Evaluating the Efficiency of the Private Healthcare Facilities in Italy: A Game Cross-Efficiency DEA Modeling Framework
Abstract
1. Introduction
1.1. Background and Main Focus
1.2. The Healthcare Regulatory Context in Italy
1.3. Measuring Efficiency in Private Healthcare Facilities
2. Materials and Methods
2.1. The DEA Game Cross-Efficiency Methodology
2.2. Sample and Data
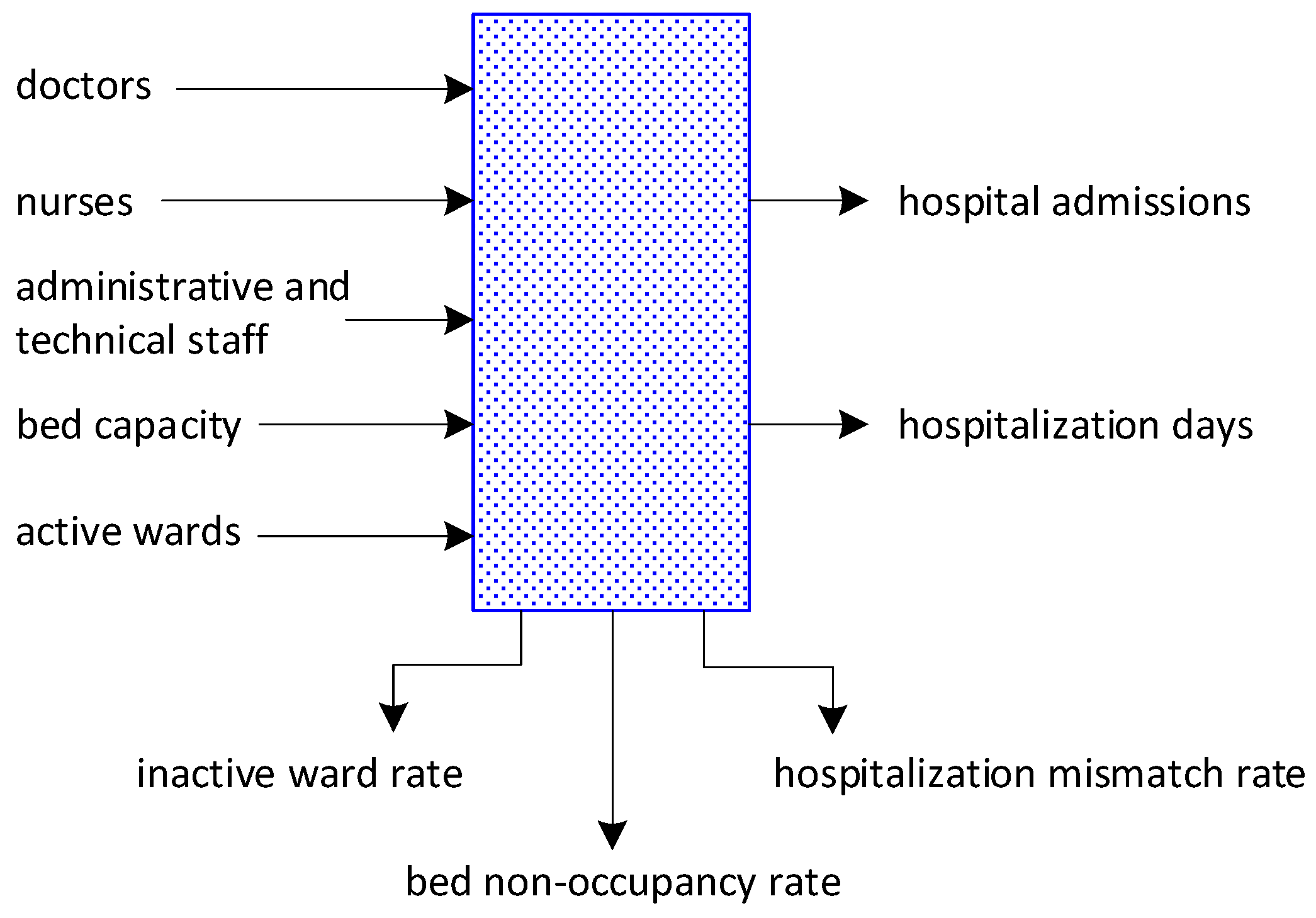
2.3. Variables of the Healthcare Facility’s Production Model
2.3.1. Endogenous Variables
2.3.2. Exogenous Variables
3. Results
3.1. Healthcare Facilities Performance
3.2. Variables Affecting the Cross-Efficiency Value of Healthcare Facilities
3.2.1. Production Model (Endogenous) Variables
3.2.2. Impact of Exogenous Variables on Efficiency
3.3. Healthcare Facility Efficiency and Regional Spending Patterns
4. Discussion
4.1. Theoretical Contributions
4.1.1. Research Focus
4.1.2. Methodological Advancement in Healthcare Efficiency Measurement
4.1.3. Reconceptualization of Scale Effects in Healthcare
4.1.4. Geographic Efficiency Patterns and Regional Healthcare Systems
4.1.5. Resource Utilization in Healthcare Settings
4.2. Policy and Managerial Implications
4.2.1. Facility-Level Management Strategies
4.2.2. Regional Healthcare Policy Implications
4.2.3. Healthcare System Design Implications
4.2.4. The Efficiency Paradox
5. Conclusions
5.1. Summary of Main Findings
5.2. Limitations and Future Research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Geographic Sub-Samples | ||
|---|---|---|---|
| N (166) | C (90) | S (188) | |
| Bed capacity | 106.01 | 91.77 | 81.24 |
| Wards | 4.75 | 3.39 | 4.49 |
| Doctors | 71.51 | 62.81 | 41.49 |
| Nurses | 69.61 | 59.84 | 43.56 |
| Administrative and technical staff | 102.86 | 91.42 | 66.72 |
| Hospital admissions | 2093.47 | 1738.84 | 1871.57 |
| Hospitalization days | 21,883.99 | 18,133.01 | 14,443.85 |
| Bed non-occupancy rate | 9.31% | 9.17% | 8.95% |
| Inactive ward rate | 9.60% | 6.11% | 7.57% |
| Hospitalization mismatch rate | 37.82% | 43.60% | 48.53% |
| Variable | Size | |||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Bed capacity | 43.84 | 69.86 | 96.09 | 160.77 |
