A Three-Dimensional Tracking Method with the Self-Calibration Functions of Coaxiality and Magnification for Single Fluorescent Nanoparticles
Abstract
Featured Application
Abstract
1. Introduction
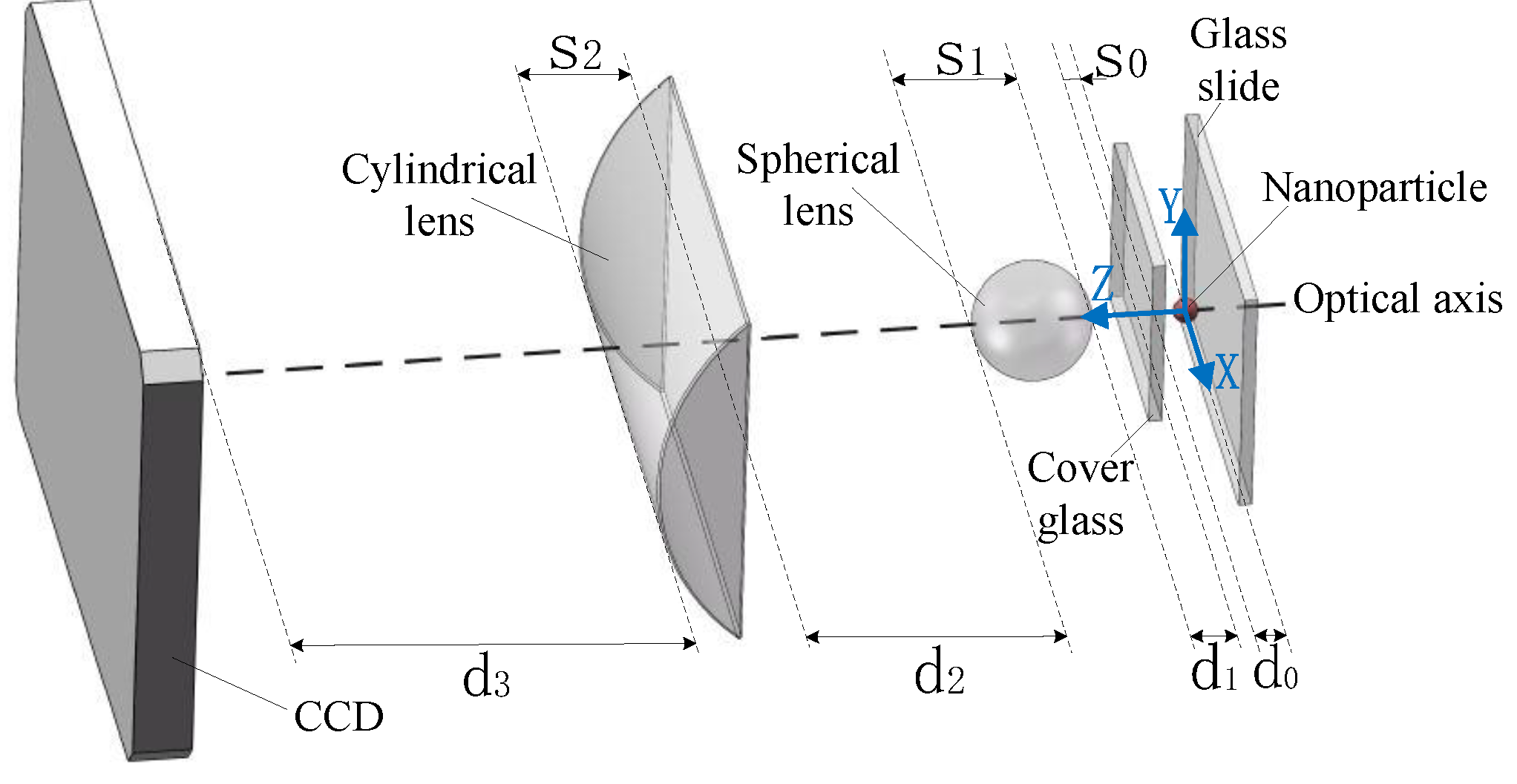
2. Micro-Imaging System
2.1. Ray Transfer Matrix of the Micro-Imaging System
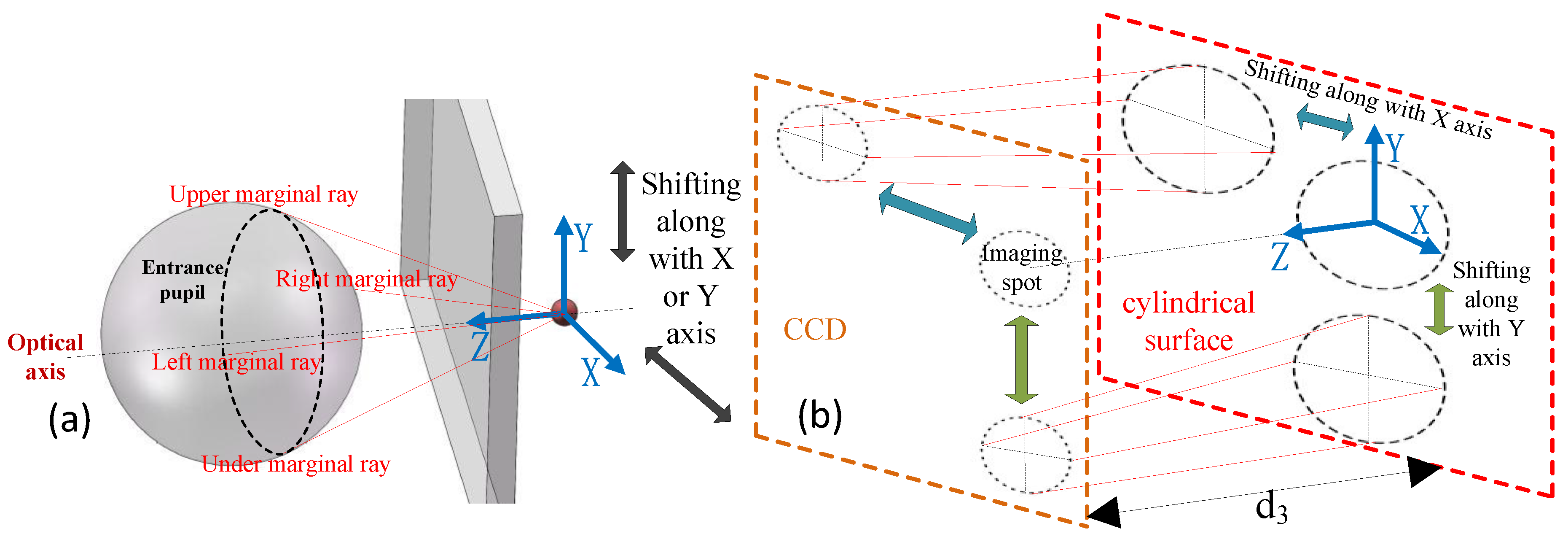
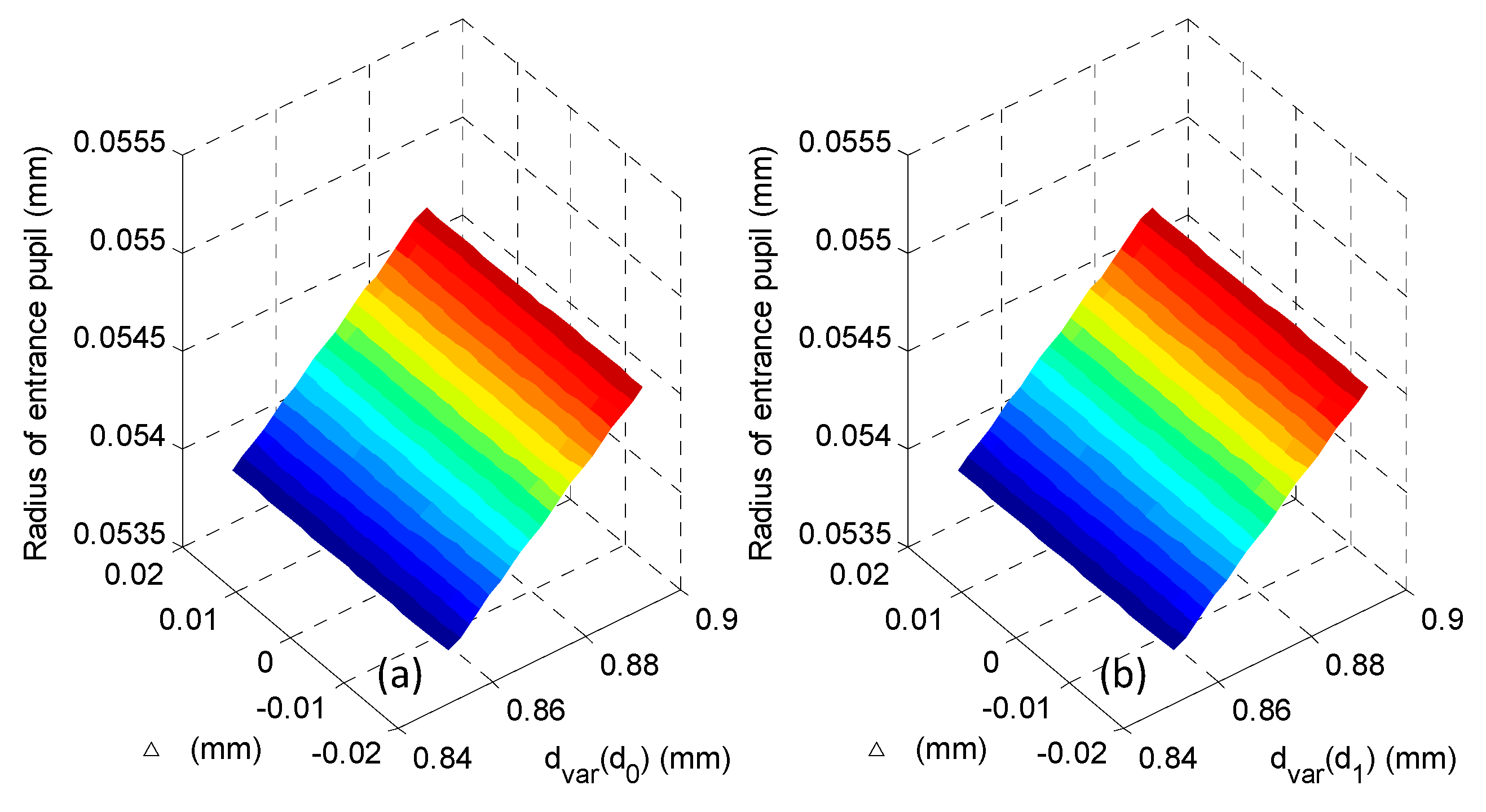
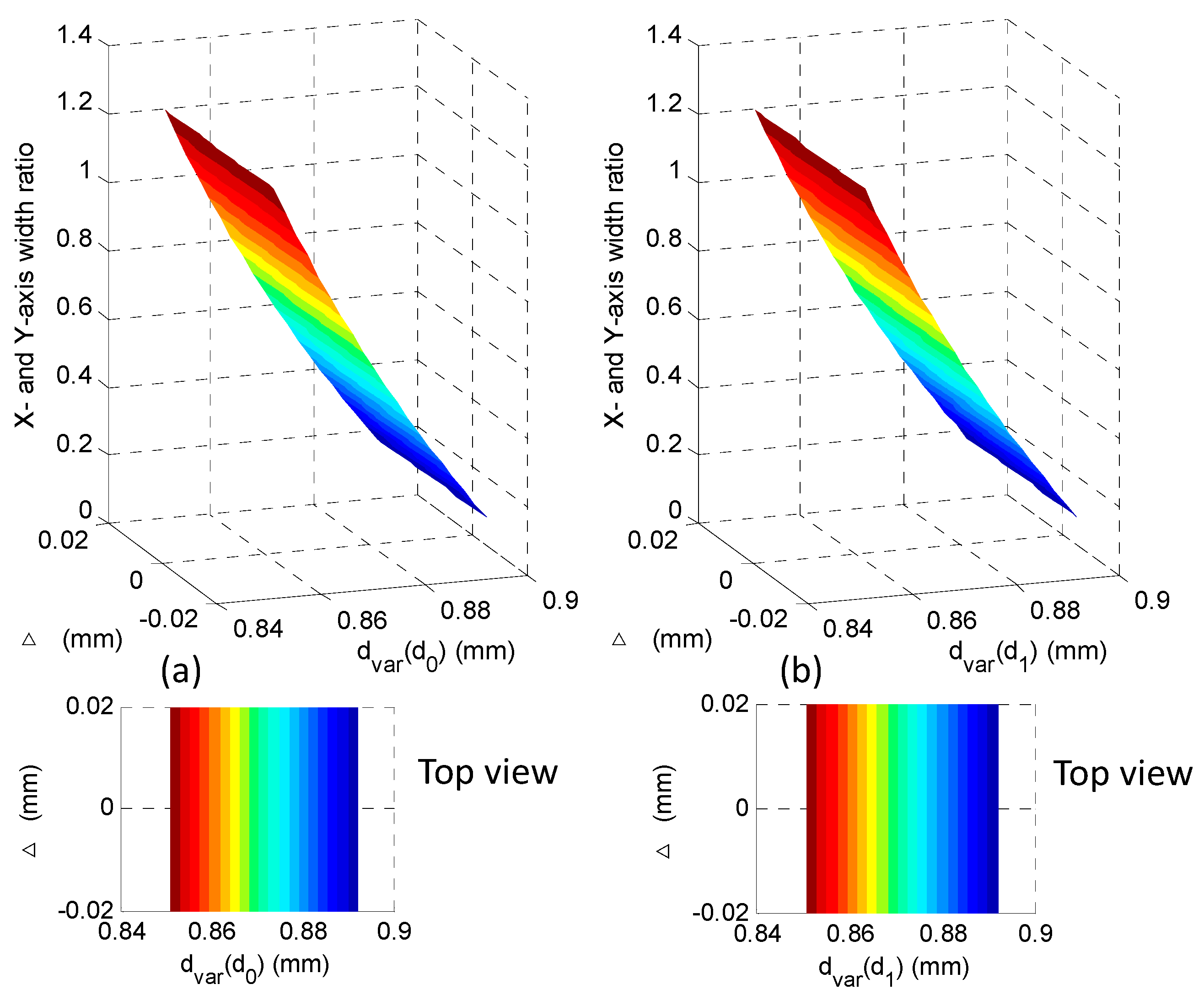
2.2. Analysis of the Micro-Imaging System
3. Layout for Achieving Micro-Imaging
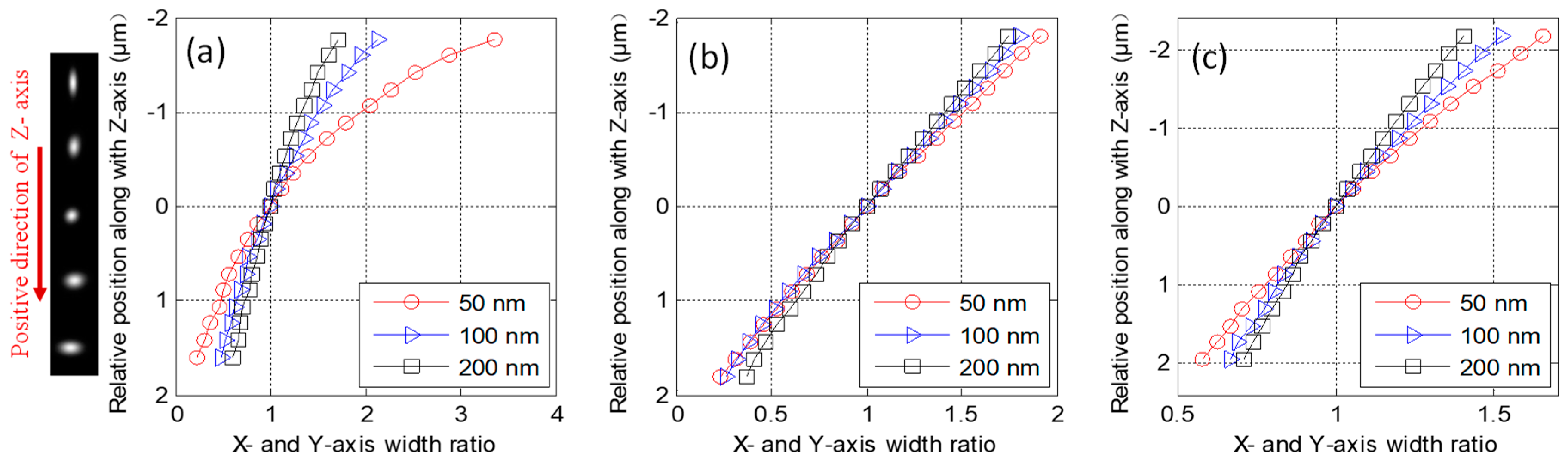
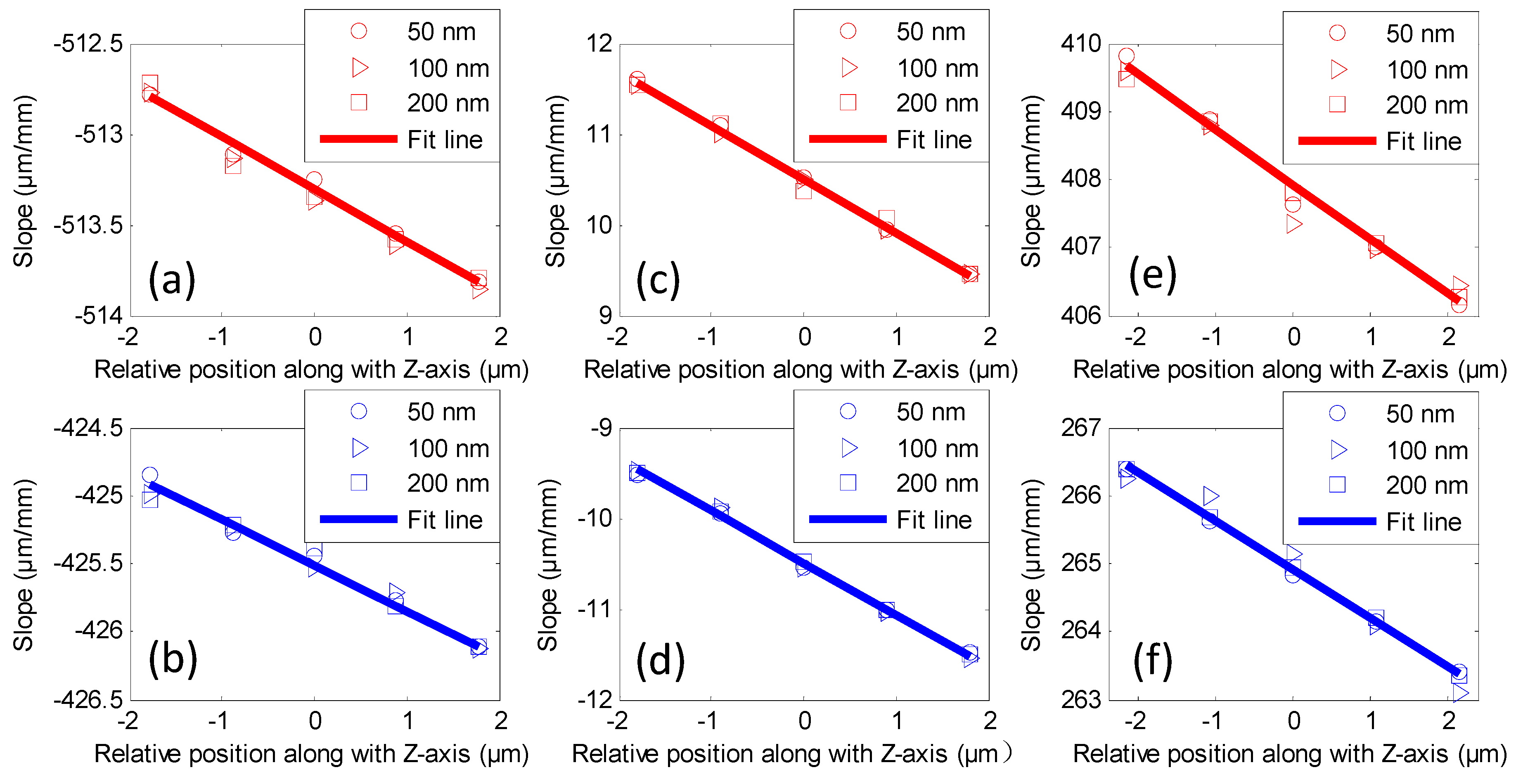
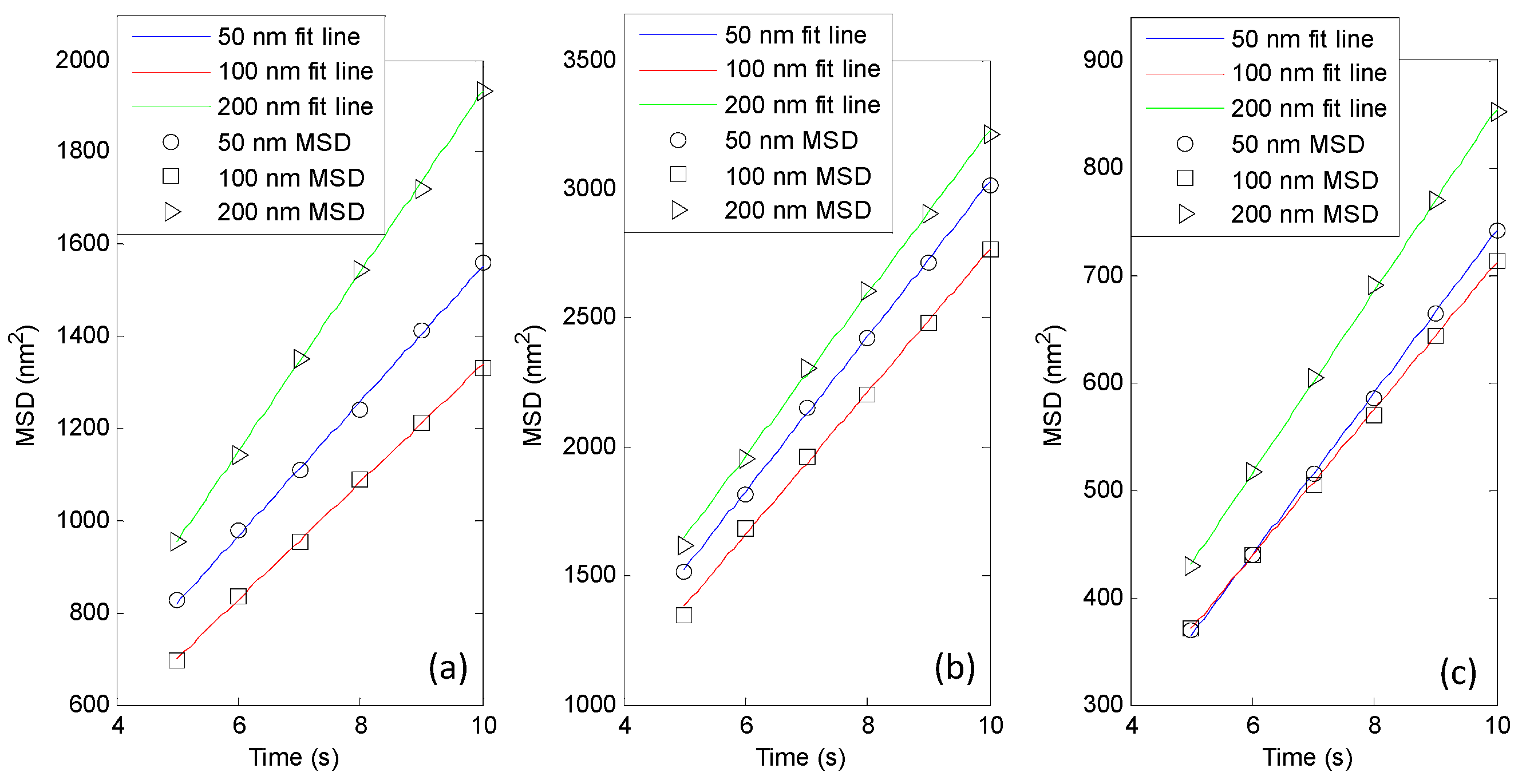
4. Experiments and Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, L.C.; Hsiao, L.C.; Doyle, P.S. Multiple particle tracking study of thermally-gelling nanoemulsions. Soft Matter 2017, 13, 6606–6619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, C.; Lv, F.; Liu, T. Real-time imaging tracking of a dual-fluorescent drug delivery system based on doxorubicin-loaded globin-polyethylenimine nanoparticles for visible tumor therapy. Colloids Surf. B Biointerfaces 2018, 170, 163–171. [Google Scholar] [CrossRef]
