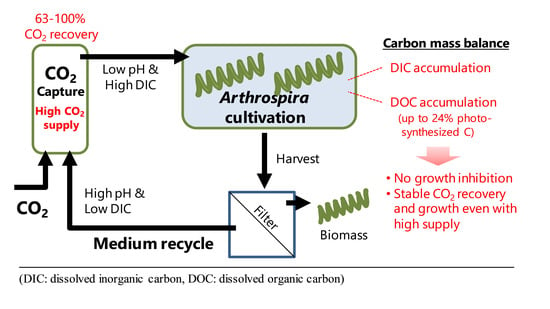
Carbon Mass Balance in Arthrospira platensis Culture with Medium Recycle and High CO2 Supply
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Strain, Growth Medium, and Inoculum Preparation
2.2. Experimental Design and Culture Conditions
2.3. Experimental Procedures
2.4. Analytical Procedures
2.5. Calculations and Statistical Analysis
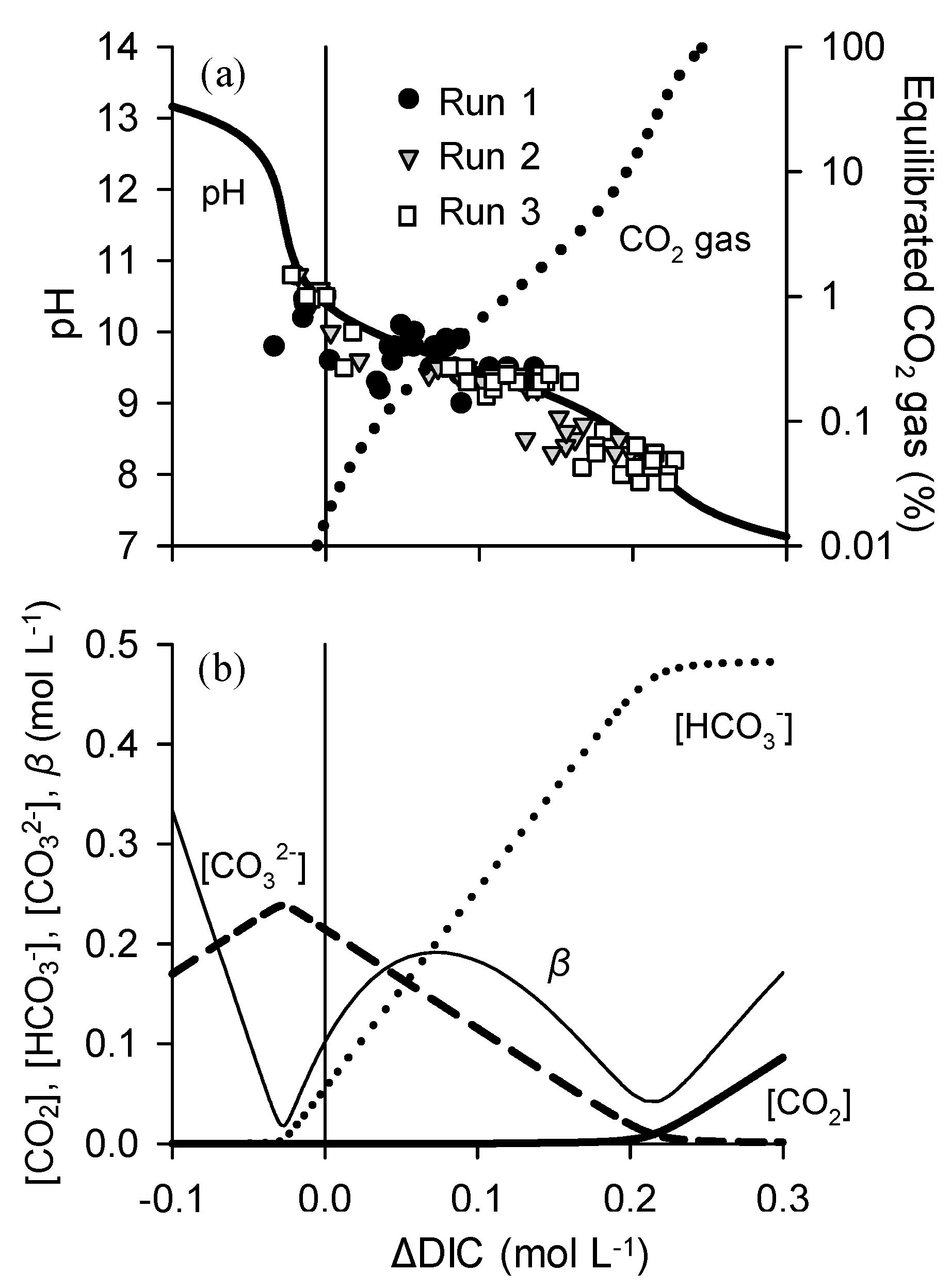
2.5.1. Modeling pH Variation with DIC Change
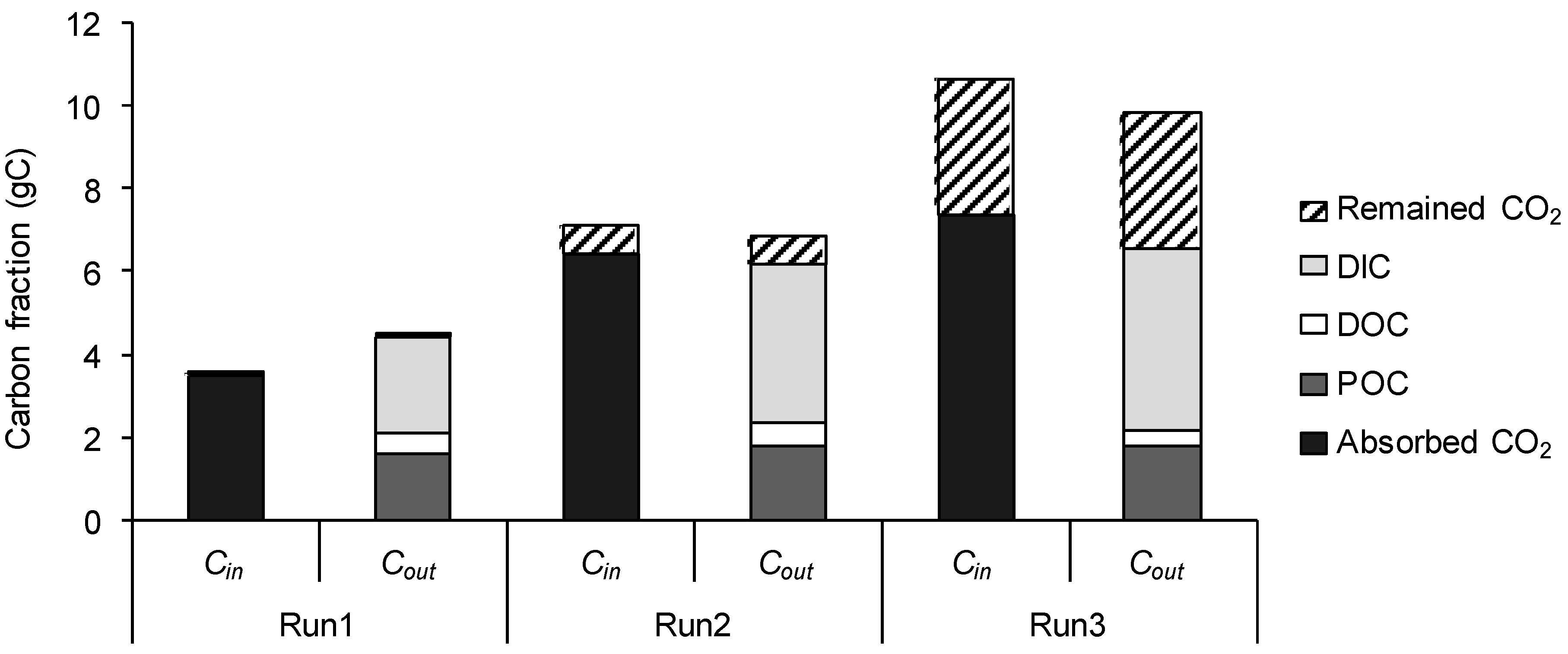
2.5.2. Biomass Production, Carbon Fixation, and Carbon Mass Balance
2.5.3. Statistical Analysis
3. Results
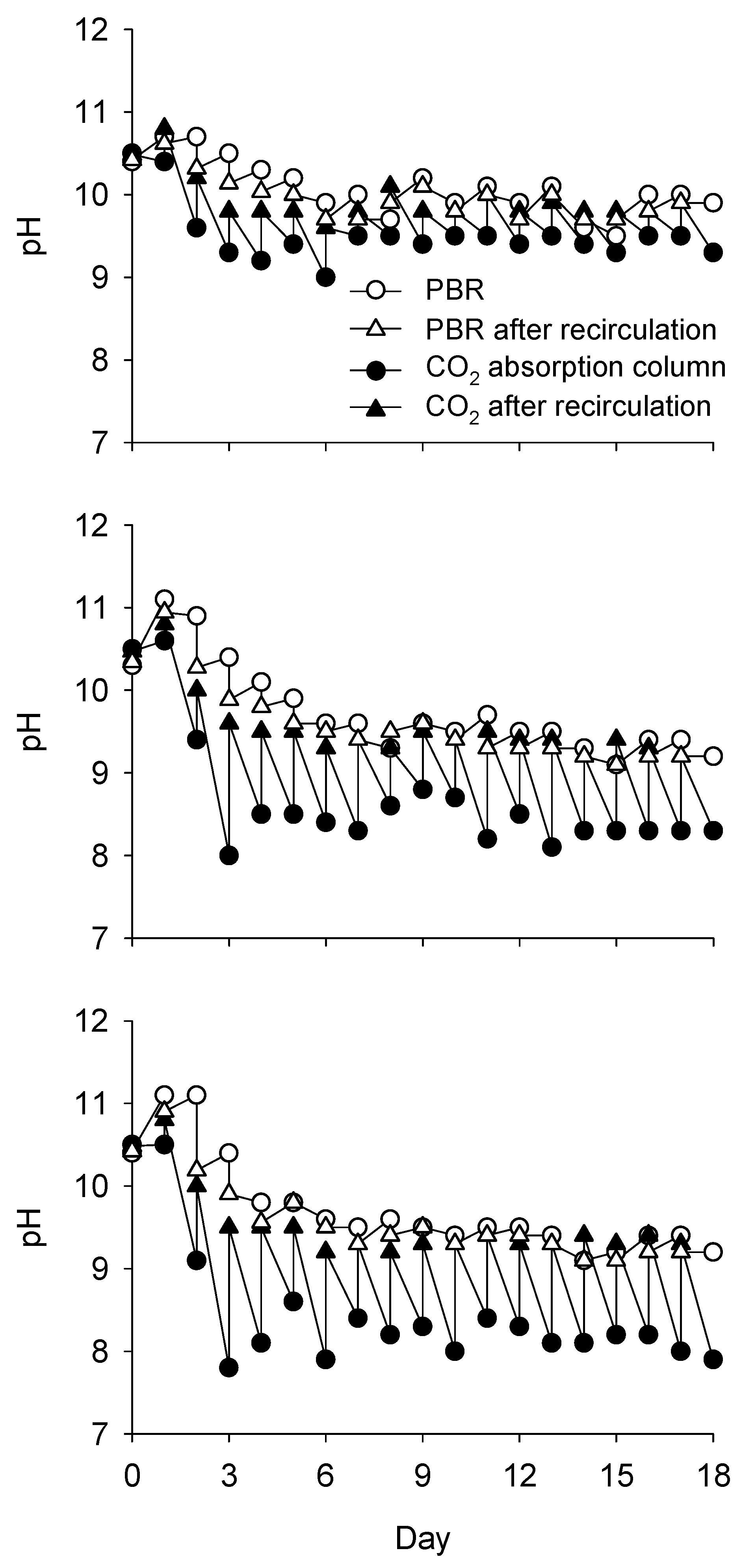
3.1. Change in pH and DIC Concentration with CO2 Absorption
3.2. Algal Growth and DOC Excretion
3.3. Carbon Mass Balance
4. Discussion
4.1. Effect of High CO2 Supply
4.2. Effect of Medium Recycling
4.3. Evaluation of CO2 Recovery with Media-Recycled Arthrospira Culture
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Furmaniak, M.A.; Misztak, A.E.; Franczuk, M.D.; Wilmotte, A.; Waleron, M.; Waleron, K.F. Edible cyanobacterial genus Arthrospira: Actual state of the art in cultivation methods, genetics, and application in medicine. Front. Microbiol. 2017, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Belay, A. Biology and industrial production of Arthrospira (Spirulina). In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 339–358. ISBN 9780470673898. [Google Scholar]
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Agustini, T.W.; Suzery, M.; Sutrisnanto, D.; Ma’ruf, W.F. Hadiyanto Comparative study of bioactive substances extracted from fresh and dried Spirulina sp. Procedia Environ. Sci. 2015, 23, 282–289. [Google Scholar] [CrossRef]
- Choi, W.Y.; Sim, J.-H.; Lee, J.-Y.; Kang, D.H.; Lee, H.Y. Increased anti-inflammatory effects on LPS-induced microglia cells by Spirulina maxima extract from ultrasonic process. Appl. Sci. 2019, 9, 2144. [Google Scholar] [CrossRef]