| Wards | 2.01 | 3.17 | 4.77 | 7.52 |
| Doctors | 18.96 | 32.89 | 51.32 | 124.97 |
| Nurses | 16.41 | 28.09 | 52.43 | 129.47 |
| Administrative and technical staff | 27.32 | 49.18 | 83.16 | 181.31 |
| Hospital admissions | 710.50 | 1166.72 | 1874.80 | 3958.50 |
| Hospitalization days | 8706.87 | 13,304.49 | 17,938.48 | 31,943.47 |
| Bed non-occupancy rate | 8.41% | 9.24% | 9.73% | 9.15% |
| Inactive ward rate | 6.66% | 9.37% | 7.26% | 8.85% |
| Hospitalization mismatch rate | 43.90% | 44.73% | 43.97% | 41.50% |
Appendix B
| Healthcare Facility | Province | Cross- Efficiency | Healthcare Facility | Province | Cross- Efficiency | Healthcare Facility | Province | Cross- Efficiency |
|---|---|---|---|---|---|---|---|---|
| CASA DI CURA VILLE AUGUSTA—S.R.L. | TO | 0.998 | ISTITUTO CLINICO BEATO MATTEO | PV | 0.896 | CASA DI CURA LIOTTI | PG | 0.977 |
| CASA DI CURA VILLA PATRIZIA—S.R.L. | TO | 0.999 | CASA DI CURA CITTADELLA SOCIALE | PV | 0.290 | CASA DI CURA VILLA AURORA SRL | PG | 0.916 |
| CASA DI CURA VILLA SERENA—S.P.A. | TO | 0.998 | IST.DI CURA CITTA’DI PAVIA | PV | 0.983 | CASA DI CURA VILLA SILVIA | AN | 1.000 |
| VILLA PAPA GIOVANNI XXIII | TO | 0.999 | CASA DI CURA SANTA MARIA | BZ | 0.973 | KOS CARE SRL—CLINICA VILLA JOLANDA | AN | 1.000 |
| CASA DI CURA VILLA IRIS SRL | TO | 0.997 | CASA DI CURA VILLA SANT’ANNA | BZ | 0.999 | CASA DI CURA ‘SAN GIUSEPPE’ | AP | 0.999 |
| CASA DI CURA MADONNA DEI BOSCHI | TO | 0.999 | CASA DI CURA BONVICINI S.R.L. | BZ | 0.999 | CDC MARCHE—RETE IMPRESA AREA VASTA 2 | AN | 0.851 |
| CLINICA EPOREDIESE | TO | 0.997 | BRIXSANA PRIVATE CLINIC GMBH/SRL | BZ | 0.959 | CDC MARCHE—RETE IMPRESA AREA VASTA 3 | MC | 0.973 |
| CASA DI CURA VILLE TURINA AMIONE | TO | 0.999 | CITY CLINIC SRL | BZ | 1.000 | CDC MARCHE—RETE IMPRESA AREA VASTA 4/5 | FM | 0.924 |
| SANTA CROCE SRL | TO | 0.775 | KOS CARE SRL—U.O. OSP. S. PANCRAZIO | TN | 0.990 | CENTRO OSPEDALIERO SANTO STEFANO | MC | 1.000 |
| VILLA GRAZIA SRL | TO | 0.998 | CASA DI CURA EREMO DI ARCO SRL | TN | 0.988 | VILLA IMMACOLATA | VT | 0.998 |
| CASA DI CURA E RIPOSO S. LUCA S.P.A. | TO | 0.772 | CASA DI CURA REGINA | TN | 1.000 | CASA DEL SOLE CLINICA TOMMASO COSTA | LT | 0.839 |
