Effect of Functional Group Density of Anion Exchange Resins on Removal of p-Toluene Sulfonic Acid from Aqueous Solution
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
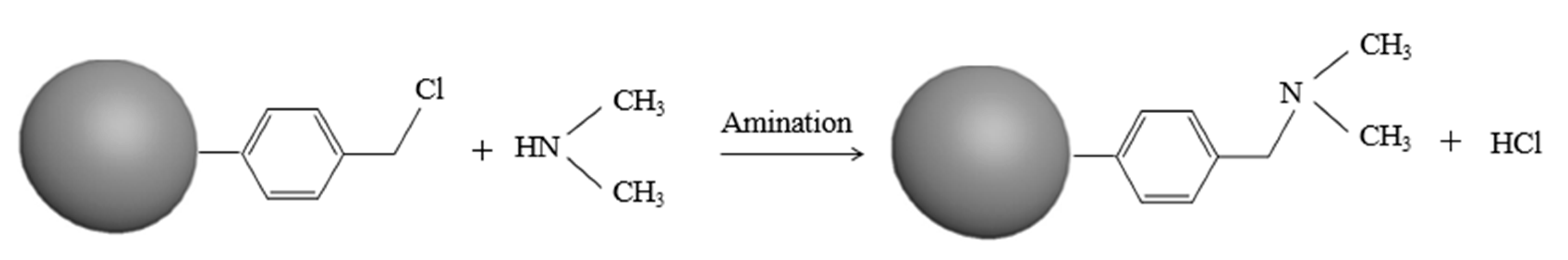
2.2. Resin Synthesis and Characterization
2.3. Batch Sorption Experiments
2.4. Desorption Experiments
2.5. Column Sorption Experiments
3. Results and Discussion
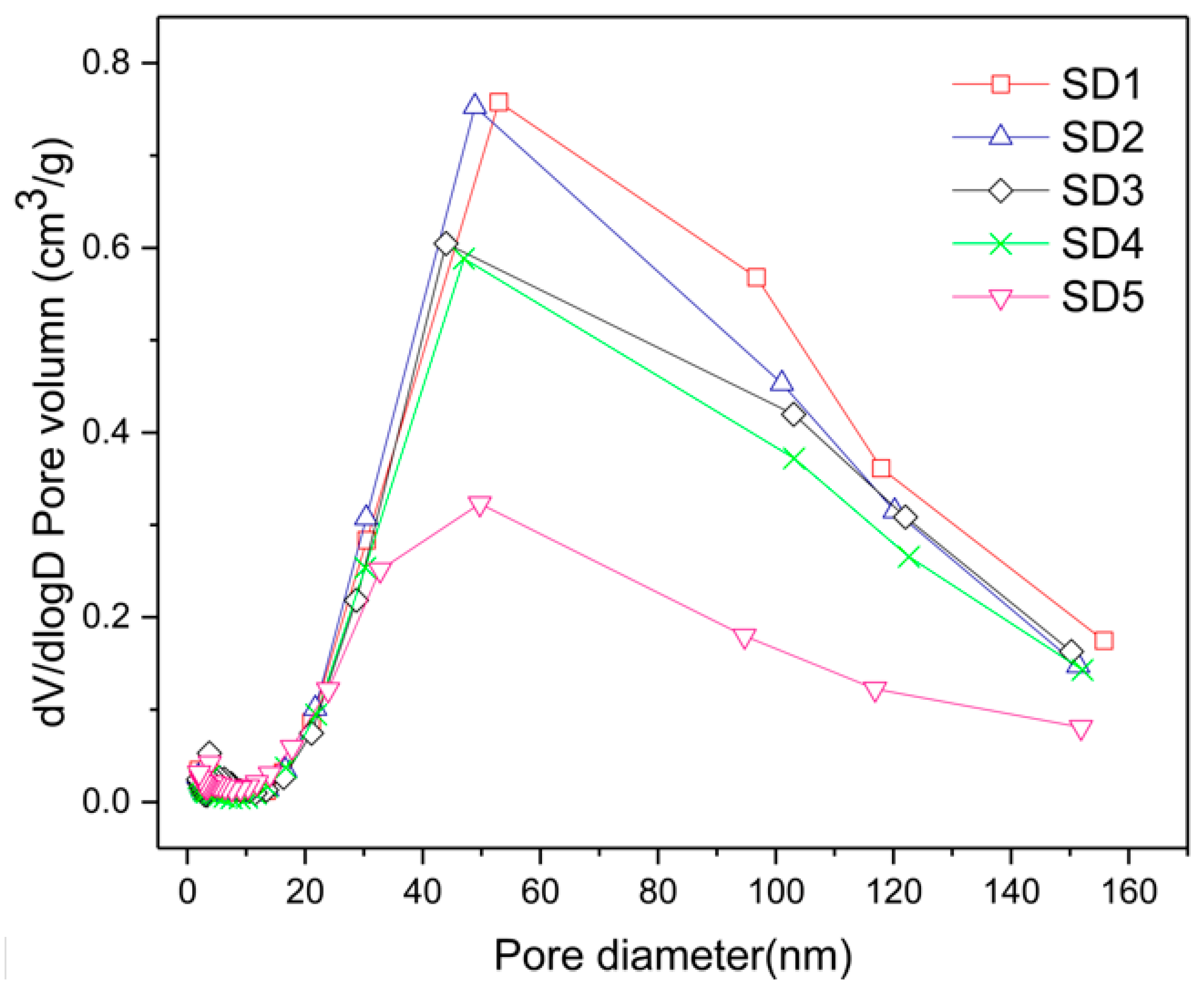
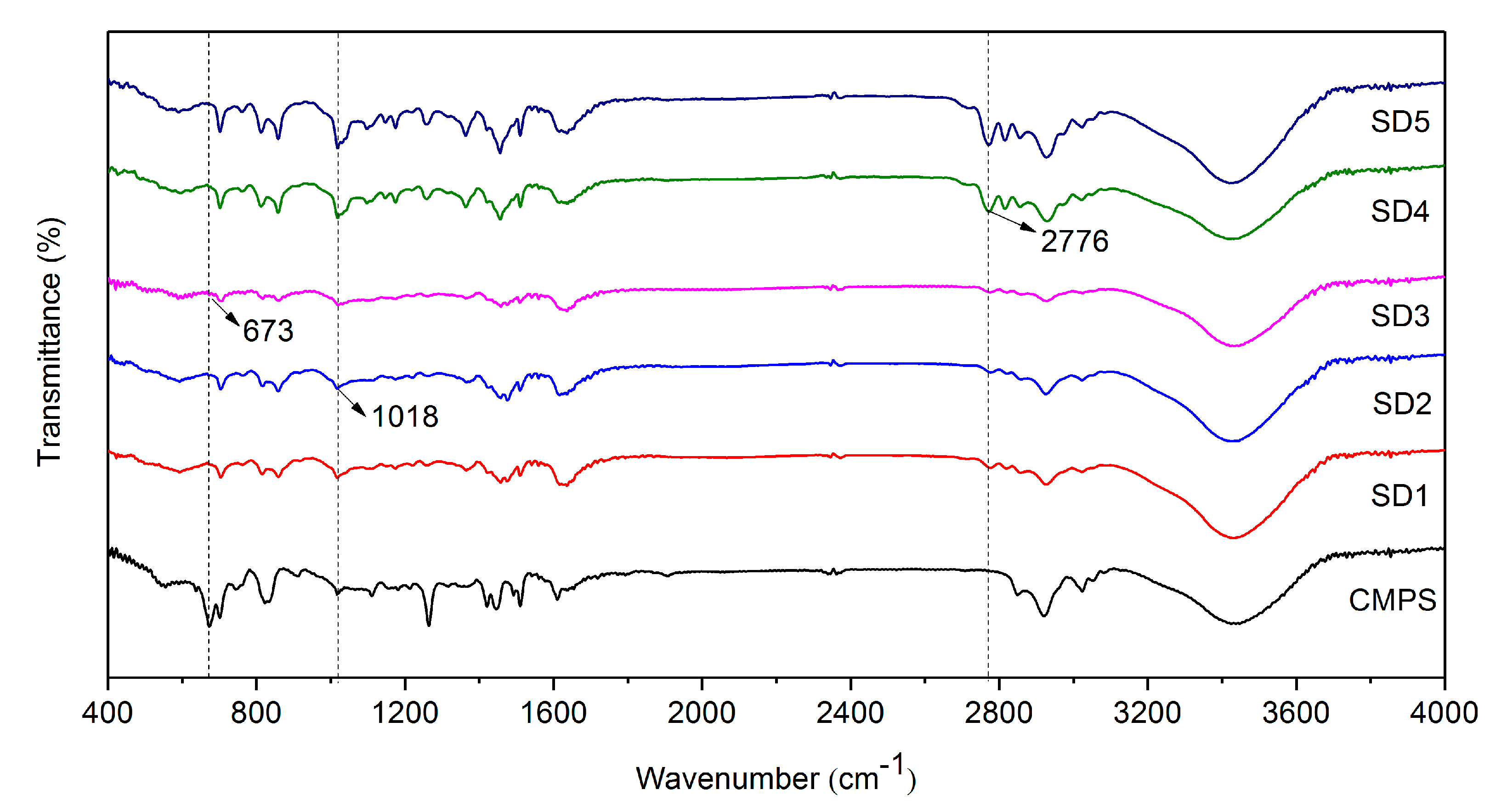
3.1. Characterization of Different Functional Group Density Resins
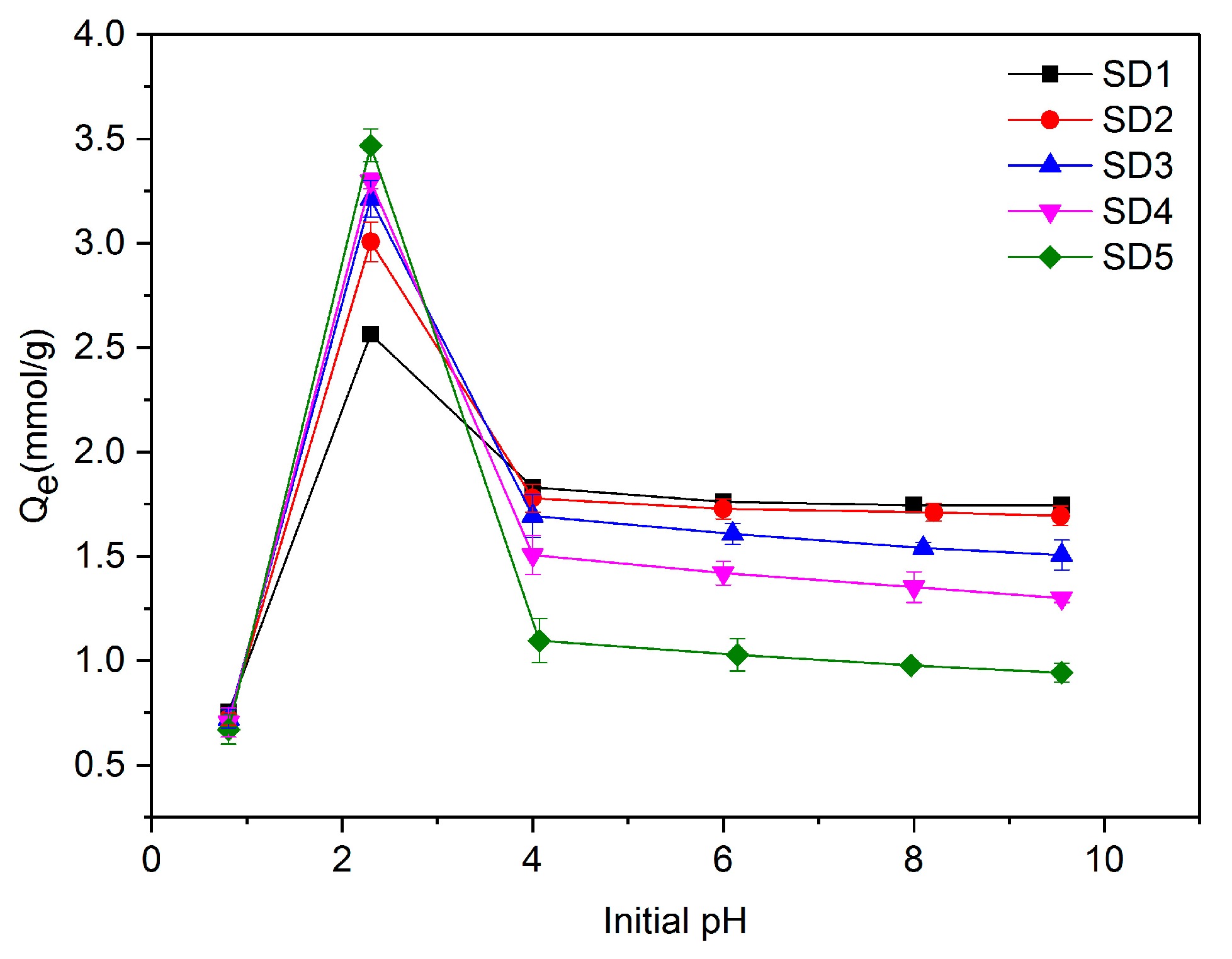
3.2. Effect of pH on Adsorption
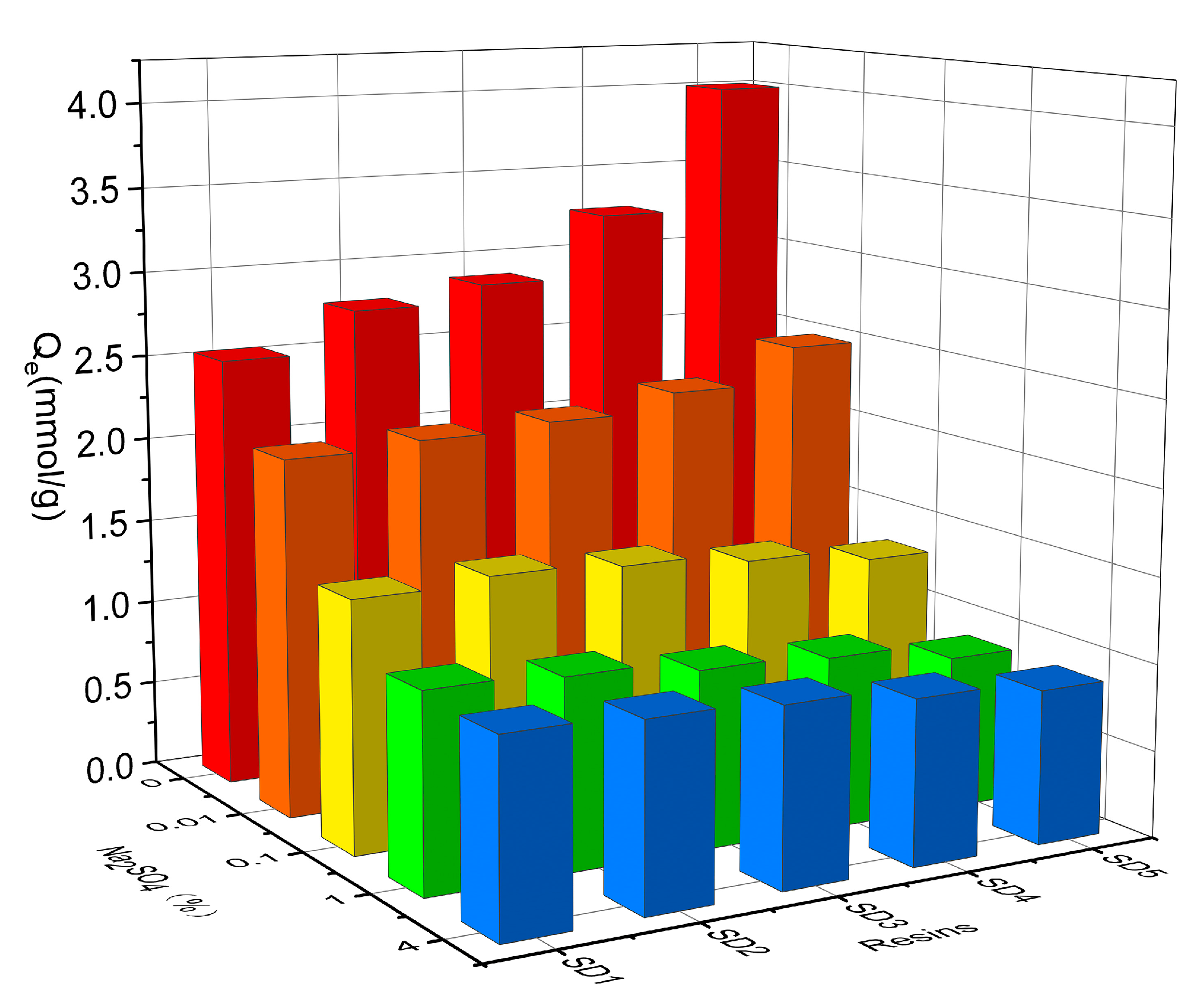
3.3. Effect of SO42− on Adsorption and Selectivity of PTSA
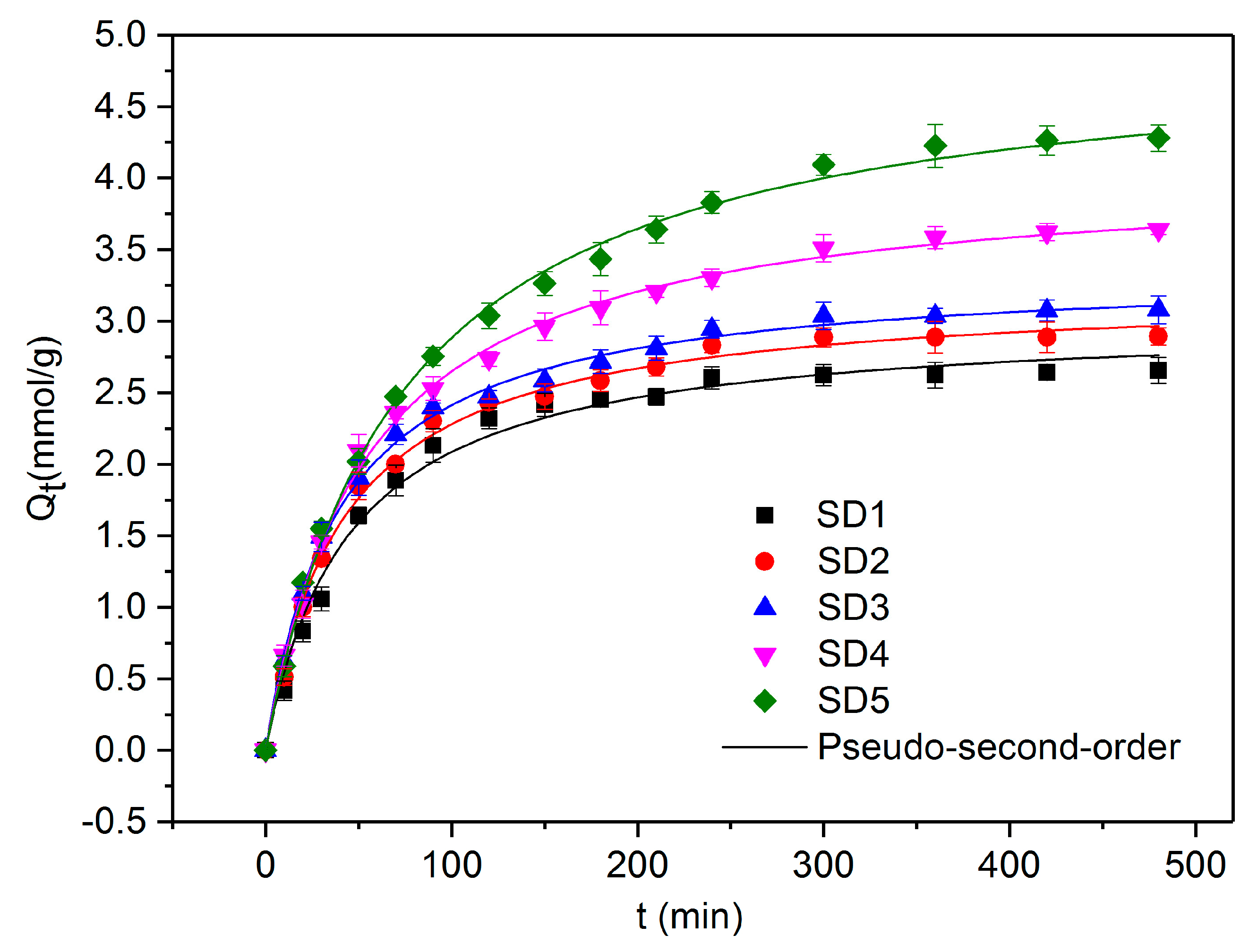
3.4. Adsorption Kinetics of PTSA
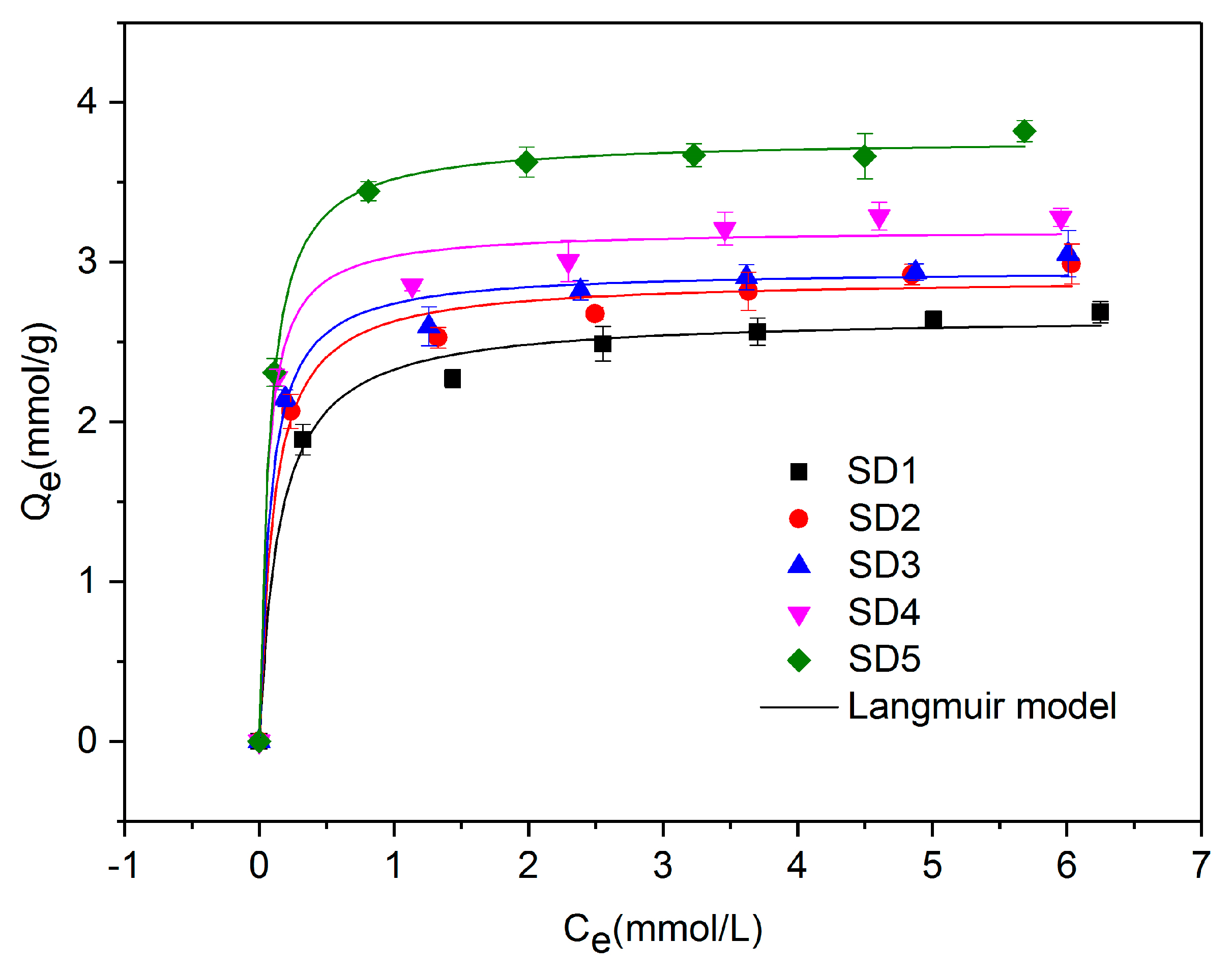
3.5. Adsorption Isotherm of PTSA
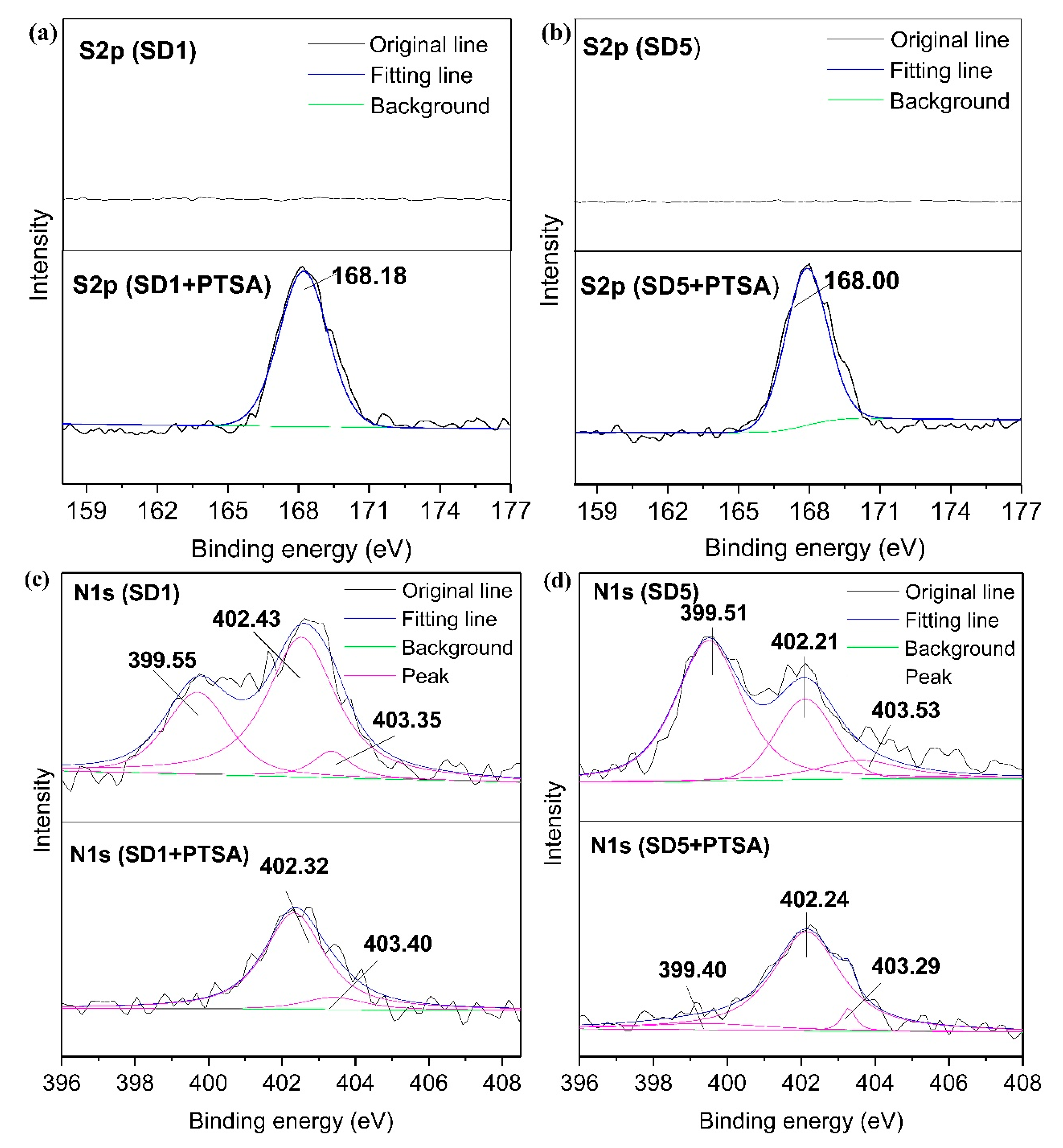
3.6. XPS Analysis before and after Adsorption
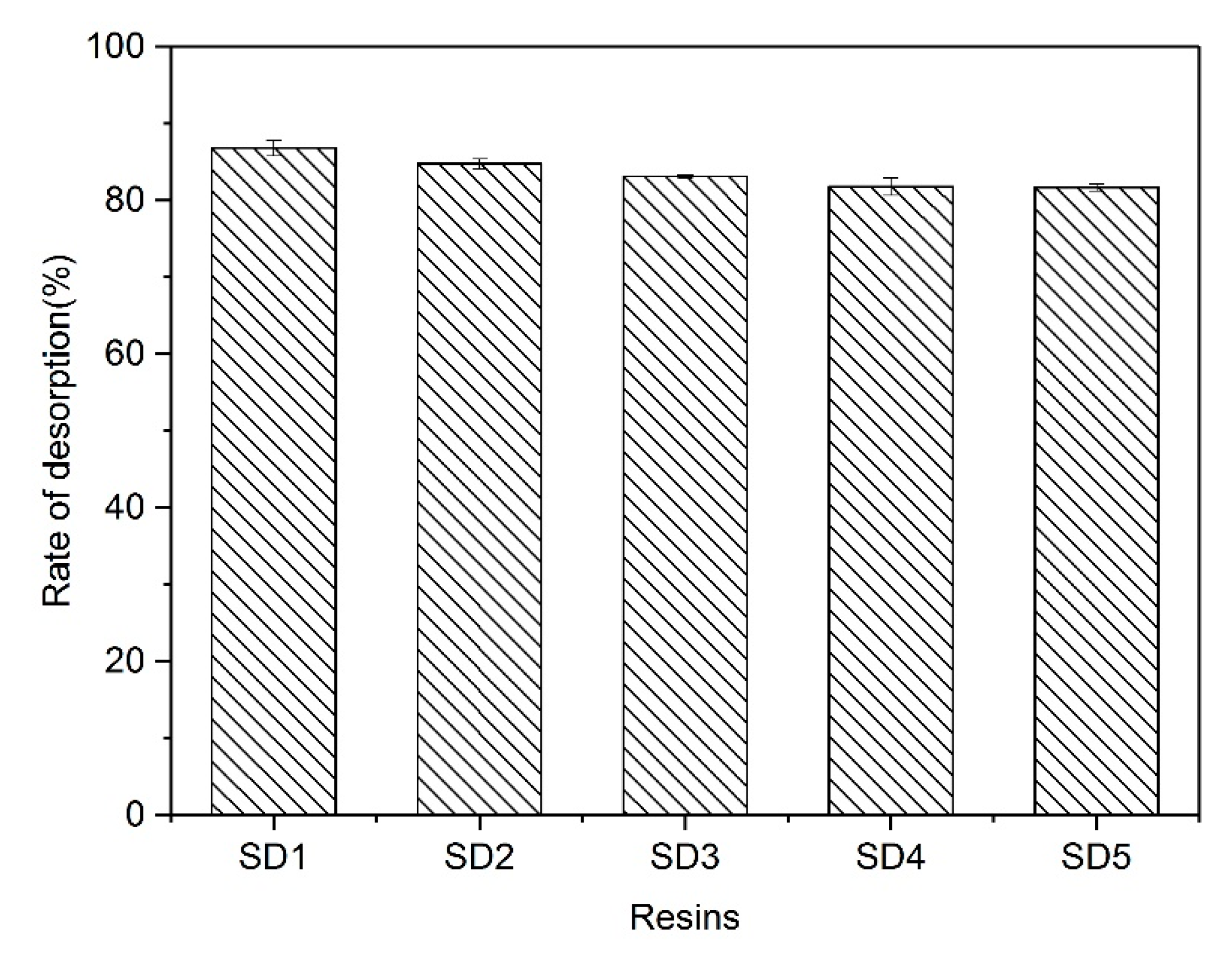
3.7. Desorption
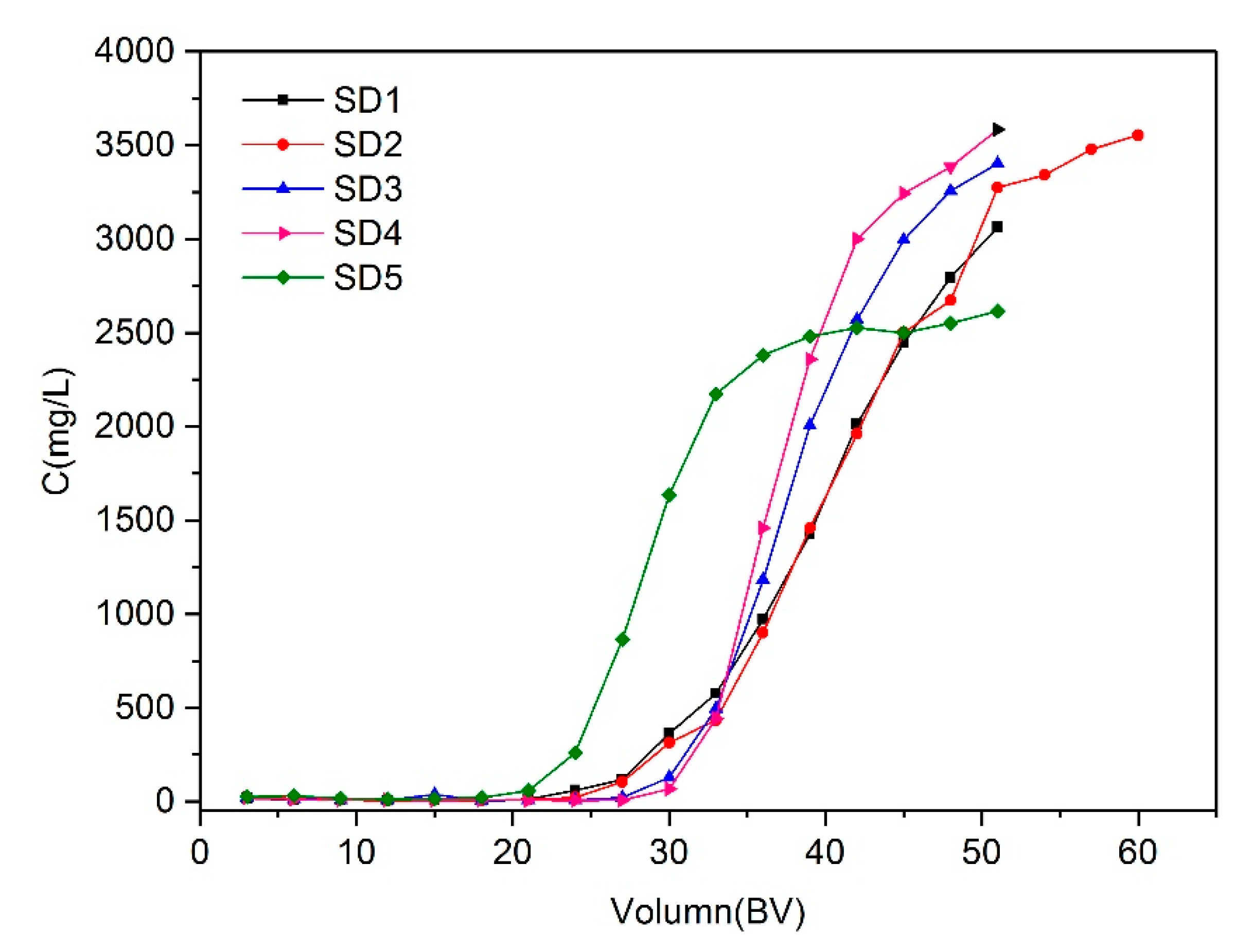
3.8. Column Sorption Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ravera, M.; Buico, A.; Gosetti, F.; Cassino, C.; Musso, D.; Osella, D. Oxidative degradation of 1,5-naphthalenedisulfonic acid in aqueous solutions by microwave irradiation in the presence of H2O2. Chemosphere 2009, 74, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Zhang, Q.; Meng, F.; Li, X.; Zhang, X.; Zheng, J.; Zhang, W.; Pan, B.; Chen, J. Sorption enhancement of aromatic sulfonates onto an aminated hyper-cross-linked polymer. Environ. Sci. Technol. 