- Choi, W.; Kang, D.; Heo, S.-J.; Lee, H. Enhancement of the neuroprotective effect of fermented Spirulina maxima associated with antioxidant activities by ultrasonic extraction. Appl. Sci. 2018, 8, 2469. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à L’étude D’une Cyanophycée: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthèse de Spirulina maxima (Setch et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Depraetere, O.; Pierre, G.; Noppe, W.; Vandamme, D.; Foubert, I.; Michaud, P.; Muylaert, K. Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res. 2015, 10, 48–54. [Google Scholar] [CrossRef]
- Mejia-da-Silva, L.d.C.; Matsudo, M.C.; Morocho-Jacome, A.L.; de Carvalho, J.C.M. Application of physicochemical treatment allows reutilization of Arthrospira platensis exhausted medium: An investigation of reusing medium in Arthrospira platensis cultivation. Appl. Biochem. Biotechnol. 2018, 186, 40–53. [Google Scholar] [CrossRef]
- Chi, Z.; O’Fallon, J.V.; Chen, S. Bicarbonate produced from carbon capture for algae culture. Trends Biotechnol. 2011, 29, 537–541. [Google Scholar] [CrossRef]
- Arroyo, C.A.; Contreras, J.L.; Zeifert, B.; Ramírez, C. CO2 capture of the gas emission, using a catalytic converter and airlift bioreactors with the microalga Scenedesmus dimorphus. Appl. Sci. 2019, 9, 3212. [Google Scholar] [CrossRef]
- Kumar, K.; Dasgupta, C.N.; Nayak, B.; Lindblad, P.; Das, D. Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour. Technol. 2011, 102, 4945–4953. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of carbon capture technology: Microalgal biorefinery concept and state-of-the-art. Front. Mar. Sci. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Klamczynska, B.; Mooney, W.D. Heterotrophic Microalgae. