Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process
Abstract
1. Introduction
2. Materials and Methods
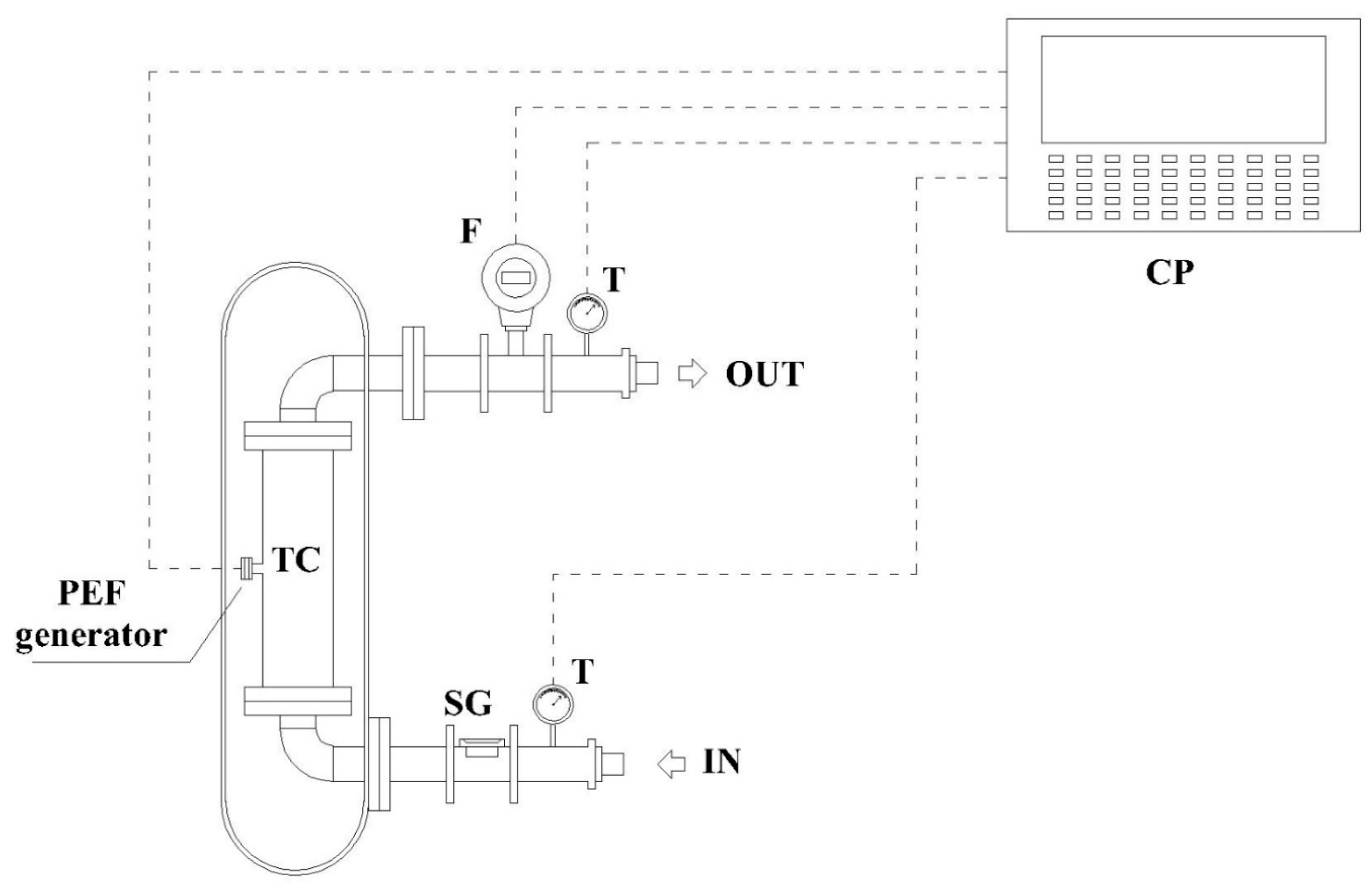
2.1. Pulsed Electric Field System
2.2. Plant for Olive Oil Extraction and Experimental Plan
2.3. Extractability and Oil Content in the Olives, Pomace, and Wastewater
2.4. Olive Oil Quality
2.4.1. Legal Quality Parameters
2.4.2. Phenolic Compounds
2.4.3. Volatile Compounds
2.5. Data Processing
3. Results and Discussion
3.1. Effect of the Pulsed Electric Field Treatment of Olive Paste Versus Extractability
3.2. Olive Oil Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbosa-Canovas, G.V.; Altunakar, B. Pulsed electric fields processing of foods: An overview. In Pulsed Electric Field Technology for Food Industry: Fundamentals and Applications; Raso, J., Heinz, V., Eds.; Springer: New York, NY, USA, 2006; pp. 153–194. [Google Scholar]
- Zimmermann, U. Electrical breakdown, electropermeabilization and electrofusion. Rev. Physiol. Biochem. Pharmacol. 1986, 105, 175–256. [Google Scholar]
- Kotnik, T.; Frey, W.; Sack, M.; Haberl Meglič, S.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–332. [Google Scholar] [CrossRef]
- Toledo, R.T.; Singh, R.K.; Kong, F. Fundamentals of Food Process Engineering, 4th ed.; Gewerbestrasse: Cham, Switzerland, 2018. [Google Scholar]
- Zhang, Q.; Barbosa-Canovas, G.V.; Swanson, B.G. Engineering aspects of pulsed electric field pasteurization. J. Food Eng. 1995, 25, 261–281. [Google Scholar] [CrossRef]
- Jeyamkondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: A review. J. Food Prot. 1999, 62, 1088–1096. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Vorobiev, E. & Miklavčič, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Lebovka, N. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldaña, G.; Raso, J. Pulsed Electric Field Treatment for Fruit and Vegetable Processing; Handbook of Electroporation; Springer: Berlin, Germany, 2016; pp. 1–21. [Google Scholar]
- Luengo, E.; Álvarez, I.; Raso, J. Improving carotenoid extraction from tomato waste by pulsed electric fields. Front. Nutr. 2014, 1, 12. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzan, O.; Luengo, E.; Alvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Enhancing the carotenoid content of tomato fruit with pulsed electric field treatments: Effects on respiratory activity and quality attributes. Postharv. Biol. Technol. 2018, 137, 113–118. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Praporscic, I.; Vorobiev, E. Effect of moderate thermal and pulsed electric field treatments on textural properties of carrots, potatoes and apples. Innov. Food Sci. Emerg. Technol. 2004, 5, 9–16. [Google Scholar] [CrossRef]
- Ignat, A.; Manzocco, L.; Brunton, N.P.; Nicoli, M.C.; Lyng, J.G. The effect of pulsed electric field pre-treatments prior to deep-fat frying on quality aspects of potato fries. Innov. Food Sci. Emerg. Technol. 2015, 29, 65–69. [Google Scholar] [CrossRef]