2005, 39, 3308–3313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Wang, C.; Yip, A.C.; Tsang, D.C. Highly effective degradation of sodium dodecylbenzene sulphonate and synthetic greywater by Fenton-like reaction over zerovalent iron-based catalyst. Environ. Technol. 2015, 36, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Olmezhanci, T.; Arslanalaton, I.; Gelegen, O. Photo-Fenton-like treatment of K-acid: Assessment of treatability, toxicity and oxidation products. Water Sci. Technol. 2014, 70, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Avetta, P.; Prevot, A.B.; Fabbri, D.; Montoneri, E.; Tomasso, L. Photodegradation of naphthalene sulfonic compounds in the presence of a bio-waste derived sensitizer. Chem. Eng. J. 2012, 197, 193–198. [Google Scholar] [CrossRef]
- de Souza, N.A.; Ramaiah, N.; Damare, S.; Furtado, B.; Mohandass, C.; Patil, A.; de Lima, M. Differential Protein Expression in Shewanella seohaensis Decolorizing Azo Dyes. Curr. Proteom. 2019, 16, 156–164. [Google Scholar] [CrossRef]
- Song, Z.; Song, L.; Shao, Y.; Tan, L. Degradation and detoxification of azo dyes by a salt-tolerant yeast Cyberlindnera samutprakarnensis S4 under high-salt conditions. World J. Microb. Biot. 2018, 34, 131. [Google Scholar] [CrossRef]
- Wan, S.; Hua, Z.; Sun, L.; Bai, X.; Liang, L. Biosorption of nitroimidazole antibiotics onto chemically modified porous biochar prepared by experimental design: Kinetics, thermodynamics, and equilibrium analysis. Process Saf. Environ. 2016, 104, 422–435. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, D.-L.; Wang, P.; Dong, Y.-H. Removal of sodium salts and chemical oxygen demand from real reactive dye wastewater by the integrated process of chemical precipitation and extraction. Desalin. Water Treat. 2015, 57, 6772–6780. [Google Scholar] [CrossRef]
