-scale cooling experiments.
The EAF at Ovako steel plant in Hofors produces about 100 tonnes of steel in each batch. One batch was fed by two scrap baskets, the second basket was added after 20 min. Normally, two batches of slag from the EAF were tapped into the same slag pot. The slag pot was cooled outside for two hours before transportation to the slag tipping site. There, the slag was tilted from a ramp onto a heap of slag from previous slag ladles. The ramp was water sprayed and cleared of slag once every 24 h. This procedure caused the slag to reheat every 2 h and the slag might retain heat for up to 24 h. The elevated temperature over time might allow for brownmillerite formation. Two of the cooling method experiments (the hold temperature and altering temperatures experiments) were created to simulate the worst case scenarios of the slag cooling process with respect to forming brownmillerite.
The slags were remelted in MgO crucibles with nitrogen running through the furnace. The slag was heated at 6 °C/min to 1600 °C and the temperature was maintained for 1 h before the cooling procedures started. The cooling methods are described below and the temperature profiles for the different cooling rates can be seen in Figure 2. In the hold temperature experiment, the slag was cooled at 6 °C/min to 700 °C and the temperature was maintained for 24 h; thereafter, the furnace was cooled to room temperature at 6 °C/min. In the altering temperatures experiment, the slag was set to cool at 6 °C/min to 1000 °C and maintained for 1 h. The temperature was alternated between 1000 and 800 °C three times at a rate of 6 °C/min, to simulate the addition of new slag onto the ramp,. The temperatures 1000 °C and 800 °C were maintained for 0.5 h, which resulted in a two-hour cycle. After the third time the temperature reached 800 °C, the furnace was cooled to room temperature at 6 °C/min. As a set cooling rate, 4 °C/min was used. For the fast cooling rate experiment, a furnace with water cooling without an inert atmosphere of nitrogen was used. The temperature dropped to about 500 °C in 1 h and the sample was then removed from the furnace and cooled to room temperature within 1 additional hour. Since the slag with a basicity of 2.1 leached below detection limit of 0.04 mg/kg, it was pointless to reduce the chromium leaching further; therefore, the slag was excluded from the experiments with a set cooling rate and a fast cooling rate. As a result of the retained heat in the refractory of the furnace, cooling took longer than described and depicted in Figure 2. The slag was crushed to less than 4 mm before autoclave treatment and the following EN 12457-2 leaching test.
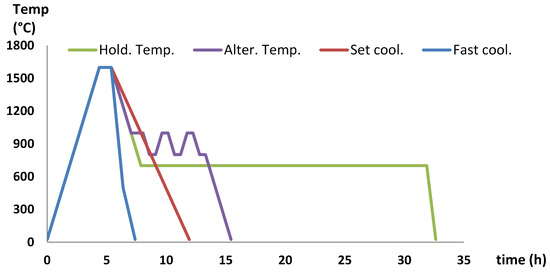
Figure 2.
Figure conceptualizing the different cooling rates used in the laboratory cooling controlled experiments.
3. Results and Discussion
3.1. Controlling Basicity
The analyses of the modified slag samples can be seen in Table 4. The basicity, CaO/SiO2 ratio, of the samples corresponded to the desired basicity. The MgO content in the samples increased from 9.1 wt% due to the slag reacting with the MgO crucible. The changes in the composition were too small to be significant and could therefore be due to variations in the slag, but the results indicate that more MgO was dissolving into the slag as the basicity decreased.

Table 4.
Composition in wt% of the modified slag samples.
Table 5 shows the minerals detected by XRD and SEM-EDS. Magnesiowüstite and spinel were found for all basicities. Larnite was not found at basicity 1.5, but merwinite was. Brownmillerite was only detected by SEM-EDS and XRD at basicity 2.4 and higher. The results from these experiments confirm the results from the thermodynamic calculations: by changing the basicity, the formation of brownmillerite can be avoided without forming merwinite.

Table 5.
Mineral analysis in XRD and SEM-EDS of the samples of different basicities (the sample with basicity 2.0 was not analyzed in SEM).