| CASA DI CURA VILLA DI SALUTE SPA | TO | 0.999 | CASA DI CURA SOLATRIX | TN | 0.990 | ISTIT CHIR ORTOP TRAUMATOLOGICO | LT | 0.909 |
| ADRIANA S.R.L. | TO | 0.998 | CASA DI CURA VILLA BIANCA | TN | 0.952 | CASA DI CURA SAN MARCO | LT | 0.974 |
| POLICLINICO DI MONZA SPA | VC | 0.997 | CASA DI CURA PARK VILLA NAPOLEON | TV | 1.000 | ISTITUTO FISIOTERAPICO C. FRANCESCHINI | LT | 0.999 |
| CASA DI CURA CENTRO R.R.F. MONS. LUIGI N | VC | 0.999 | CASA DI CURA GIOVANNI XXIII | TV | 0.976 | CASA DI CURA CITTA` DI APRILIA | LT | 0.894 |
| CASA DI CURA SAN GIORGIO SRL | BI | 1.000 | CASA DI CURA POLICLINICO SAN MARCO SPA | VE | 0.889 | CASA DI CURA ‘VILLA SILVANA’ | LT | 0.976 |
| POLICLINICO DI MONZA S.P.A. PRESIDIO CLI | BI | 0.667 | CASA DI CURA SILENO E ANNA RIZZOLA | VE | 0.902 | CASA DI CURA PRIV. S. ANNA S.R.L CASSINO | FR | 0.744 |
| POLICLINICO DI MONZA SPA | NO | 0.997 | CASA DI CURA CITTÀ DI ROVIGO | RO | 0.731 | CASA DI CURA PRIV. VILLA SERENA CASSINO | FR | 1.000 |
| CASA DI CURA S. CARLO DI ARONA | NO | 0.999 | CASA DI CURA VILLA MARIA SPA | PD | 0.924 | CASA DI CURA PRIV. SANTA TERESA ISOLA L. | FR | 0.606 |
| HABILITA S.P.A.—PRESIDIO I CEDRI | NO | 0.721 | CASA DI CURA ABANO TERME POLISPEC. E TER | PD | 0.981 | CASA CURA PRIVATA’SAN RAFFAELE’ CASSINO | FR | 0.987 |
| CASA DI CURA VILLA CRISTINA | NO | 0.999 | CASA DI CURA PARCO DEI TIGLI | PD | 0.999 | I.N.I. DIV.DISTACCAT CITTA` BIANCA | FR | 0.997 |
| CASA DI CURA L’EREMO DI MIAZZINA | VB | 0.998 | CASA DI CURA ERETENIA | VI | 0.748 | CASA DI CURA SRL ‘SORA’ | FR | 0.516 |
| EX ISTITUTO CLIMATICO DI ROBILANTE DEL D | CN | 0.663 | CASA DI CURA VILLA BERICA | VI | 0.855 | CASA DI CURA VILLA DOMELIA S.R.L. | RM | 0.999 |
| CASA DI CURA MONTESERRAT S.R.L | CN | 0.999 | CASA DI CURA VILLA MARGHERITA | VI | 0.988 | CASA DI CURA POLICLINICO ITALIA | RM | 0.975 |
| FONDAZIONE ORIZZONTE SPERANZA—ONLUS CA | CN | 0.999 | CASA DI CURA CENTRO RIABILIT VERONESE | VR | 0.999 | CASA DI CURA MARCO POLO | RM | 0.936 |
| CASA DI CURA SAN MICHELE | CN | 0.998 | CASA DI CURA VILLA SANTA CHIARA | VR | 1.000 | CASA DI CURA SANTA FAMIGLIA | RM | 0.978 |