- Gai, H.; Zhong, C.; Qiao, L.; Chen, S.; Xiao, M.; Song, H. Extraction of 1-amino-2-Naphthol-4-Sulfonic acid from wastewater using trioctylamine (N, N-dioctyloctan-1-amine) in methyl isobutyl ketone. J. Clean. Prod. 2018, 201, 774–782. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Zhang, Y.; Pan, C.; Liu, Y.-N. Resorcinol modified hypercrosslinked poly(styrene-co-divinlybenzene) resin and its adsorption equilibriums, kinetics and dynamics towards p-hydroxylbenzaldehyde from aqueous solution. Chem. Eng. J. 2013, 219, 238–244. [Google Scholar] [CrossRef]
- de Abreu Domingos, R.; da Fonseca, F.V. Evaluation of adsorbent and ion exchange resins for removal of organic matter from petroleum refinery wastewaters aiming to increase water reuse. J. Environ. Manag. 2018, 214, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, M.; Ahmadpour, A.; Rohani-Bastami, T.; Dabir, H. Synthesis and application of diethanolamine-functionalized polystyrene as a new sorbent for the removal of p-toluenesulfonic acid from aqueous solution. J. Ind. Eng. Chem. 2015, 30, 281–288. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Thitame, P.V.; Shukla, S.R. Adsorptive removal of naphthalenesulfonic acids using wild almond shell activated carbon from aqueous solution. Environ. Prog. Sustain. 2017, 36, 38–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Yang, W.; Yang, Z.; Li, A. A comparative study on the adsorption of 8-amino-1-naphthol-3,6-disulfonic acid by a macroporous amination resin. Chem. Eng. J. 2016, 283, 1522–1533. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Al-Mohaimeed, A.M.; Alwarthan, A. Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ. Res. 2019, 170, 389–397. [Google Scholar] [CrossRef]