- Germann, J.A.; Davis, L.M. Three-dimensional tracking of a single fluorescent nanoparticle using four-focus excitation in a confocal microscope. Opt. Express 2014, 22, 5641–5650. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Torge, A.; Schaefer, U.F.; Schneider, M.; Lehr, C.-M.; Wagner, C. A foam model highlights the differences of the macro- and microrheology of respiratory horse mucus. J. Mech. Behav. Biomed. Mater. 2017, 71, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Lewis, J.S.; Oijen, A.M.V. Shining a Spotlight on DNA: Single-Molecule Methods to Visualise DNA. Molecules 2019, 24, 491. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Bae, Y.M.; Park, Y.I.; Kim, J.H.; Kim, H.M.; Choi, J.S.; Lee, K.T.; Hyeon, T.; Suh, Y.D. Long-TermReal-Time Tracking of Lanthanide Ion Doped Upconverting Nanoparticles in Living Cells. Angew. Chem. Int. Ed. 2011, 123, 6217–6221. [Google Scholar] [CrossRef]
- Jo, H.L.; Song, Y.H.; Park, J.; Jo, E.-J.; Goh, Y.; Shin, K.; Kim, M.-G.; Lee, K.T. Fast and background-free three-dimensional (3D) live-cell imaging with lanthanide-doped upconverting nanoparticles. Nanoscale 2015, 7, 19397–19402. [Google Scholar] [CrossRef]
- Goh, Y.; Song, Y.H.; Lee, G.; Bae, H.; Mahata, M.K.; Lee, K.T. Cellular uptake efficiency of nanoparticles investigated by three-dimensional imaging. Phys. Chem. Chem. Phys. 2018, 20, 11359–11368. [Google Scholar] [CrossRef]
- Shin, K.; Song, Y.H.; Goh, Y.; Lee, K.T. Two-Dimensional and Three-Dimensional Single Particle Tracking of Upconverting Nanoparticles in Living Cells. Int. J. Mol. Sci. 2019, 20, 1424. [Google Scholar] [CrossRef]
- Sio, S.D.; July, C.; Dhonta, J.K.G.; Lang, P.R. Near wall dynamics of a spherical particle in crowded suspensions of colloidal rods–dynamic information from TIRM revisited. Soft Matter 2018, 14, 9232–9242. [Google Scholar] [CrossRef]