| CASA DI CURA CITTA’ DI BRA | CN | 0.971 | CASA DI CURA PRIVATA ‘S. GIORGIO’ SPA | PN | 0.688 | CASA DI CURA S.RITA DA CASCIA | RM | 1.000 |
| CASA DI CURA “LA RESIDENZA” | CN | 0.998 | POLICLINICO CITTA` DI UDINE—CC PRIVATA | UD | 0.950 | CASA DI CURA NUOVA VILLA CLAUDIA | RM | 0.971 |
| CASA DI CURA S. ANNA SPA | AT | 0.999 | SANATORIO TRIESTINO S.P.A. | TS | 0.802 | CASA DI CURA SAN FELICIANO | RM | 0.990 |
| CASA DI CURA SANT’ANNA | AL | 0.617 | POLICLINICO TRIESTINO SPA | TS | 0.888 | AURELIA HOSPITAL | RM | 0.931 |
| VILLA MARIA PIA HOSPITAL | TO | 0.851 | CASA DI CURA SAN MICHELE—ENNE S.R.L. | SV | 1.000 | CASA DI CURA VILLA VERDE | RM | 0.999 |
| CASA DI CURA KOELLIKER OSPEDALINO | TO | 0.963 | VILLA SERENA S.P.A. | GE | 0.998 | VILLA TIBERIA HOSPITAL | RM | 0.816 |
| CASA DI CURA CELLINI | TO | 0.766 | ISTITUTO CARDIOVASCOLARE CAMOGLI | GE | 0.992 | CASA DI CURA VILLA AURORA | RM | 0.961 |
| ISAV SPA | AO | 0.994 | ICLAS S.R.L. | GE | 0.633 | CASA DI CURA VILLA BETANIA | RM | 0.969 |
| CASA DI CURA DEL POLICLINICO—MILANO | MI | 0.973 | CASA DI CURA ‘ALMA MATER’ | SP | 0.623 | CASA DI CURA SALUS INFIRMORUM | RM | 0.990 |
| ISTITUTO CLINICO CITTA’ STUDI—MILANO | MI | 0.958 | CENTRO RIABILITAZIONE DON CARLO GNOCCHI | SP | 0.979 | C.DI C. AUXOLOGICO ROMA—BUON PASTORE | RM | 0.989 |
| CASA DI CURA IGEA—MILANO | MI | 0.735 | CASA DI CURA PRIVATA PIACENZA S.P.A. | PC | 0.996 | CASA DI CURA PRIVATA `DON CARLO GNOCCHI` | RM | 1.000 |
| CASA DI CURA PALAZZOLO-FOND.DON GNOCCHI | MI | 0.998 | CASA DI CURA S. GIACOMO S.R.L. | PC | 0.995 | N.C.L. ISTITUTO DI NEUROSCIENZE S.R.L. | RM | 0.975 |
| CASA DI CURA S. GIOVANNI—MILANO | MI | 0.682 | CASA DI CURA CITTA’ DI PARMA | PR | 0.856 | CASA DI CURA VILLA FULVIA SRL | RM | 0.975 |
| IST.CLINICO S. AMBROGIO SPA-MILANO | MI | 0.758 | HOSPITAL PICCOLE FIGLIE | PR | 0.828 | CASA DI CURA GUARNIERI SPA | RM | 0.977 |
| CLINICA POLISPECIALISTICA SAN CARLO—S. | MI | 0.828 | CASA DI CURA VILLA IGEA | PR | 0.999 | C.D.C. MATER MISERICORDIAE | RM | 0.999 |
| CASA DI CURA S. PIO X—MILANO | MI | 0.932 | HOSPITAL VAL PARMA | PR | 0.751 | CLINICA LATINA | RM | 1.000 |
| ISTITUTO STOMATOLOGICO ITALIANO—MILANO | MI | 0.929 | FONDAZIONE DON CARLO GNOCCHI ONLUS | PR | 0.986 | CONCORDIA HOSPITAL | RM | 0.983 |