- Zang, Y.; Yue, Q.; Kan, Y.; Zhang, L.; Gao, B. Research on adsorption of Cr(VI) by Poly-epichlorohydrin-dimethylamine (EPIDMA) modified weakly basic anion exchange resin D301. Ecotoxicol. Environ. Saf. 2018, 161, 467–473. [Google Scholar] [CrossRef]
- Sun, Y.; Zuo, P.; Luo, J.; Singh, R.P. Adsorption behavior of benzenesulfonic acid by novel weakly basic anion exchange resins. J. Environ. Sci. 2017, 54, 40–47. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, Q.; Pan, B.; Zhang, W.; Du, W.; Ren, H. Removal of aromatic sulfonates from aqueous media by aminated polymeric sorbents: Concentration-dependent selectivity and the application. Microporous Mesoporous Mater. 2008, 116, 63–69. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, B.; Li, A. Investigation of the anti-fouling performance of an aminated resin. Chem. Eng. J. 2012, 193–194, 139–145. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Prez, F.E.D. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Huang, J.; Jin, X.; Mao, J.; Yuan, B.; Deng, R.; Deng, S. Synthesis, characterization and adsorption properties of diethylenetriamine-modified hypercrosslinked resins for efficient removal of salicylic acid from aqueous solution. J. Hazard. Mater. 