- Schoch, R.L.; Barel, I.; Brown, F.L.H.; Haran, G. Lipid diffusion in the distal and proximal leaflets of supported lipid bilayer membranes studied by single particle tracking. J. Chem. Phys. 2018, 148, 123333. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gu, Y.; Sun, W.; Dong, B.; Wang, G.; Fan, X.; Xia, T.; Fang, N. Characteristic rotational behaviors of rod-shaped cargo revealed by automated five-dimensional single particle tracking. Nat. Commun. 2017, 8, 887. [Google Scholar] [CrossRef] [PubMed]
- Cang, H.; Xu, C.S.; Montiel, D.; Yang, H. Guiding a confocal microscope by single fluorescent nanoparticles. Opt. Lett. 2007, 32, 2729–2731. [Google Scholar] [CrossRef] [PubMed]
- Backlund, M.P.; Lew, M.D.; Backer, A.S. The role of molecular dipole orientation in single-molecule fluorescence microscopy and implications for super-resolution imaging. Chem. Phys. Chem. 2014, 15, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, J.; McGorty, R.; Huang, B. 4Pi fluorescence detection and 3D particle localization with a single objective. Opt. Express 2013, 21, 19701–19708. [Google Scholar] [CrossRef]
- Gallatin, G.M.; Berglund, A.J. Optimal laser scan path for localizing a fluorescent particle in two or three dimensions. Opt. Express 2012, 20, 16381–16393. [Google Scholar] [CrossRef]
- Adams, M.C.; Salmon, W.C.; Gupton, S.L.; Cohan, C.S.; Wittmann, T.; Prigozhina, N.; Waterman-storer, C.M. A high-speed multispectral spinning-disk confocal microscope system for fluorescent speckle microscopy of living cells. Methods 2003, 29, 29–41. [Google Scholar] [CrossRef]
- Nakano, A. Spinning-disk confocal microscopy—A cutting-edge tool for imaging of membrane traffic. Cell Struct. Funct. 2002, 27, 349–355. [Google Scholar] [CrossRef]
- Axelrod, D. Total Internal Reflection Fluorescence Microscopy in Cell Biology. Traffic 2001, 2, 764–774. [Google Scholar] [CrossRef]
- Webb, R.H. Confocal optical microscopy. Rep. Prog. Phys. 1996, 59, 427–471. [Google Scholar] [CrossRef]
- Schermelleh, L.; Carlton, P.M.; Haase, S.; Shao, L.; Winoto, L.; Kner, P.; Burke, B.; Cardoso, M.C.; Agard, D.A.; Gustafsson, M.G.L.; et al. Subdiffraction Multicolor Imaging of the Nuclear Periphery