Combined Effect of Ferrous Ion and Biochar on Cadmium and Arsenic Accumulation in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pot Experiments
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Effective Tiller Number
3.2. Soil pH
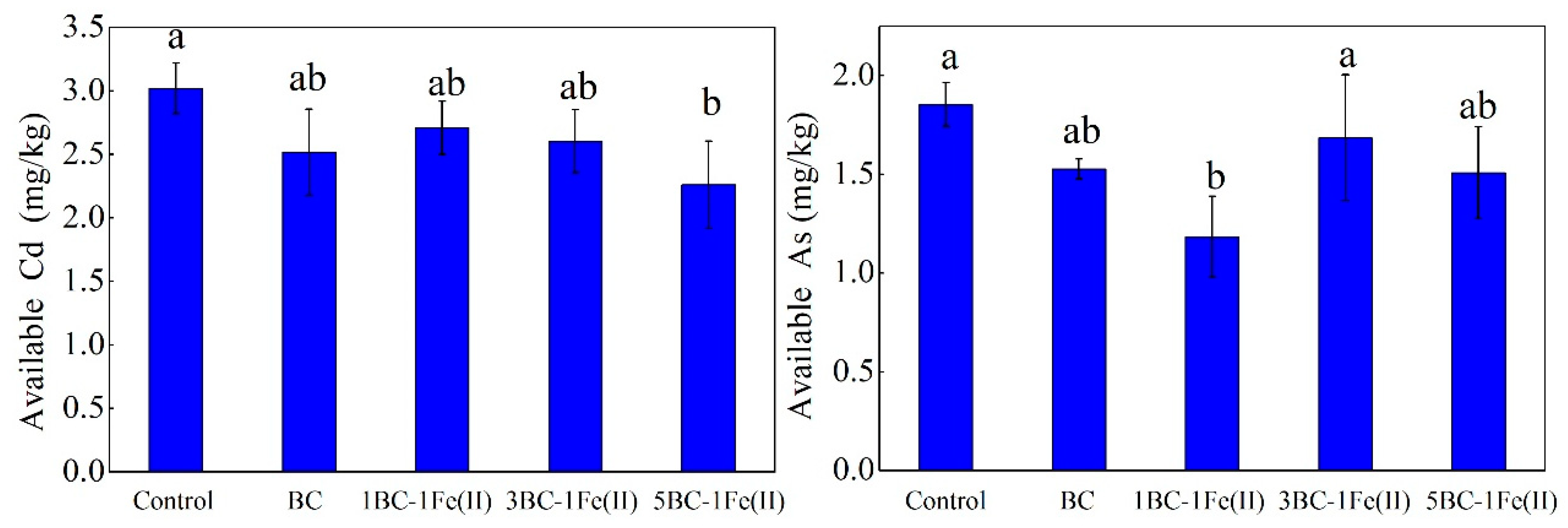
3.3. Available Cd and As
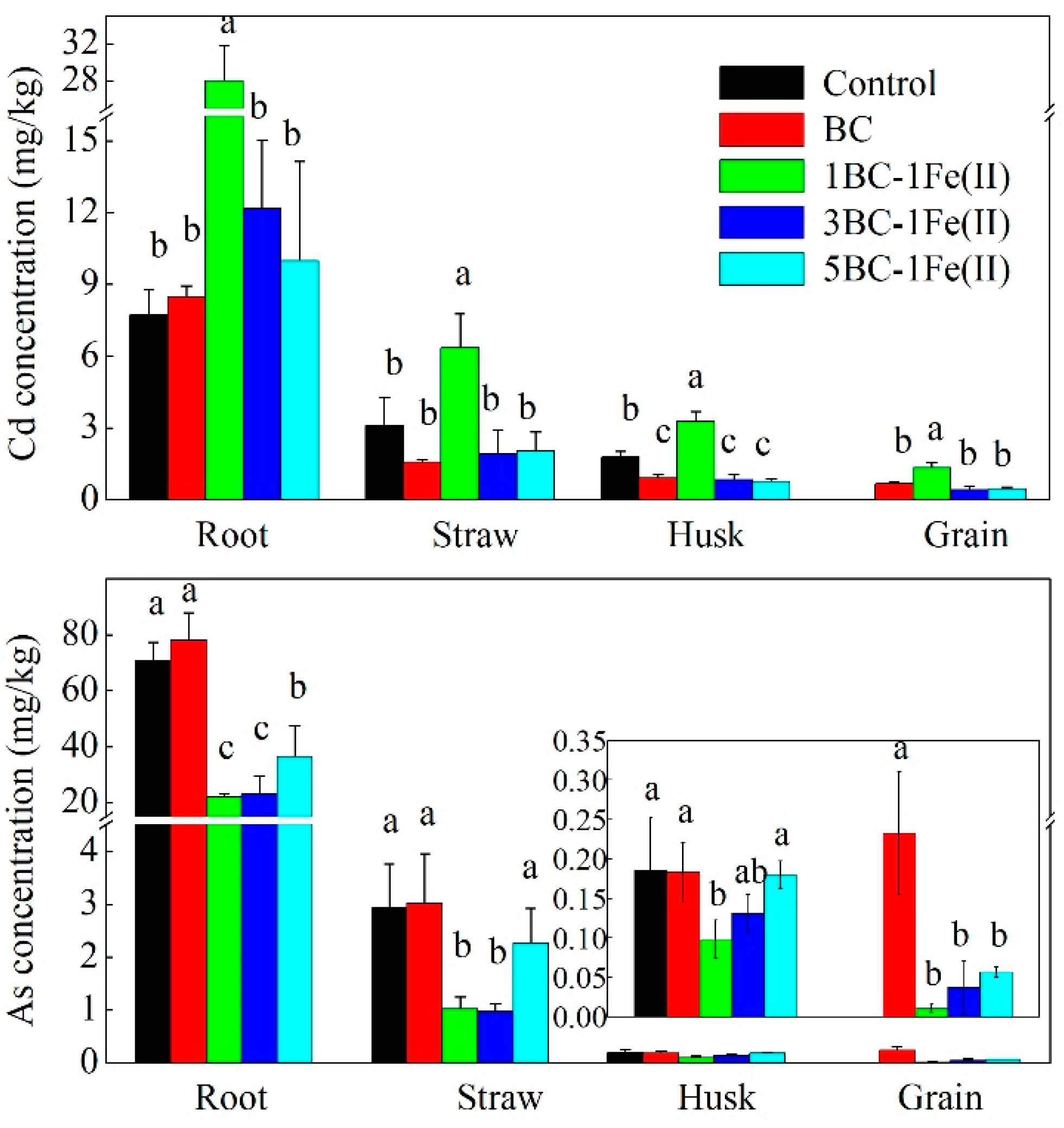
3.4. Cd and As Contents in Rice Tissues
3.5. Comparison of Cd and As Translocation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Tian, G.; Jiang, D.; Zhang, C.; Kong, L. Cadmium (Cd) distribution and contamination in chinese paddy soils on national scale. Environ. Sci. Pollut. Res. 2016, 23, 17941–17952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; Mcgrath, S.P. Soil contamination in china: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Tang, Z.; Wang, P.; Zhao, F.J. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in chinese rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Yang, X.P.; Wang, P.; Wang, Z.X.; Li, M.; Zhao, F.J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in xiangtan, southern china. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; Frohne, T. Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere 2016, 142, 41–47. [Google Scholar] [CrossRef]
- Hu, P.; Ouyang, Y.; Wu, L.; Shen, L.; Luo, Y.; Christie, P. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar. J. Environ. Sci. 2015, 27, 225–231. [Google Scholar] [CrossRef]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Peng, X.; Deng, Y.E.; Peng, Y.; Yue, K. Effects of biochar addition on toxic element concentrations in plants: A meta-analysis. Sci. Total Environ. 2018, 616, 970–977. [Google Scholar] [CrossRef]
- He, E.K.; Yang, Y.X.; Xu, Z.B.; Qiu, H.; Yang, F.; Peijnenburg, W.J.G.M.; Zhang, W.H.; Qiu, R.L.; Wang, S.Z. Two years of aging influences the distribution and lability of metal(loid)s in a contaminated soil amended with different biochars. Sci. Total Environ. 2019, 673, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Bian, P.Y.; Zhang, J.J.; Zhang, C.L.; Huang, H.; Rong, Q.; Wu, H.X.; Li, X.; Xu, M.M.; Liu, Y.; Ren, S.W. Effects of silk-worm excrement biochar combined with different iron-based materials on the speciation of cadmium and lead in soil. Appl. Sci. 2018, 8, 1999. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.L.; Zhang, P.; Cao, M.Z.; Xu, G.P.; Wu, H.X.; Zhang, J.J.; Li, C.Z.; Rong, Q. Effects of biochar amendment on the sorption and degradation of atrazine in different soils. Soil Sediment Contam. 2018, 27, 643–657. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Marchiol, L.; Delle Vedove, G.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef]