The diffractograms for the slags of different basicities can be seen in Figure 3. For basicity 2.3, the brownmillerite peaks could almost not be distinguished from the background noise. At basicity 1.5, the larnite was exchanged for merwinite.
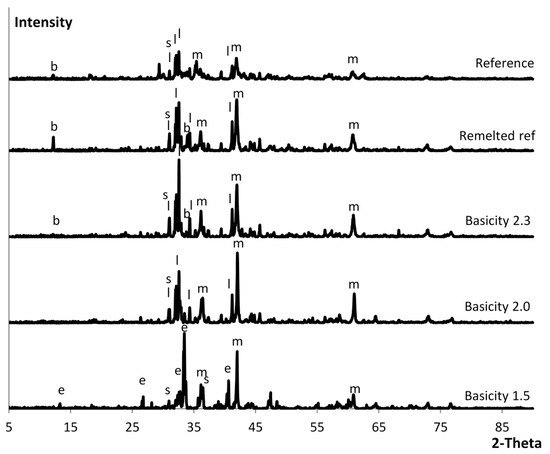
Figure 3.
XRD diffractograms of the basicity modified slag. The letters represent the minerals found in the analysis—s: spinel; b: brownmillerite; m: magnesiowüstite; l: larnite; e: merwinite.
Figure 4 shows the chromium leaching of the reference and the autoclaved slag samples. The aged reference slag leached 2.2 mg/kg originally. Remelting the reference slag decreased the leaching of chromium to less than 0.04 mg/kg, which is not shown in Figure 4. Autoclave treatment returned the chromium leaching to 1.8 mg/kg, the same magnitude as the aged slag. Henceforth, only leaching from autoclaved samples were presented and discussed, since autoclave treatment causes slag to leach as aged slag. The decrease in basicity from 2.7 to 2.3 resulted in significantly less chromium leaching, and the leaching decreased continuously as the basicity was lowered to 2.0. As a result of brownmillerite being absent at basicity 2.0 and below, decreasing the basicity further than 2.0 did not result in significantly less chromium leaching. The chromium leaching decreased with decreasing brownmillerite content, indicating that chromium leaching and brownmillerite content were related. The chromium that usually is included in brownmillerite relocates to another chromium-containing mineral when brownmillerite is not present. If the only minerals chromium can enter at this point do not dissolve in water, there can be no chromium leaching. Thereby, it is possible to prevent most of the chromium leaching by having a basicity at which neither brownmillerite nor merwinite are formed.
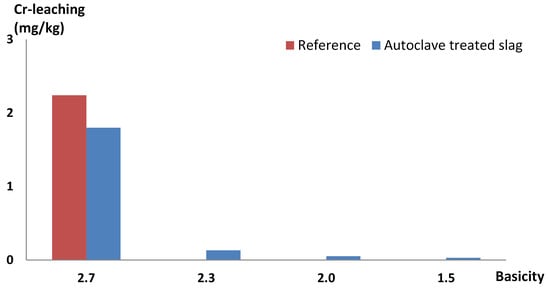
Figure 4.
Chromium leaching of low-alloy EAF slag remelted with and without the addition of SiO2 to achieve different basicities.
3.2. Controlling Cooling
Brownmillerite was not detected in the slag samples of basicity 2.1, but was detected in every sample of basicity 2.8, by both XRD and SEM-EDS analysis, as seen in Table 6.

Table 6.
The major minerals discovered in XRD and SEM-EDS analysis of the samples of different cooling rates. No SEM analyses were performed on the references.
The SEM images in Figure 5 show the slags from the cooling experiments. The hold temperature and altering temperature experiments have the largest spinels. The magnesiowüstite in most samples contains darker dots, representing spinels that are beginning to form. The fast cooling rate experiment shows the smallest particle size since the mineral phases had the shortest time to grow. In addition to the phases seen in Figure 5, a phase with chemical composition fitting pleochroite, Ca6Al8MgSiO21 was found in the slag at basicity 2.8. The pleochroite did not contain chromium.