- Wiktor, A.; Schulz, M.; Voigt, E.; Witrowa-Rajchert, D.; Knorr, D. The effect of pulsed electric field treatment on immersion freezing, thawing and selected properties of apple tissue. J. Food Eng. 2015, 146, 8–16. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Siemer, C.; Toepfl, S.; Mykhailykc, V.; Gondekb, E.; Rybakb, K.; Witrowa-Rajchertb, D.; Parniakova, O. The effects of pulsed electric fields on the quality parameters of freeze-dried apples. J. Food Eng. 2019, 252, 36–43. [Google Scholar] [CrossRef]
- Kantar, S.E.; Boussetta, N.; Lebovka, N.; Foucart, F.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Juliano, P.; Tamborrino, A. Use of a mixing-coil heat exchanger combined with microwave and ultrasound technology in an olive oil extraction process. Innov. Food Sci. Emerg. Technol. 2018, 50, 66–72. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Abuagela, M.O.; Marshall, S.M.; Yagiz, Y.; Goodrich-Schneider, R.; Baker, G.L., IV; Welt, B.A.; Marshall, M.R. Combining high power ultrasound pre-treatment with malaxation oxygen control to improve quantity and quality of extra virgin olive oil. J. Food Eng. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Tamborrino, A.; Romaniello, R.; Caponio, F.; Squeo, G.; Leone, A. Combined industrial olive oil extraction plant using ultrasounds, microwave, and heat exchange: Impact on olive oil quality and yield. J. Food Eng. 2019, 245, 124–130. [Google Scholar] [CrossRef]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef]
- Tamborrino, A.; Romaniello, R.; Zagaria, R.; Leone, A. Microwave-assisted treatment for continuous olive paste conditioning: Impact on olive oil quality and yield. Biosyst. Eng. 2014, 127, 92–102. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Zagaria, R.; Sabella, E.; Romaniello, R. Plant innovation in the olive oil extraction process: A comparison of efficiency and energy consumption between microwave treatment and traditional malaxation of olive pastes. J. Food Eng. 2015, 146, 44–52. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Tamborrino, A.; Xu, X.-Q.; Juliano, P. Microwave and megasonics combined technology for a continuous olive oil process with enhanced extractability Innovative. Food Sci. Emerg. Technol. 2017, 42, 56–63. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Tamborrino, A.; Urbani, S.; Servili, M.; Amarillo, M.; Grompone, M.A.; Gambaro, A.; Juliano, P. Application of Microwaves and Megasound to Olive Paste in an Industrial Olive Oil Extraction Plant: Impact on Virgin Olive Oil Quality and Composition European. J. Lipid Sci. Technol. 2018, 120, 1700261. [Google Scholar] [CrossRef]
- Amarillo, M.; Pérez, N.; Blasina, F.; Gambaro, A.; Leone, A.; Romaniello, R.; Xu, X.-Q.; Juliano, P. Impact of sound attenuation on ultrasound-driven yield improvements during olive oil extraction. Ultrason. Sonochem. 2019, 53, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Andreou, V.; Alexandrakis, G.D.Z.; Katsaros, G.; Oikonomou, D.; Toepfl, S.; Heinz, V.; Taoukis, P. Shelf-life evaluation of virgin olive oil extracted from olives subjected to nonthermal pretreatments for yield increase. Innov. Food Sci. Emerg. Technol. 2017, 40, 52–57. [Google Scholar] [CrossRef]