- Yue, L.; Lian, F.; Han, Y.; Bao, Q.L.; Wang, Z.Y.; Xing, B.S. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: Implications for agronomic benefits and potential risk. Sci. Total Environ. 2019, 656, 9–18. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Wang, Y.; Zhao, D.Y.; Pan, G. Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles. Chin. Sci. Bull. 2010, 55, 365–372. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.Q.; Yu, H.Y.; Li, F.B.; Liu, T.X.; Wu, W.J.; Liu, C.P.; Liu, C.S.; Zhang, X.Q. Enhanced immobilization of arsenic and cadmium in a paddy soil by combined applications of woody peat and Fe(NO3)3: Possible mechanisms and environmental implications. Sci. Total Environ. 2019, 649, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Subacz, J.L.; Barnett, M.O.; Jardine, P.M.; Stewart, M.A. Decreasing arsenic bioaccessibility/bioavailability in soils with iron amendments. J. Environ. Sci. Eng. A 2007, 42, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Wang, L.L.; Chen, J.; Xu, S.Q.; Hou, H.J.; Shi, Y.; Zhang, J.D.; Ma, M.; Tsang, D.C.W.; et al. Transformation of arsenic during realgar tailings stabilization using ferrous sulfate in a pilot-scale treatment. Sci. Total Environ. 2019, 668, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Komarek, M.; Vanek, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Li, F.B.; Yuan, C.L.; Li, B.; Liu, T.X.; Liu, C.S.; Du, Y.H.; Liu, C.P. The translocation of antimony in soil-rice system with comparisons to arsenic: Alleviation of their accumulation in rice by simultaneous use of fe(ii) and no3. Sci. Total Environ. 2019, 650, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.N.; Zhang, S.; Duan, D.C.; Liang, X.Q.; Shi, J.Y.; Xu, J.M.; Tang, X.J. Effects of ferrous sulfate amendment and water management on rice growth and metal(loid) accumulation in arsenic and lead co-contaminated soil. Environ. Sci. Pollut. Res. 2018, 25, 8888–8902. [Google Scholar] [CrossRef] [PubMed]