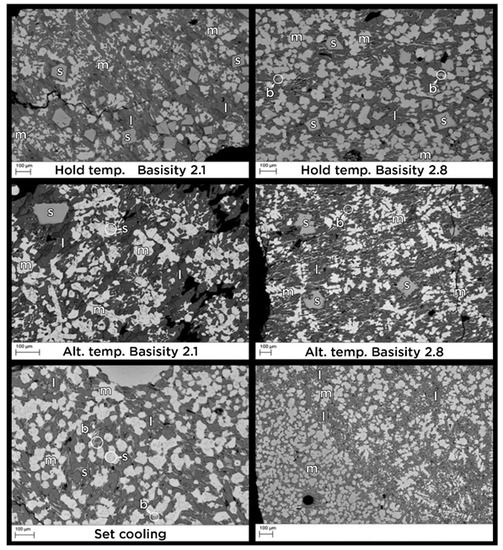
Figure 5.
SEM image of the slag at basicity 2.1 and 2.8 cooled by different methods. The letters representing the minerals found in the analysis—s: spinel; b: brownmillerite; m: magnesiowüstite; l: larnite.
The XRD diffractograms for the different cooling methods can be seen in Figure 6. All slag samples were completely crystalline. The only difference between the samples of basicity 2.1 and basicity 2.8 is the presence of brownmillerite in the latter. Otherwise there were no differences between the different cooling methods at the same basicity, except that there was no strong peak corresponding to spinel in the fast cooling rate sample.
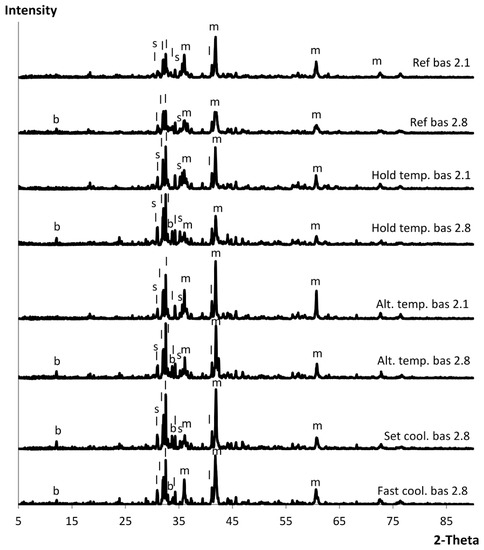
Figure 6.
XRD diffractograms of the slag at basicity 2.1 and 2.8 cooled by different methods. The letters representing the minerals found in the analysis—s: spinel; b: brownmillerite; m: magnesiowüstite; l: larnite.
The cooling rates affected chromium leaching in both basicities, as seen in Figure 7. The highest leaching of the two different basicities was achieved by different cooling methods. At basicity 2.1, alternating the temperature between 800 and 1000 °C gave the highest chromium leaching of 0.65 mg/kg; however, at basicity 2.8, holding the temperature at 700 °C gave the highest leaching of 15.2 mg/kg. As the temperature of the slag in the tipping site at Ovako Hofors is unknown, the methods hold temperature and alternating temperature represent the worst-case scenarios in terms of slag handling with respect to brownmillerite formation. It was therefore anticipated that these methods should cause an increase in chromium leaching. Even with increased chromium leaching at basicity 2.1, brownmillerite was neither seen in SEM-EDS nor XRD, which is still to be explained. Either the amount of brownmillerite was below the detection limit or another mineral was responsible for the chromium leaching.
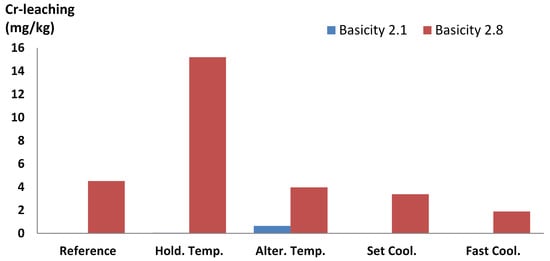
Figure 7.
Chromium leaching from autoclave-treated slag with basicity 2.1 and 2.8 cooled by different methods. The leaching of slag at basicity 2.1 is below detection limit in the reference and hold temperature experiment.