| CASA DI CURA AMBROSIANA SPA-CESANO B. | MI | 0.920 | CENTRO CARDINAL FERRARI S.R.L. | PR | 0.982 | C.D.C. FABIA MATER | RM | 0.955 |
| POLO GERIATRICO RIABILITATIVO-CINISELLO | MI | 0.999 | SALUS HOSPITAL (CASA DI CURA PRIVATA) | RE | 0.831 | CASA DI CURA NUOVA ITOR | RM | 0.903 |
| FONDAZIONE EUROPEA DI RICERCA BIOMEDICA- | MI | 0.999 | CASA DI CURA PRIVATA POLISPECIALISTICA V | RE | 0.879 | NUOVA CLINICA ANNUNZIATELLA | RM | 0.799 |
| RESIDENZA ANNI AZZURRI MIRASOLE—OPERA | MI | 0.999 | HESPERIA HOSPITAL MODENA S.R.L. | MO | 0.902 | CASA DI CURA KAROL WOJTYLA HOSPITAL | RM | 0.978 |
| CENTRO CLINICO NEMO—FOND. SERENA—MIL | MI | 0.982 | PROF. FOGLIANI CASA DI CURA S.R.L. | MO | 0.624 | POLICLINICO CASILINO | RM | 0.972 |
| CASA DI CURA SANTA MARIA—CASTELLANZA | VA | 0.828 | OSPEDALE PRIVATO “VILLA IGEA S.P.A.” | MO | 0.991 | CASA DI CURA VILLA PIA | RM | 0.890 |
| CASA DI CURA MATER DOMINI—CASTELLANZA | VA | 0.946 | VILLA PINETA S.R.L. | MO | 0.986 | CASA DI CURA VILLA SANDRA | RM | 0.987 |
| IST.CLINICO VILLA APRICA SPA-COMO | CO | 0.871 | OSPEDALE PRIVATO ACCREDITATO VILLA CHIAR | BO | 0.795 | CASA DI CURA CITTA` DI ROMA | RM | 0.997 |
| C.O.F. LANZO HOSPITAL—ALTA VALLE INTEL | CO | 0.991 | CASA DI CURA PROF. NOBILI S.P.A. | BO | 0.990 | EUROPEAN HOSPITAL | RM | 0.997 |
| CASA DI CURA VILLA S. BENEDETTO-ALBESE | CO | 1.000 | CASA DI CURA VILLA ERBOSA OSPEDALE PRIVA | BO | 0.999 | CASA DI CURA MERRY HOUSE/C.GERIATRICO RO | RM | 0.999 |
| CASA DI CURA VILLA S. GIUSEPPE-ANZANO DE | CO | 0.994 | OSPEDALE PRIVATO ACCREDITATO NIGRISOLI | BO | 0.999 | CASA DI CURA VILLA MARIA IMMACOLATA | RM | 1.000 |
| FOND. GAETANO E PIERA BORGHI—BREBBIA | VA | 0.999 | OSPEDALE PRIVATO ACCREDITATO VILLA TORRI | BO | 0.649 | C. DI C. ‘ISTITUTO CLINICO CARDIOLOGICO’ | RM | 0.998 |
| CASA DI CURA LE TERRAZZE—CUNARDO | VA | 0.998 | OSPEDALE PRIVATO ACCREDITATO VILLA BARUZ | BO | 0.999 | POLICLINICO LUIGI DI LIEGRO | RM | 0.887 |
| CASA DI CURA BEATO L. TALAMONI-LECCO | LC | 0.630 | OSPEDALE PRIVATO ACCREDITATO VILLA BELLO | BO | 1.000 | CASA DI CURA S. RAFFAELE PORTUENSE | RM | 0.981 |