with 3D Structured Illumination Microscopy. Science 2008, 320, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Huisken, J.; Swoger, J.; Bene, F.D.; Wittbrodt, J.; Stelzer, E.H.K. Live Embryos by Selective Plane Illumination Microscopy. Science 2004, 305, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.P.; Verkman, A.S. Tracking of single fluorescent particles in three dimensions: Use of cylindrical optics to encode particle position. Biophys. J. 1994, 67, 1291–1300. [Google Scholar] [CrossRef]
- Holtzer, L.; Meckel, T.; Schmidt, T. Nanometric three-dimensional tracking of individual quantum dots in cells. Appl. Phys. Lett. 2007, 90, 053902. [Google Scholar] [CrossRef]
- Pavani, S.R.; Thompson, M.A.; Biteen, J.S. Three-dimensional single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. Proc. Natl. Acad. Sci. USA 2009, 106, 2995–2999. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Mao, S.; Tan, J.B. Compensation of errors due to incident beam drift in a 3 DOF measurement system for linear guide motion. Opt. Express 2015, 23, 28389–28401. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Hu, P.; Ding, X.; Tan, J. Parameter correction method for dual position-sensitive-detector-based unit. Appl. Opt. 2016, 55, 4073–4078. [Google Scholar] [CrossRef]
- Kato, H.; Ouchi, N.; Nakamura, A. Simultaneous measurement of size and density of spherical particles using two-dimensional particle tracking analysis method. Powder Technol. 2017, 315, 68–72. [Google Scholar] [CrossRef]
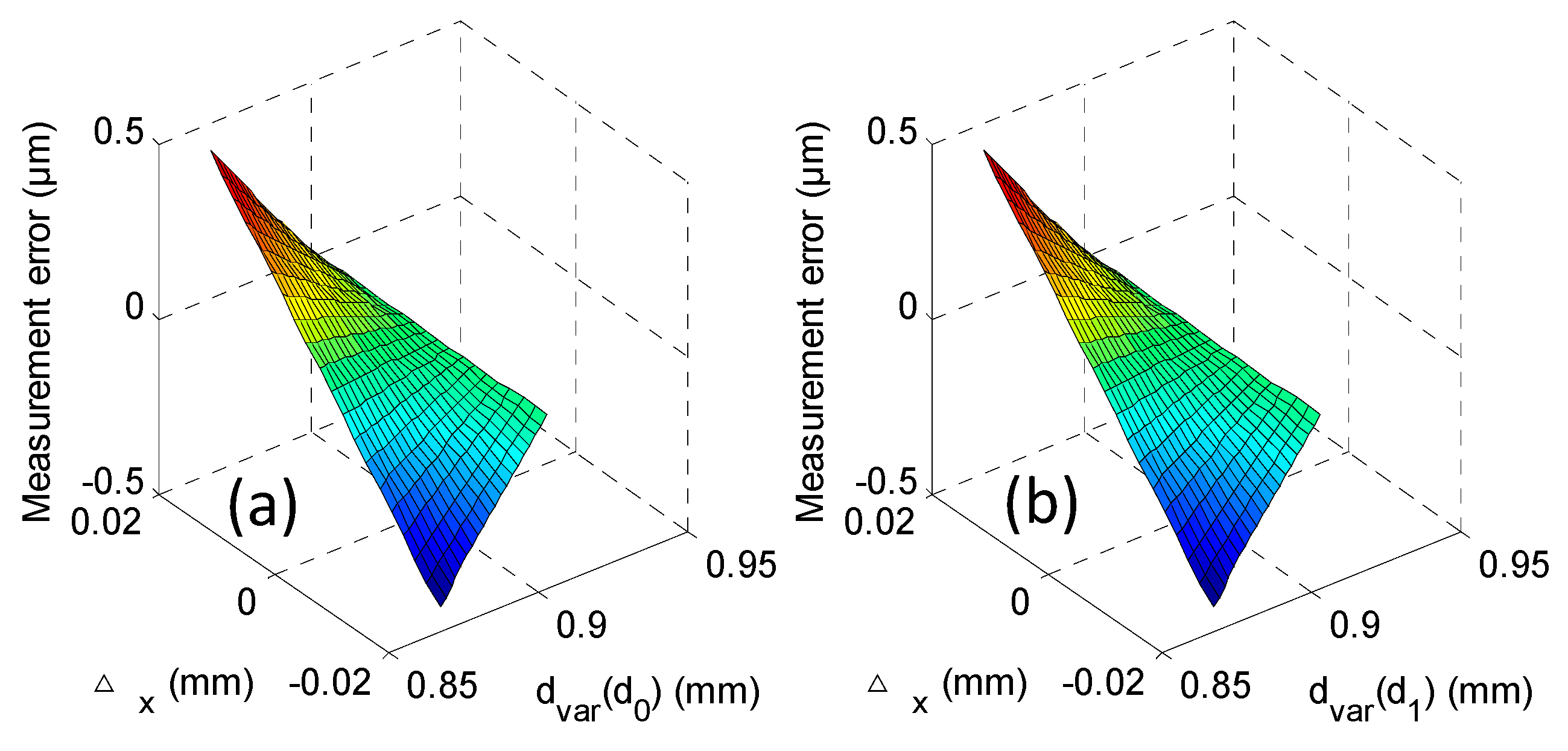
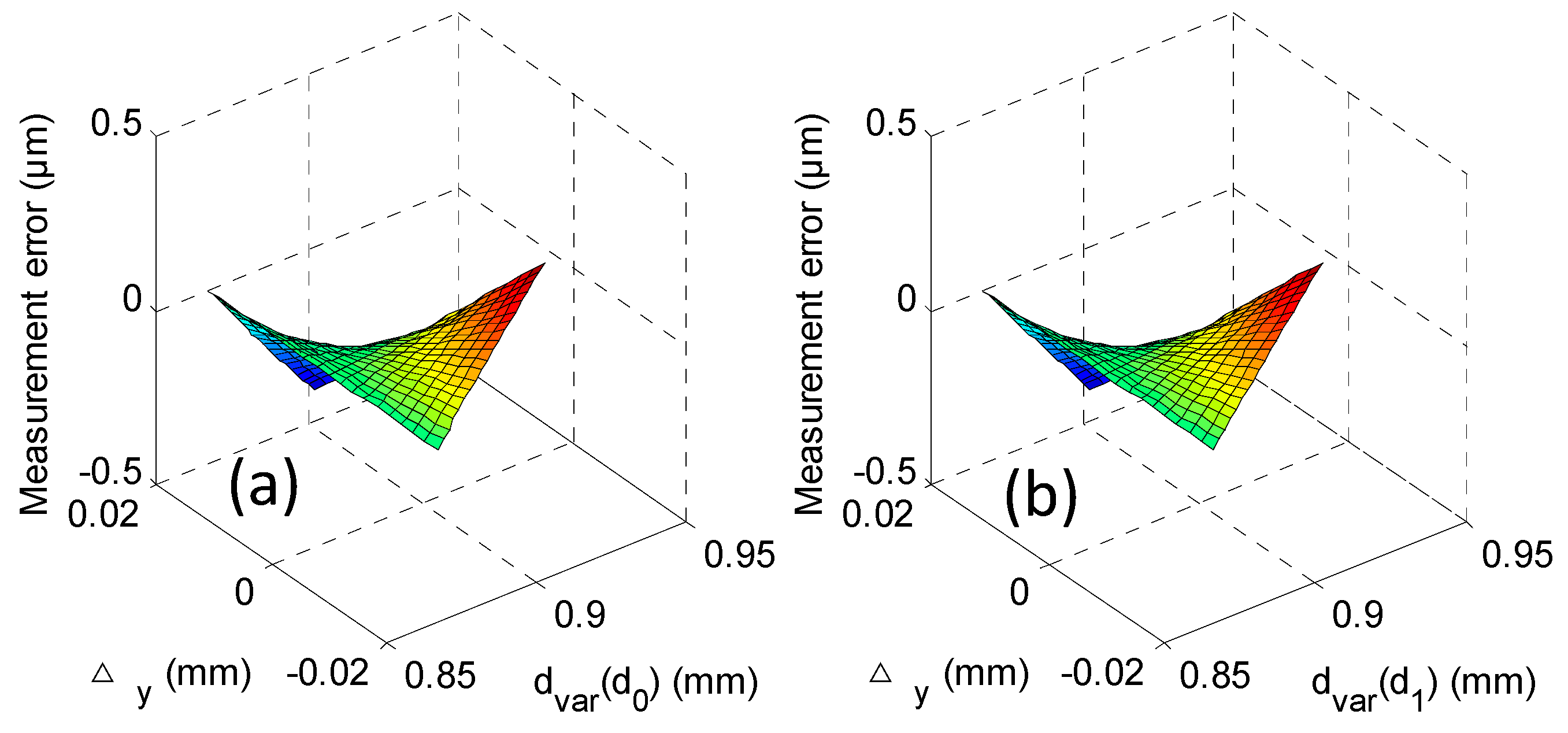
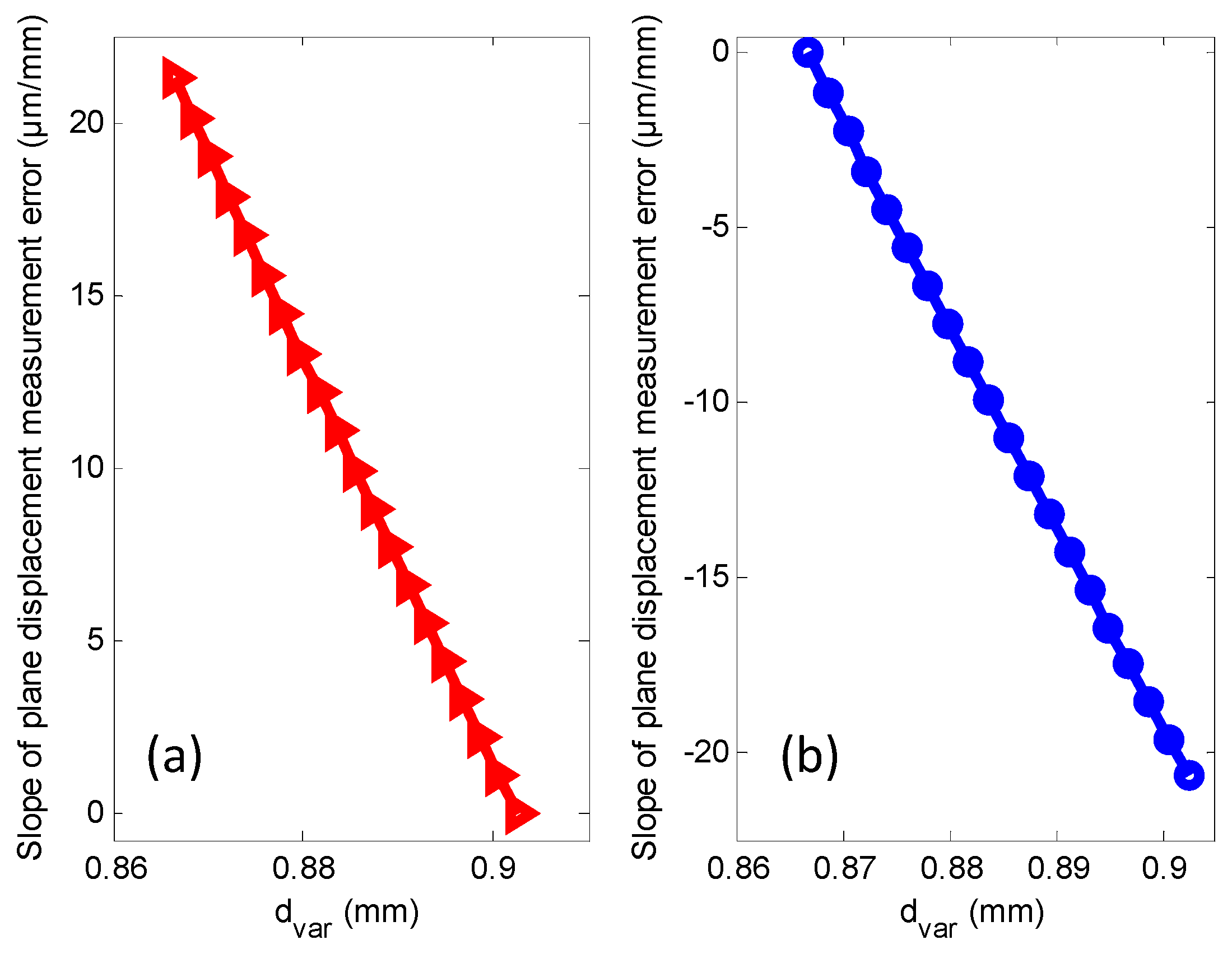
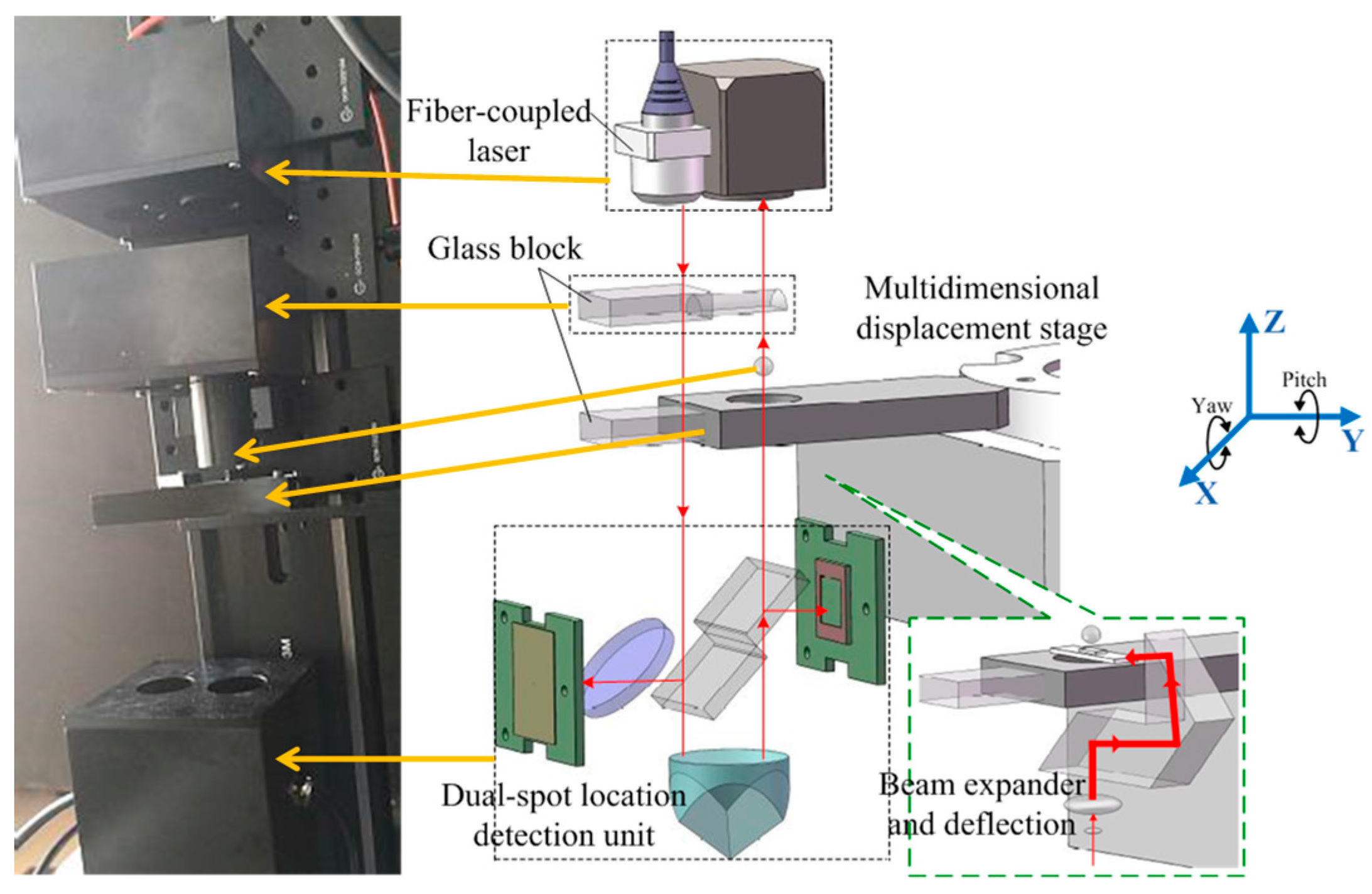
| Probe Ø | M1 | M2 | M3 |
|---|---|---|---|
| 50 nm | σ = 25.6 nm2, Ø = 43.6 nm | σ = − 12.1 nm2, Ø = 47.3 nm | σ = 69.4 nm2, Ø = 41.5 nm |
| 100 nm | σ = 91.4 nm2, Ø = 81.8 nm | σ = 63.5 nm2, Ø = 102.8 nm | σ = −21.8 nm2, Ø = 67.1 nm |
| 200 nm | σ = − 11.3 nm2, Ø = 174.3 nm | σ = 29.5 nm2, Ø = 192.1 nm | σ = 75.3 nm2, Ø = 154.8 nm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, S.; Shen, J.; Wang, Y.; Liu, W.; Pan, J. A Three-Dimensional Tracking Method with the Self-Calibration Functions of Coaxiality and Magnification for Single Fluorescent Nanoparticles. Appl. Sci. 2020, 10, 131. https://doi.org/10.3390/app10010131
Mao S, Shen J, Wang Y, Liu W, Pan J. A Three-Dimensional Tracking Method with the Self-Calibration Functions of Coaxiality and Magnification for Single Fluorescent Nanoparticles. Applied Sciences. 2020; 10(1):131. https://doi.org/10.3390/app10010131
Chicago/Turabian StyleMao, Shuai, Jin Shen, Yajing Wang, Wei Liu, and Jinfeng Pan. 2020. "A Three-Dimensional Tracking Method with the Self-Calibration Functions of Coaxiality and Magnification for Single Fluorescent Nanoparticles" Applied Sciences 10, no. 1: 131. https://doi.org/10.3390/app10010131
APA StyleMao, S., Shen, J., Wang, Y., Liu, W., & Pan, J. (2020). A Three-Dimensional Tracking Method with the Self-Calibration Functions of Coaxiality and Magnification for Single Fluorescent Nanoparticles. Applied Sciences, 10(1), 131. https://doi.org/10.3390/app10010131