The thermodynamic calculations of the slag at basicity 2.8 showed that 700 °C was close to the temperature of maximum brownmillerite formation. The 24-h holding period at this temperature giving sufficient time for brownmillerite formation may explain the exceptionally high chromium leaching of 15.2 mg/kg. The lowest chromium leaching, at 1.9 mg/kg, in the slag at basicity 2.8, was seen in the fast cooling rate. Faster cooling of high-basicity slag reduced the chromium leaching but did not guarantee leaching below the limit of inert landfill. Fast cooling resulting in amorphous material should be avoided, as noted by Tossavainen et al. [32] who stated that amorphous EAF slag could leach more chromium than fast-cooled crystalline EAF slag. The leaching from the different samples also confirmed that chromium leaching is connected to mineralogy [15,33] and not chromium content, as the chromium content in basicity 2.1 was 4.1 wt% and in basicity 2.8 the chromium content was 2.9 wt%.
4. Full-Scale Production Verifications
4.1. Controlling Basicity in Full-Scale Experiments
Full-scale trials targeting basicity 2.2 were conducted at the EAF in Hofors. The SiO2-based sand, composition shown in Table 7, was added in the second scrap basket to the EAF. The sand used as a SiO2 carrier was not optimal, as it contained about 13 wt% Al2O3. Additional Al2O3 content in the slag may promote the formation of brownmillerite or increase the Al2O3 content in brownmillerite. Increased Al2O3 content in brownmillerite produces a more reactive brownmillerite [25], the opposite of what was intended. The SiO2 sand was added directly to the furnace instead of during slag tapping to promote an even distribution and integration of the slag and the sand. Since the scrap composition is not fixed, the basicity varies; therefore, different amounts of SiO2-sand addition, from 300 to 500 kg, were tested. Single batches of slag were collected and were kept isolated to avoid contamination by ordinary slag batches. Therefore, the modified slag batches did not experience the normal cooling and reheating cycle. The sampling of cooled slag was done from different areas of the slag pile after the slag had been transported off tipping site. The samples of the same slag were mixed and crushed to less than 4 mm before autoclave treatment and the following EN 12457-2 leaching test.

Table 7.
Composition in wt% of the SiO2 sand used to modify the slag in full scale.
The basicity of the collected modified batches was below the targeted 2.2. The highest chromium leaching from the single batches was 0.23 mg/kg (see Figure 8) and therefore leached below the limit of inert material of 0.5 mg/kg.
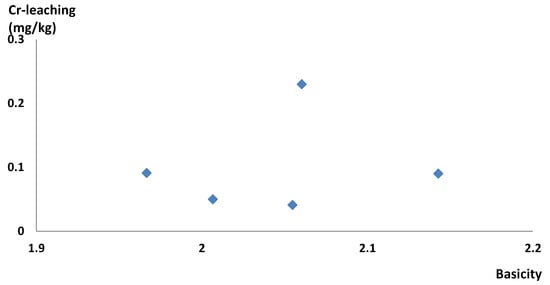
Figure 8.
Chromium leaching from autoclave-treated slag from full-scale single batches from Ovako Hofors EAF.
Brownmillerite was detected by XRD analysis in the batch with the highest chromium leaching (see Figure 9) where the second brownmillerite peak in was the largest in that diffractogram. This was not seen earlier when brownmillerite was detected in the other slag samples. In Figure 3 and Figure 6 the second brownmillerite peak was in the same magnitude as the first brownmillerite peak at 12 2-theta. There is a possibility that another mineral was present and adding intensity to the two larger brownmillerite peaks seen in the diffractogram. The presence of another mineral needs to be confirmed by SEM-EDS analysis. The Al2O3 content in the slag could not be connected to the formation of brownmillerite or the leaching of chromium. The Al2O3 content of the slag that leached 0.23 mg/kg chromium was at 9.1 wt%, which was close to the 8.3 wt% Al2O3 of the slag that leached 0.04 mg/kg. The slag leaching 0.09 mg/kg had an Al2O3 content of 5.2 wt% but a similar diffractogram to the slag leaching 0.04 mg/kg. The results from the full-scale single batches verified the results of the laboratory-scale tests. Decreased basicity led to less brownmillerite formation, which resulted in less chromium leaching.