- Fresno, T.; Moreno, J.E.; Peñalosa, J.M. Assessing the combination of iron sulfate and organic materials as amendment for an arsenic and copper contaminated soil. A chemical and ecotoxicological approach. Chemosphere 2016, 165, 539–546. [Google Scholar] [CrossRef]
- Fresno, T.; Moreno, J.E.; Zornoza, P.; Peñalosa, J.M.; Fresno, T.; Moreno, J.E.; Zornoza, P.; Peñalosa, J.M.; Fresno, T.; Moreno, J.E. Aided phytostabilisation of As- and Cu-contaminated soils using white lupin and combined iron and organic amendments. J. Environ. Manag. 2017, 205, 142–150. [Google Scholar] [CrossRef]
- Lu, M.X.; Li, F.Y.; Zhng, C.L.; Li, C.Z.; Yang, W.W.; Zhang, J.J.; Huang, H.; Wu, H.H. Effects of different soil amendments on the immobilization and remediation of cadmium and arsenic in soil. J. Guangxi Univ. 2016, 41, 1667–1675. (In Chinese) [Google Scholar]
- Bao, S.D. Soil Agro-Chemistrical Analysis (Third Edition); China Agriculture Press: Beijing, China, 2018; pp. 39–83. (In Chinese) [Google Scholar]
- DIN ISO. ISO-14870, 2011: Soil Quality—Extraction of Trace Elements by Buffered Dtpa Solution, Standardization; Din ISO: Berlin, Germany, 2001. [Google Scholar]
- Woolson, E.A.; Axley, J.H.; Kearney, P.C. Correlation between available soil arsenic, estimated by six methods, and response of corn (Zea mays l.)1. Soil Sci. Soc. Am. J. 1971, 35, 101–105. [Google Scholar] [CrossRef]
- Huang, G.X.; Ding, C.F.; Hu, Z.Y.; Cui, C.H.; Zhang, T.L.; Wang, X.X. Topdressing iron fertilizer coupled with pre-immobilization in acidic paddy fields reduced cadmium uptake by rice (Oryza sativa l.). Sci. Total Environ. 2018, 636, 1040–1047. [Google Scholar] [CrossRef]
- Liu, C.P.; Luo, C.L.; Gao, Y.; Li, F.B.; Lin, L.W.; Wu, C.A.; Li, X.D. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern china. Environ. Pollut. 2010, 158, 820–826. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.Y. Understanding the molecular bases of agronomic trait improvement in rice. Plant Cell 2019, 31, 1416–1417. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets – Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Zhang, J.L.; Christie, P.; Zhang, F.S. Influence of iron plaque on uptake and accumulation of cd by rice (Oryza sativa L.) seedlings grown in soil. Sci. Total Environ. 2008, 394, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Vromman, D.; Lutts, S.; Lefevre, I.; Somer, L.; De Vreese, O.; Slejkovec, Z.; Quinet, M. Effects of simultaneous arsenic and iron toxicities on rice (Oryza sativa L.) development, yield-related parameters and as and fe accumulation in relation to as speciation in the grains. Plant. Soil. 2013, 371, 199–217. [Google Scholar] [CrossRef]