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 327–339. ISBN 9780128027769. [Google Scholar]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Kishi, M.; Toda, T. Carbon fixation properties of three alkalihalophilic microalgal strains under high alkalinity. J. Appl. Phycol. 2017, 30, 1–10. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef]
- Tripathi, R.; Singh, J.; Thakur, I.S. Characterization of microalga Scenedesmus sp. ISTGA1 for potential CO2 sequestration and biodiesel production. Renew. Energy 2015, 74, 774–781. [Google Scholar] [CrossRef]
- Chi, Z.; Xie, Y.; Elloy, F.; Zheng, Y.; Hu, Y.; Chen, S. Bicarbonate-based Integrated Carbon Capture and Algae Production System with alkalihalophilic cyanobacterium. Bioresour. Technol. 2013, 133, 513–521. [Google Scholar] [CrossRef]
- González-López, C.V.; Acién Fernández, F.G.; Fernández-Sevilla, J.M.; Sánchez Fernández, J.F.; Molina Grima, E. Development of a process for efficient use of CO2 from flue gases in the production of photosynthetic microorganisms. Biotechnol. Bioeng. 2012, 109, 1637–1650. [Google Scholar] [CrossRef]
- Canon-Rubio, K.A.; Sharp, C.E.; Bergerson, J.; Strous, M.; De la Hoz Siegler, H. Use of highly alkaline conditions to improve cost-effectiveness of algal biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 1611–1622. [Google Scholar] [CrossRef]
- Cui, H.; Yang, Z.; Lu, Z.; Wang, Q.; Liu, J.; Song, L. Combination of utilization of CO2 from flue gas of biomass power plant and medium recycling to enhance cost-effective Spirulina production. J. Appl. Phycol. 2019, 31, 2175–2185. [Google Scholar] [CrossRef]
- Ogawa, T.; Terui, G. Studies on the growth of Spirulina platensis: (I) On the pure culture of Spirulina platensis. J. Ferment. Technol. 1970, 48, 361–367. [Google Scholar]