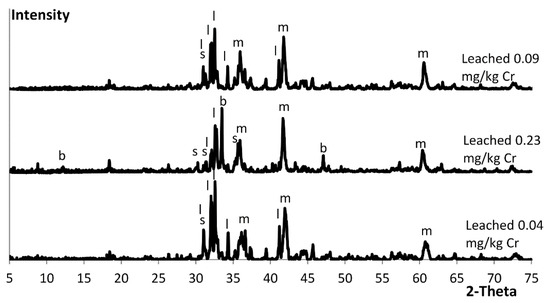
Figure 9.
XRD diffractograms of the modified slag batches. The letters representing the minerals found in the analysis—s: spinel; b: brownmillerite; m: magnesiowüstite; l: larnite.
4.2. Controlling Cooling in Full-Scale Experiments
Two different cooling rates were tested on a single batch of slag with a basicity of 2.8 at Ovako Hofors. One part of the slag was air cooled and the other was cooled by water spraying. The slag was crushed to less than 4 mm before autoclave treatment and the following EN 12457-2 leaching test.
The air-cooled slag showed chromium leaching of 1.4 mg/kg after autoclave treatment. When water cooling was applied, the chromium leaching decreased to 0.29 mg/kg after autoclave treatment, as seen in Figure 10. Since both the samples were from the same slag ladle, the only difference was the cooling procedure. The result of this full-scale trial reflected the results from the laboratory-scale experiments: faster cooling led to less leaching of chromium. The basicity of the samples was at 2.8, proving a decrease in chromium leaching could be achieved in slag outside of the optimum basicity range by increasing the cooling rate. Water cooling was effective to reduce the chromium leaching but comes with some risk: if the water does not evaporate completely before the next batch is tipped, the remaining water can be trapped in the slag and gasified, resulting in a gas explosion. Other less efficient but safer methods exist for cooling slag faster, for example, spreading the slag over a larger surface and refraining from dumping slag from different batches on top of each other.
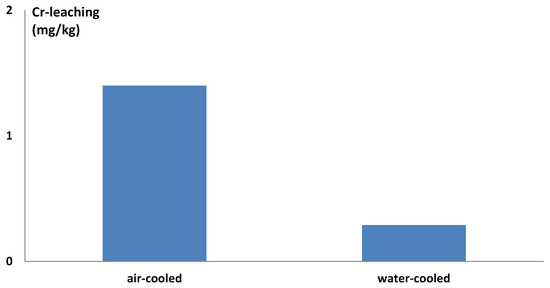
Figure 10.
Chromium leaching of air- and water-cooled autoclave-treated slag from the full-scale experiments.
5. Conclusions
Both decreasing the basicity, the CaO/SiO2 ratio, and increasing the cooling rate resulted in decreased chromium leaching. Decreasing basicity was more effective than increasing the cooling rate. Changing the basicity in laboratory-scale experiments showed that brownmillerite could be decreased below detection limit of XRD and consequently resulted in chromium leaching close to the detection limit of 0.04 mg/kg. On the basis of the results, brownmillerite seemed to be the main mineral responsible for leaching chromium in the low-alloy EAF slag and should thereby be avoided to prevent chromium leaching. Decreasing the basicity in full-scale production can be done, but as the scrap composition varies, it is difficult to maintain the basicity at a certain value. Decreasing the basicity may result in less chromium leaching, even if optimal basicity is not reached; this was shown in the laboratory experiments, a decrease from basicity 2.7–2.3 resulted in significantly reduced chromium leaching. Faster cooling decreased the leaching of chromium but did not eliminate it.
A combination of the methods, aiming at a basicity between 2.0 and 2.2 and preventing slag from maintaining heat is recommended. It may not be possible to control slag in full-scale production to achieve the perfect composition or have the best cooling procedure, but by improving these parameters, chromium leaching can be decreased.