- Yi, H.Z.; Zhu, Y.G.; Li, M.; Zhang, L.G.; Cao, Z.H.; Smith, E.A. Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ. Pollut. 2007, 147, 387–393. [Google Scholar]
- Hooda, P.S.; Alloway, B.J. Cadmium and lead sorption behaviour of selected english and indian soils. Geoderma 1998, 84, 121–134. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic transformations in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719. [Google Scholar] [CrossRef]
- Chen, L.J.; Jiang, Y.J.; Liang, C.; Luo, Y.; Xu, Q.S.; Han, C.; Zhao, Q.G.; Sun, B. Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 2019, 7, 77. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of as, cr, cu, pb and zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef]
- Cui, Y.S.; Xin, D.; Weng, L.P.; Riemsdijk, W.H.V. Assessment of in situ immobilization of lead (pPb) and arsenic (As) in contaminated soils with phosphate and iron: Solubility and bioaccessibility. Water Air Soil Pollut. 2010, 213, 95–104. [Google Scholar] [CrossRef]
- Olowe, A.A.; Génin, J.M.R. The mechanism of oxidation of ferrous hydroxide in sulphated aqueous media: Importance of the initial ratio of the reactants. Corros Sci. 1991, 32, 965–984. [Google Scholar] [CrossRef]
- Lin, R.G.; Spicer, R.L.; Tungate, F.L.; Davis, B.H. A study of the oxidation of ferrous hydroxide in slightly basic solution to produce γ-FeOOH. Colloids Surf. A 1996, 113, 79–96. [Google Scholar] [CrossRef]
- Wang, R.Z.; Wei, S.; Jia, P.H.; Liu, T.; Hou, D.D.; Xie, R.H.; Lin, Z.; Ge, J.; Qiao, Y.B.; Chang, X.Y.; et al. Biochar significantly alters rhizobacterial communities and reduces cd concentration in rice grains grown on cd-contaminated soils. Sci. Total Environ. 2019, 676, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Zouboulis, A.I. Application of biological processes for the removal of arsenic from groundwaters. Water Res. 2004, 38, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.; Fleming, P.; Wang, B.Q.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Bolan, N.S.; Naidu, R.; Syers, J.K.; Tillman, R.W. Surface charge and solute interactions in soils. Adv. Agron. 1999, 67, 87–140. [Google Scholar]