2012, 217–218, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shuang, C.; Wang, Y.; Wang, J.; Su, Y.; Li, A. Effect of pore structure on the removal of clofibric acid by magnetic anion exchange resin. Chemosphere 2018, 191, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A.; Delacôte, C. Mercury(II) binding to thiol-functionalized mesoporous silicas: Critical effect of pH and sorbent properties on capacity and selectivity. Anal. Chim. Acta 2005, 547, 3–13. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J.; Hu, J.; Liu, H. The effect of grafted amine group on the adsorption of CO2 in MCM-41: A molecular simulation. Catal. Today 2012, 194, 53–59. [Google Scholar] [CrossRef]
- Ling, Z. Study on Wastewater Treatment and Resource Utilization of Doxycycline Production. Master’s Thesis, Henan University, Kaifeng, Henan, 2006. [Google Scholar]
- Deepatana, A.; Valix, M. Steric hindrance effect on adsorption of metal–organic complexes onto aminophosphonate chelating resin. Desalination 2008, 218, 297–303. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.D.; Choo, K.-H. The removal of perchlorate using amine-functionalized mesoporous anion-exchange resins with different number of ligands. Korean J. Chem. Eng. 2013, 30, 898–905. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, B.; Zhang, W.; Pan, B.; Zhang, Q.; Ren, H. Arsenate Removal from Aqueous Media by Nanosized Hydrated Ferric Oxide (HFO)-Loaded Polymeric Sorbents: Effect of HFO Loadings. Ind. Eng. Chem. Res. 2008, 47, 3957–3962. [Google Scholar] [CrossRef]
- Zhao, H.C.; Guo, J.L.; Li, J.T.; Gao, L.L.; Bian, C.C. Synthesis and Thermal Property of Linear Chloromethylated Polystyrene. Adv. Mater. Res. 2010, 150–151, 1504–1507. [Google Scholar] [CrossRef]
- Siddiqi, H.M.; Siraj, A.; Khalid, N.; Akhtar, Z.; Haq, M.Z.U. Thermally stable epoxy polymers from new tetraglycidyl amine-based resin. J. Therm. Anal. Calorim. 2018, 132, 205–214. [Google Scholar] [CrossRef]