Author Contributions
Conceptualization, I.S., K.P. and F.E.; Methodology, I.S., K.P. and F.E.; Formal Analysis, A.A., J.O. and I.S.; Investigation, A.A., J.O. and I.S.; Resources, K.P. and I.S.; Data Curation, I.S.; Writing—Original Draft Preparation, I.S.; Writing—Review and Editing, F.E., C.S., A.A., J.O., A.L. and K.P.; Visualization, I.S.; Supervision, F.E.; Project Administration, I.S., K.P. and F.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MiMeR-lab, VINNOVA, the Swedish Steel Producers’ Association (TO 55) via MISTRA and the Centre for Advanced Mining & Metallurgy (CAMM).
Acknowledgments
We wish to thank MiMeR-lab, VINNOVA, the Swedish Steel Producers’ Association (TO 55) via MISTRA and the Centre for Advanced Mining & Metallurgy (CAMM) for financial support and commitment. We also express our gratitude to colleagues and company members with special thanks to Christoffer Schmidt, Johan Lyttbacka, Johan Stenman and Pernilla Sellfors-Forsling.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Produktion Jernkontoret. Available online: http://www.jernkontoret.se/sv/stalindustrin/branschfakta-och-statistik/produktion/ (accessed on 31 January 2018).
- Pålsson, K.; Stemne, J.; Johansson, L.; Blixt, E. Stålindustrin Gör Mer än Stål—Handbok för Restprodukter; Jernkontoret: Stockholm, Sweden, 2012. [Google Scholar]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Juckes, L.M. The volume stability of modern steelmaking slags. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2003, 112, 177–197. [Google Scholar] [CrossRef]
- Durinck, D.; Arnout, S.; Mertens, G.; Boydens, E.; Jones, P.T.; Elsen, J.; Blanpain, B.; Wollants, P. Borate distribution in stabilized stainless-steel slag. J. Am. Ceram. Soc. 2008, 91, 548–554. [Google Scholar] [CrossRef]
- Mudersbach, D.; Kühn, M.; Geisler, J.; Koch, K. Chrome immobilisation in EAF-slags from high-alloy steelmaking: tests at FEhS institute and development of an operational slag treatment process. In Proceedings of the 1st International Slag Valorisation Symposium, Leuven, Belgium, 6–7 April 2009. [Google Scholar]
- Pillay, K.; Von Blottnitz, H.; Petersen, J. Ageing of chromium(III)-bearing slag and its relation to the atmospheric oxidation of solid chromium(III)-oxide in the presence of calcium oxide. Chemosphere 2003, 52, 1771–1779. [Google Scholar] [CrossRef]
- Fakta och Nyckeltal. 2019. Available online: https://www.jernkontoret.se/sv/stalindustrin/branschfakta-och-statistik/fakta-och-nyckeltal/ (accessed on 8 October 2019).
- Pålsson, K.; Stemne, J.; Ruist, G.; Blixt, E. Stålindustrin Gör Mer än Stål—Handbok för Restprodukter 2018; Jernkontoret: Stockholm, Sweden, 2018. [Google Scholar]
- Jansson, Å. Minimal Återverkan på Ljusbågsugnsslagg på Miljön; Bergsskolan i Filipstad: Filipstad, Sweden, 2000. [Google Scholar]
- Nilsson, N. Inverkan av MgO på Ljusbågsugnsslaggens Lakningsegenskaper; Luleå University of Technology: Luleå, Sweden, 2002. [Google Scholar]
- Lindström, B. Inverkan av Processparametrar på Ljusbågsugsslaggens Kromlakningsegenskaper; Luleå University of Technology: Luleå, Sweden, 2004. [Google Scholar]
- Lindström, B. Arbetet med att Förbättra Ljusbågugnsslaggens Kromlakningsegenskaper vid Ovakos Anläggning i Hofors; Ovako Steel: Hofors, Sweden, 2006. [Google Scholar]
- Fällman, A. Leaching of chromium and barium from steel slag in laboratory and field tests—A solubility controlled process? Waste Manag. 2000, 20, 149–154. [Google Scholar] [CrossRef]
- Aldrian, A.; Raith, J.G.; Höllen, D.; Pomberger, R. Influence of chromium containing spinels in an electric arc furnace slag on the leaching behaviour. J. Solid Waste Technol. Manag. 2015, 41, 357–365. [Google Scholar] [CrossRef]