- Zeebe, R.; Wolf-Gladrow, D. CO2 in Seawater-Equilibrium, Kinetics, Isotopes, 1st ed.; Halpern, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 978-0-444-50946-8. [Google Scholar]
- Camacho Rubio, F.; Acién Fernández, F.G.; Sánchez Pérez, J.A.; García Camacho, F.; Molina Grima, E. Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnol. Bioeng. 1999, 62, 71–86. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1993; Volume 3, ISBN 0471511854. [Google Scholar]
- Zengling, M.; Kunshan, G.; Teruo, W. Effects of dissolved organic matter on the growth and pigments synthesis of Spirulina platensis (Arthospira). Prog. Nat. Sci. 2006, 16, 50–54. [Google Scholar] [CrossRef]
- Sha, J.; Lu, Z.; Ye, J.; Wang, G.; Hu, Q.; Chen, Y.; Zhang, X. The inhibition effect of recycled Scenedesmus acuminatus culture media: Influence of growth phase, inhibitor identification and removal. Algal Res. 2019, 42, 101612. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Jiménez, C.; Figueroa, F.L.; Niell, F.X. Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J. Appl. Phycol. 1998, 10, 461–469. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Chen, W.; Shen, H.; Han, D.; Li, Y.; Jones, H.D.T.; Timlin, J.A.; Hu, Q. Basic culturing and analytical measurement techniques. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 37–68. ISBN 9781118567166. [Google Scholar]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Li, M.; Xu, Z.; Zhu, W. Effects of Ca and Mg levels on colony formation and EPS content of cultured M. aeruginosa. Procedia Environ. Sci. 2011, 10, 1452–1458. [Google Scholar] [CrossRef]
- Subba Rao, D. Cultivation, growth media, division rates and applications of Dunaliella species. In The Alga Dunaliella; Ben-Amotz, A., Polle, J.E.W., Subba Rao, D.V., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 45–89. ISBN 9781578085453. [Google Scholar]