- Pan, B.C.; Xiong, Y.; Su, Q.; Li, A.M.; Chen, J.L.; Zhang, Q.X. Role of amination of a polymeric adsorbent on phenol adsorption from aqueous solution. Chemosphere 2003, 51, 953–962. [Google Scholar] [CrossRef]
- Tawde, S.; Mukesh, D.; Yakhmi, J.V. Redox behavior of polyaniline as influenced by aromatic sulphonate anions: Cyclic voltammetry and molecular modeling. Synth. Met. 2001, 125, 401–413. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, W.; Pan, B.; Qiu, H.; Zhang, Q.; Zhang, Q.; Zheng, S. Efficient removal of aromatic sulfonates from wastewater by a recyclable polymer: 2-naphthalene sulfonate as a representative pollutant. Environ. Sci. Technol. 2008, 42, 7411–7416. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.B.; Li, A.M.; Fu, C.; Fan, J.; Zhang, Q.X. Adsorption mechanism of aromatic sulfonates onto resins with different matrices. Ind. Eng. Chem. Res. 2007, 46, 6971–6977. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.; Jing, X.; Ling, P.; Li, A. Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel IDA-chelating resin: Isotherm and kinetic modeling. Water Res. 2011, 45, 1177–1188. [Google Scholar] [CrossRef]
- Maity, N.; Payne, G.F.; Chipchosky, J.L. Adsorptive separations based on the differences in solute-sorbent hydrogen-bonding strengths. Ind. Eng. Chem. Res. 1991, 30, 2456–2463. [Google Scholar] [CrossRef]
- Sun, Y.; Li, A.; Zhang, Q.; Chen, J.; Fu, D.; Wang, S. Adsorptive separation of tannic acid from aqueous solution by polymeric resins. Sep. Sci. Technol. 2008, 43, 389–402. [Google Scholar] [CrossRef]