- Strandkvist, I.; Björkman, B.; Engström, F. Synthesis and dissolution of slag minerals—A study of β-dicalcium silicate, pseudowollastonite and monticellite. Can. Metall. Q. 2015, 54, 446–454. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Di Cecca, C.; Le Saout, G.; Garcia-Diaz, E. The effect of microstructure on the leaching behaviour of electric arc furnace (EAF) carbon steel slag. Process Saf. Environ. Prot. 2016, 102, 810–821. [Google Scholar] [CrossRef]
- Strandkvist, I. Inverkan av Järnoxid på Ljusbågsugnsslaggens Kromlakningsegenskaper; Luleå University of Technology: Luleå, Sweden, 2010. [Google Scholar]
- Roininen, J.; Vaara, N.; Ylimaunu, J. Quality Control for Stainless Steel Slag Products. In Proceedings of the 4th European Slag Conference, Oulu, Finland, 20–21 June 2005; pp. 199–210. [Google Scholar]
- Geiseler, J.; Schlösser, R.; Scheel, R.; Koch, K.; Janke, D. Untersuchungen zum Hydratationsverhalten von synthetischen Magnesiowüstiten. Steel Res. 1987, 58, 210–214. [Google Scholar] [CrossRef]
- Strandkvist, I.; Sandström, Å.; Engström, F. Effect of FeO/MgO Ratio on Dissolution and Leaching of Magnesiowüstite. Steel Res. Int. 2017, 88, 1600322. [Google Scholar] [CrossRef]
- Qian, G.R.; Sun, D.D.; Tay, J.H.; Lai, Z.Y. Hydrothermal reaction and autoclave stability of Mg bearing RO phase in steel slag. Br. Ceram. Trans. 2002, 101, 159–164. [Google Scholar] [CrossRef]
- Schlösser, R.; Steffes, B. Commission of the European Communities. In Untersuchungen an Stahlwerksschlacken, Insbesondere im Hinblick auf Ihre Verwendung im Strassenbau; Kommission der Europäischen Gemeinschaften: Luxemburg, 1985. [Google Scholar]
- Mombelli, D.; Mapelli, C.; Di Cecca, C.; Barella, S.; Gruttadauria, A. Electric arc furnace slag: Study on leaching mechanisms and stabilization treatments. [Scorie da forno elettrico ad arco: Studio sui meccanismi di rilascio e trattamenti di stabilizzazione]. Metall. Ital. 2016, 108, 5–17. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd.: London, UK, 1997. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melancon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The efficiency of quartz addition on electric arc furnace (EAF) carbon steel slag stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef]
- Kilau, H.W.; Shah, I.D. Preventing Chromium Leaching from Waste Slag Exposed to Simulated Acid Precipitation: A Laboratory Study; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1984.
- Neuhold, S.; Van Zomeren, A.; Dijkstra, J.J.; Van der Sloot, H.A.; Drissen, P.; Algermissen, D.; Mudersbach, D.; Schüler, S.; Griessacher, T.; Raith, J.G.; et al. Investigation of possible leaching control mechanisms for chromium and vanadium in electric arc furnace (EAF) slags using combined experimental and modeling approaches. Minerals 2019, 9, 525. [Google Scholar] [CrossRef]
- Engström, F.; Adolfsson, D.; Samuelsson, C.; Sandström, Å.; Björkman, B. A study of the solubility of pure slag minerals. Miner. Eng. 2013, 41, 46–52. [Google Scholar] [CrossRef]
- Strandkvist, I.; Björkman, B.; Engström, F.; Pålsson, K. Chromium leaching from low-alloy EAF slag—Influence of ageing and FeO content. In Proceedings of the 14th ISIJ-VDEh Seminar, the 8th Japan-Nordic Countries Joint Symposium on Science and Technology of Process Metallurgy, ISIJ-VDeh-Jernkontoret Joint Symposium, Osaka, Japan, 15–16 April 2013; p. 57. [Google Scholar]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R., II. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).