- Zheng, Y.; Li, T.; Yu, X.; Bates, P.D.; Dong, T.; Chen, S. High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl. Energy 2013, 108, 281–287. [Google Scholar] [CrossRef]


| Run | CO2 Supply Rate | Headspace | Headspace CO2 | CO2 in Gasbag | N2 in Gasbag a | |
|---|---|---|---|---|---|---|
| (gC L-PBR−1 d−1) | (gC L-Abs−1 d−1) | (L) | (%) | (L) | (L) | |
| 1 | 0.20 | 0.49 | 0.73 | 55 | 0 | 0.40 |
| 2 | 0.39 | 0.98 | 0.72 | 100 | 0.07 | 0.74 |
| 3 | 0.59 | 1.5 | 0.74 | 100 | 0.45 | 0.75 |
| Run | CO2 Supply Rate | CO2 Recovery Rate, RCO2 | CO2 Removal Efficiency | pH before CO2 Supply | pH after CO2 Supply |
|---|---|---|---|---|---|
| (gC L-PBR−1 d−1) | (gC L-PBR−1 d−1) | (%) | |||
| 1 | 0.20 | 0.227 ± 0.052 | 115 b | 9.85 ± 0.08 | 9.43 ± 0.09 |
| 2 | 0.39 | 0.369 ± 0.058 | 94 | 9.35 ± 0.11 | 8.33 ± 0.17 |
| 3 | 0.59 | 0.369 ± 0.055 | 63 | 9.34 ± 0.05 | 8.13 ± 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishi, M.; Yamada, Y.; Katayama, T.; Matsuyama, T.; Toda, T. Carbon Mass Balance in Arthrospira platensis Culture with Medium Recycle and High CO2 Supply. Appl. Sci. 2020, 10, 228. https://doi.org/10.3390/app10010228
Kishi M, Yamada Y, Katayama T, Matsuyama T, Toda T. Carbon Mass Balance in Arthrospira platensis Culture with Medium Recycle and High CO2 Supply. Applied Sciences. 2020; 10(1):228. https://doi.org/10.3390/app10010228
Chicago/Turabian StyleKishi, Masatoshi, Yukina Yamada, Tomoyo Katayama, Tatsushi Matsuyama, and Tatsuki Toda. 2020. "Carbon Mass Balance in Arthrospira platensis Culture with Medium Recycle and High CO2 Supply" Applied Sciences 10, no. 1: 228. https://doi.org/10.3390/app10010228
APA StyleKishi, M., Yamada, Y., Katayama, T., Matsuyama, T., & Toda, T. (2020). Carbon Mass Balance in Arthrospira platensis Culture with Medium Recycle and High CO2 Supply. Applied Sciences, 10(1), 228. https://doi.org/10.3390/app10010228