- Ji, C.; Song, S.; Wang, C.; Sun, C.; Qu, R.; Wang, C.; Chen, H. Preparation and adsorption properties of chelating resins containing 3-aminopyridine and hydrophilic spacer arm for Hg(II). Chem. Eng. J. 2010, 165, 573–580. [Google Scholar] [CrossRef]
- Lei, P.; Lucy, C.A. Insight into the stability of poly(diallydimethylammoniumchloride) and polybrene poly cationic coatings in capillary electrophoresis. J. Chromatogr. A 2014, 1365, 226–233. [Google Scholar]
| Resins | SD1 | SD2 | SD3 | SD4 | SD5 |
|---|---|---|---|---|---|
| BET surface area (m2/g) | 50.43 | 49.01 | 47.45 | 42.17 | 37.96 |
| Pore volume (cm3/g) | 0.45 | 0.43 | 0.39 | 0.36 | 0.22 |
| Mean pore diameter (nm) | 34.66 | 35.97 | 32.70 | 34.47 | 23.64 |
| Total exchange capacity (mmol/g) | 1.38 | 1.81 | 2.12 | 2.57 | 3.27 |
| Weakly basic exchange capacity (mmol/g) | 1.31 | 1.74 | 2.03 | 2.42 | 3.08 |
| Strongly basic exchange capacity (mmol/g) | 0.07 | 0.07 | 0.09 | 0.15 | 0.19 |
| Adsorbents | Initial Concentration of PTSA (mg/L)/SO42− (mg/L) | ||
|---|---|---|---|
| 100/100 | 500/500 | 1000/1000 | |
| SD1 | 3.2010 | 0.8078 | 0.5074 |
| SD2 | 2.9223 | 0.7786 | 0.4628 |
| SD3 | 2.3752 | 0.6774 | 0.4014 |
| SD4 | 2.1478 | 0.6490 | 0.3455 |
| SD5 | 1.3690 | 0.5812 | 0.3003 |
| Adsorbents | Qe,exp | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| Qe,cal | k1 | R2 | Qe,cal | k2 | R2 | ||
| (mmol/g) | (mmol/g) | (1/min) | (mmol/g) | (g/mmol·min) | |||
| SD1 | 2.8419 | 2.6114 | 0.0184 | 0.9976 | 3.0208 | 0.0073 | 0.9888 |
| SD2 | 3.0869 | 2.8045 | 0.0195 | 0.9883 | 3.2218 | 0.0075 | 0.9952 |
| SD3 | 3.2943 | 2.9373 | 0.0202 | 0.9819 | 3.3630 | 0.0075 | 0.9974 |