- Harvey, O.R.; Herbert, B.E.; Rhue, R.D.; Kuo, L.J. Metal interactions at the biochar-water interface: Energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef]
- Fresno, T.; Penalosa, J.M.; Flagmeier, M.; Moreno-Jimenez, E. Aided phytostabilisation over two years using iron sulphate and organic amendments: Effects on soil quality and rye production. Chemosphere 2020, 240, 124827. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.G.; Liu, W.J.; Meharg, A.A. Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol. 2005, 165, 91–97. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kasuga, J.; Taiki, N.; Makino, T.; Arao, T. Inhibition of arsenic accumulation in japanese rice by the application of iron and silicate materials. Catena 2015, 135, 328–335. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ohkura, T.; Takahashi, Y.; Maejima, Y.; Arao, T. Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ. Sci. Technol. 2014, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Fujiwara, T. Arabidopsis nip1;1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol. 2009, 50, 1977–1981. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2018, 115, 4950–4951. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. In Annual Review of Plant Biology; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 61, pp. 535–559. [Google Scholar]
- Tanaka, K.; Fujimaki, S.; Fujiwara, T.; Yoneyama, T.; Hayashi, H. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (oryza sativa l.). Soil Sci. Plant Nutr. 2007, 53, 72–77. [Google Scholar] [CrossRef]
- Kato, M.; Ishikawa, S.; Inagaki, K.; Chiba, K.; Hayashi, H.; Yanagisawa, S.; Yoneyama, T. Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (oryza sativa L.). Soil Sci. Plant Nutr. 2010, 56, 839–847. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kasuga, J.; Makino, T.; Arao, T. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic. Environ. Exp. Bot. 2016, 125, 42–51. [Google Scholar] [CrossRef]


| Item | pH | Total Organic Carbon (TOC) % | Cation Exchange Capacity (CEC) cmol/kg | Available N mg/kg | Available P mg/kg | Available K mg/kg | Total As mg/kg | Total Cd mg/kg | Available As mg/kg | Available Cd mg/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 7.6 | 14.6 | 9.25 | 131.5 | 26.5 | 122.0 | 27.3 | 7.7 | 1.8 | 3.0 |
| Biochar | 9.4 | 227.8 | 71.59 | ND | ND | ND | ND | ND | ND | ND |
| Treatments | Effective Tiller Number (per Plant) |
|---|---|
| Control | 4.1 ± 0.2 c |