- Abenoza, M.; Benito, M.; Saldaña, G.; Álvarez, I.; Raso, J.; Sánchez-Gimeno, A.C. Effects of pulsed electric field on yield extraction and quality of olive oil. Food Bioprocess Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Puértolas, E.; Martínez de Marañón, I. Olive oil pilot-production assisted by pulsed electric field: Impact on extraction yield, chemical parameters and sensory properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Zagaria, R.; Sabella, E.; De Bellis, L.; Tamborrino, A. Machining effects of different mechanical crushers on pit particle size and oil drop distribution in olive paste. Eur. J. Lipid Sci. Technol. 2015, 117, 1271–1279. [Google Scholar] [CrossRef]
- Cherubini, C.; Migliorini, M.; Mugelli, M.; Viti, P.; Berti, A.; Cini, E.; Zanonia, B. Towards a technological ripening index for olive oil fruits. J. Sci. Food Agric. 2009, 89, 671–682. [Google Scholar] [CrossRef]
- Official Journal of the European Community (OJEC). Commission Delegated Regulation (EU) 2015/1830 of 8 July 2015 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. OJL 2015, 266, 9–13. [Google Scholar]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Servili, M. Optimization of the temperature and oxygen concentration conditions in the malaxation during the oil mechanical extraction process of four Italian olive cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Selvaggini, R.; Servili, M.; Urbani, S.; Esposto, S.; Taticchi, A.; and Montedoro, G.F. Evaluation of phenolic compounds in virgin olive oil by direct injection in high-performance liquid chromatography with fluorometric detection. J. Agric. Food Chem. 2006, 54, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Servili, M. Flash thermal conditioning of olive pastes during the oil mechanical extraction process: Cultivar impact on the phenolic and volatile composition of virgin olive oil. J. Agric. Food Chem. 2015, 63, 6066–6074. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, L. Pulsed electric field-assisted extraction. In Enhancing Extraction Processes in the Food Industry; Lebovka, L., Vorobiev, E., Chemat, F., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Di Maio, I.; Servili, M. Cooling treatment of olive paste during the oil processing: Impact on the yield and extra virgin olive oil quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef]
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-frequency, high-power ultrasound treatment at different pressures for olive paste: Effects on olive oil yield and quality. Ultrason. Sonochem. 2019, 59, 104747. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Sordini, B.; Lorefice, A.; Daidone, L.; Pagano, M.; Tomasone, R.; Servili, M. Extra-virgin olive oil extracted using pulsed electric field technology: Cultivar impact on oil yield and quality. Front. Nutr. 2019, 6, 134. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
| Test Conditions | Variety | Pomace | Extractability | |
|---|---|---|---|---|
| Moisture (%) | Oil (% Dry Basis) | (%) | ||
| Control | Nocellara del Belice | 60.6 ± 1.1 a | 10.4 ± 0.7 a | 79.5 ± 0.8 b |
| PEF | Nocellara del Belice | 60.2 ± 0.5 a | 7.4 ± 0.7 b | 85.5 ± 1.5 a |
| Test Conditions | Variety | Free Acidity (%) | Peroxide Value (meq O2/kg) | K232 | K270 | ΔK | |
|---|---|---|---|---|---|---|---|