| SD4 | 3.8598 | 3.4670 | 0.0157 | 0.9828 | 4.0619 | 0.0046 | 0.9975 |
| SD5 | 4.3795 | 4.1325 | 0.0123 | 0.9838 | 4.5570 | 0.0028 | 0.9975 |
| Adsorbents | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mmol/g) | KL (L/mmol) | R2 | KF | n | R2 | |
| SD1 | 2.7589 | 9.5222 | 0.9906 | 2.3169 | 8.4754 | 0.9972 |
| SD2 | 2.9988 | 11.1786 | 0.9920 | 2.5355 | 8.3759 | 0.9982 |
| SD3 | 3.2578 | 9.6817 | 0.9925 | 2.7110 | 7.5409 | 0.9928 |
| SD4 | 3.4916 | 13.3726 | 0.9935 | 2.9623 | 7.9107 | 0.9930 |
| SD5 | 4.2401 | 13.1659 | 0.9939 | 3.5859 | 6.9177 | 0.9804 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, X.; Zheng, W.; Ding, X.; Singh, R.P. Effect of Functional Group Density of Anion Exchange Resins on Removal of p-Toluene Sulfonic Acid from Aqueous Solution. Appl. Sci. 2020, 10, 1. https://doi.org/10.3390/app10010001
Sun Y, Li X, Zheng W, Ding X, Singh RP. Effect of Functional Group Density of Anion Exchange Resins on Removal of p-Toluene Sulfonic Acid from Aqueous Solution. Applied Sciences. 2020; 10(1):1. https://doi.org/10.3390/app10010001
Chicago/Turabian StyleSun, Yue, Xiao Li, Weisheng Zheng, Xinchun Ding, and Rajendra Prasad Singh. 2020. "Effect of Functional Group Density of Anion Exchange Resins on Removal of p-Toluene Sulfonic Acid from Aqueous Solution" Applied Sciences 10, no. 1: 1. https://doi.org/10.3390/app10010001
APA StyleSun, Y., Li, X., Zheng, W., Ding, X., & Singh, R. P. (2020). Effect of Functional Group Density of Anion Exchange Resins on Removal of p-Toluene Sulfonic Acid from Aqueous Solution. Applied Sciences, 10(1), 1. https://doi.org/10.3390/app10010001