| BC | 3.6 ± 0.7 c |
| 1BC-1Fe(II) | 9.2 ± 0.2 a |
| 3BC-1Fe(II) | 7.1 ± 1.1 b |
| 5BC-1Fe(II) | 4.7 ± 0.6 c |
| Treatment | BAFroot | BAFstraw | BAFhusk | BAFgrain | TFrs | TFsg |
|---|---|---|---|---|---|---|
| Control | 1.05 ± 0.24 c | 0.41 ± 0.17 b | 0.23 ± 0.04 b | -- | 0.37 ± 0.09 a | -- |
| BC | 1.09 ± 0.09 bc | 0.20 ± 0.11 c | 0.12 ± 0.03 c | 0.09 ± 0.01 b | 0.19 ± 0.02 b | 0.47 ± 0.05 a |
| 1BC-1Fe(II) | 3.62 ± 0.65 a | 0.84 ± 0.20 a | 0.43 ± 0.07 a | 0.17 ± 0.03 a | 0.24 ± 0.07 b | 0.25 ± 0.06 b |
| 3BC-1Fe(II) | 1.64 ± 0.46 b | 0.29 ± 0.14 bc | 0.11 ± 0.03 c | 0.06 ± 0.02 c | 0.17 ± 0.04 b | 0.21 ± 0.02 b |
| 5BC-1Fe(II) | 1.54 ± 0.30 bc | 0.26 ± 0.12 bc | 0.11 ± 0.04 c | 0.06 ± 0.01 c | 0.21 ± 0.03 b | 0.29 ± 0.08 b |
| Treatment | BAFroot | BAFstraw | BAFhusk (×10−2) | BAFgrain (×10−2) | TFrs | TFsg |
|---|---|---|---|---|---|---|
| Control | 2.69 ± 0.29 a | 0.12 ± 0.03 a | 0.64 ± 0.21 a | -- | 0.04 ± 0.01 b | -- |
| BC | 2.86 ± 0.44 a | 0.11 ± 0.04 a | 0.66 ± 0.15 ab | 0.90 ± 0.30 a | 0.04 ± 0.01 b | 0.13 ± 0.04 a |
| 1BC-1Fe(II) | 0.83 ± 0.10 b | 0.04 ± 0.01 b | 0.38 ± 0.10 b | 0.04 ± 0.02 c | 0.04 ± 0.01 b | 0.02 ± 0.01 b |
| 3BC-1Fe(II) | 0.86 ± 0.29 b | 0.04 ± 0.01 b | 0.52 ± 0.17 ab | 0.20 ± 0.10 b | 0.04 ± 0.01 b | 0.03 ± 0.01 b |
| 5BC-1Fe(II) | 1.46 ± 0.44 c | 0.08 ± 0.03 c | 0.60 ± 0.14 ab | 0.20 ± 0.01 b | 0.06 ± 0.01 a | 0.03 ± 0.02 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Q.; Zhong, K.; Li, F.; Huang, H.; Li, C.; Nong, X.; Zhang, C. Combined Effect of Ferrous Ion and Biochar on Cadmium and Arsenic Accumulation in Rice. Appl. Sci. 2020, 10, 300. https://doi.org/10.3390/app10010300
Rong Q, Zhong K, Li F, Huang H, Li C, Nong X, Zhang C. Combined Effect of Ferrous Ion and Biochar on Cadmium and Arsenic Accumulation in Rice. Applied Sciences. 2020; 10(1):300. https://doi.org/10.3390/app10010300
Chicago/Turabian StyleRong, Qun, Kai Zhong, Fangyuan Li, He Huang, Chuanzhang Li, Xinyu Nong, and Chaolan Zhang. 2020. "Combined Effect of Ferrous Ion and Biochar on Cadmium and Arsenic Accumulation in Rice" Applied Sciences 10, no. 1: 300. https://doi.org/10.3390/app10010300
APA StyleRong, Q., Zhong, K., Li, F., Huang, H., Li, C., Nong, X., & Zhang, C. (2020). Combined Effect of Ferrous Ion and Biochar on Cadmium and Arsenic Accumulation in Rice. Applied Sciences, 10(1), 300. https://doi.org/10.3390/app10010300