| Legal limit EVOO | <0.8 | ≤20 | ≤2.50 | ≤0.22 | ≤0.01 | ||
| Treatment on paste | Control | Nocellara del Belice | 0.59 ± 0.02 a | 6.78 ± 0.39 a | 1.51 ± 0.20 a | 0.17 ± 0.01 a | 0.001 ± 0.001 a |
| PEF | Nocellara del Belice | 0.57 ± 0.02 a | 7.13 ± 0.24 a | 1.54 ± 0.20 a | 0.17 ± 0.01 a | 0.001 ± 0.002 a |
| Phenolic Compounds | Nocellara del Belice | |
|---|---|---|
| Control | PEF-Treated | |
| 3,4-DHPEA | 2.6 ± 2.6 a | 1.5 ± 0.3 a |
| p-HPEA | 2.1 ± 2.1 a | 1.3 ± 0.3 a |
| Vanillic acid | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| 3,4-DHPEA-EDA | 97.8 ± 9.8 b | 114.8 ± 2.2 a |
| p-HPEA-EDA | 27.8 ± 0.5 b | 30.1 ± 1.5 a |
| (+)-1-acetoxypinoresinol | 3.1 ± 0.2 a | 3.0 ± 0.3 a |
| (+)-pinoresinol | 5.7 ± 1.7 a | 4.4 ± 0.4 a |
| 3,4-DHPEA-EA | 40.0 ± 4.4 a | 41.8 ± 9.5 a |
| Ligstroside aglycone | 4.8 ± 0.7 a | 5.4 ± 0.3 a |
| Total phenols | 184.0 ± 11.4 a | 202.3 ± 9.9 a |
| Heading | Nocellara Del Belice | |
|---|---|---|
| Control | PEF-Treated | |
| Aldehydes | ||
| Pentanal | 51.8 ± 16.6 b | 206.5 ± 13.4 a |
| (E)-2-Pentenal | 40.0 ± 2.8 a | 19.0 ± 2.1 b |
| Hexanal | 468.0 ± 53.7 b | 871.5 ± 24.0 a |
| (E)-2-Hexenal | 5647.0 ± 618.0 a | 5878.3 ± 1258.3 a |
| (E,E)-2,4-Hexadienal | 274.3 ± 23.0 a | 199.3 ± 19.4 b |
| 2,4-Hexadienal (i) | 575.8 ± 54.1 a | 426.3 ± 37.8 b |
| ΣAldehydes | 7056.8 ± 623.3 a | 7600.8 ± 1259.3 a |
| Alcohols | ||
| 1-Pentanol | 30.8 ± 0.4 b | 71.8 ± 9.5 a |
| 1-Penten-3-ol | 223.0 ± 30.4 a | 198.3 ± 11.0 a |
| (E)-2-Penten-1-ol | 110.8 ± 8.1 a | 91.5 ± 9.9 b |
| (Z)-2-Penten-1-ol | 211.8 ± 30.8 a | 127.8 ± 7.4 b |
| 1-Hexanol | 1052.8 ± 188.4 a | 792.3 ± 148.1 a |
| (E)-2-Hexen-1-ol | 1000.5 ± 127.3 a | 961.8 ± 117.0 a |
| (Z)-2-Hexen-1-ol | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| (E)-3-Hexen-1-ol | 106.8 ± 26.5 a | 55.0 ± 8.5 b |
| (Z)-3-Hexen-1-ol | 3735.8 ± 22.3 a | 2558.5 ± 383.3 b |
| ΣAlcohols | 6472.0 ± 305.2 a | 4856.8 ± 437.2 b |
| Esters | ||
| Hexyl acetate | 921.5 ± 106.8 a | 850.3 ± 151.0 a |
| (Z)-3-Hexenyl acetate | 3423.3 ± 308.7 a | 3282.8 ± 264.8 b |
| (E)-2-Hexenyl acetate | 35.8 ± 1.1 a | 17.0 ± 2.1 b |
| ΣEsters | 4380.5 ± 326.6 a | 4150.0 ± 304.8 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamborrino, A.; Urbani, S.; Servili, M.; Romaniello, R.; Perone, C.; Leone, A. Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process. Appl. Sci. 2020, 10, 114. https://doi.org/10.3390/app10010114
Tamborrino A, Urbani S, Servili M, Romaniello R, Perone C, Leone A. Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process. Applied Sciences. 2020; 10(1):114. https://doi.org/10.3390/app10010114
Chicago/Turabian StyleTamborrino, Antonia, Stefania Urbani, Maurizio Servili, Roberto Romaniello, Claudio Perone, and Alessandro Leone. 2020. "Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process" Applied Sciences 10, no. 1: 114. https://doi.org/10.3390/app10010114
APA StyleTamborrino, A., Urbani, S., Servili, M., Romaniello, R., Perone, C., & Leone, A. (2020). Pulsed Electric Fields for the Treatment of Olive Pastes in the Oil Extraction Process. Applied Sciences, 10(1), 114. https://doi.org/10.3390/app10010114