| CASA DI CURA G.B. MANGIONI—LECCO | LC | 0.991 | CASA DI CURA QUISISANA S.R.L. | FE | 0.859 | SANTO VOLTO | RM | 1.000 |
| CASA DI CURA POLICLINICO—MONZA | MB | 0.985 | CASA DI CURA SALUS S.R.L. | FE | 0.797 | I.N.I. SRL DIVISIONE MEDICUS HOTEL | RM | 0.997 |
| ISTITUTI CLINICI ZUCCHI SPA-MONZA | MB | 0.970 | OSPEDALE PRIVATO DOMUS NOVA S.P.A. | RA | 0.742 | I.N.I. SRL DIVISIONE VILLA DANTE | RM | 0.990 |
| CASA DI CURA ZUCCHI—CARATE BRIANZA | MB | 0.994 | VILLA MARIA CECILIA HOSPITAL | RA | 0.868 | NOMENTANA HOSPITAL SRL | RM | 0.996 |
| CASA DI CURA VILLA BIANCA—LIMBIATE | MB | 0.998 | OSPEDALE PRIVATO “SAN PIER DAMIANO HOSPI | RA | 0.989 | CASA DI CURA VILLA LUANA | RM | 0.999 |
| FOND. MB PER IL BAMBINO E LA SUA MAMMA— | MB | 0.991 | CASA DI CURA PRIVATA VILLA AZZURRA | RA | 0.999 | VILLA DELLE QUERCE-POLIGEST | RM | 0.905 |
| HUMANITAS GAVAZZENI | BG | 0.939 | OSPEDALE PRIVATO ACCREDITATO VILLA IGEA | FC | 0.672 | C.D.C. MADONNA DELLE GRAZIE | RM | 0.981 |
| CASA DI CURA BEATO PALAZZOLO—BERGAMO | BG | 0.982 | VILLA SERENA | FC | 0.780 | CASA DI CURA S.ANNA POMEZIA | RM | 0.865 |
| HUMANITAS CASTELLI | BG | 0.432 | MALATESTA NOVELLO | FC | 0.987 | C.D.C. I.N.I. SRL | RM | 0.983 |
| CASA DI CURA S. FRANCESCO—BERGAMO | BG | 0.993 | CASA DI CURA PRIVATA SAN LORENZINO S.P.A | FC | 0.861 | C.D.C SAN RAFFAELE MONTECOMPATRI | RM | 0.978 |
| POLICLINICO SAN MARCO—OSIO SOTTO | BG | 0.863 | SOL ET SALUS | RN | 0.978 | VILLA DEI PINI ASA SRL | RM | 0.990 |
| POLICLINICO SAN PIETRO—PONTE S. PIETR | BG | 0.929 | CASA DI CURA VILLA MARIA | RN | 0.978 | CASA DI CURA ‘DI LORENZO’ | AQ | 0.919 |
| ISTITUTO CLINICO QUARENGHI SRL | BG | 0.997 | LUCE SUL MARE | RN | 0.979 | CASA DI CURA L’IMMACOLATA | AQ | 0.925 |
| OSPEDALE S. ISIDORO—TRESCORE B. | BG | 0.999 | VILLA SALUS S.R.L. | RN | 0.999 | CASA DI CURA ‘S RAFFAELE SPA’ | AQ | 0.999 |
| NEPHROCARE S.P.A.—SERIATE | BG | 1.000 | CASA DI CURA PROF. E. MONTANARI | RN | 0.859 | CASA DI CURA PRIVATA VILLA LETIZIA | AQ | 0.972 |
| ISTITUTO CLINICO HABILITA—CISERANO | BG | 0.982 | CASA DI CURA S. RITA | PT | 0.971 | NOVA SALUS SRL | AQ | 1.000 |
| FERB-ONLUS C.TRO ALZHEIMER-GAZZANIGA | BG | 1.000 | CASA DI CURA VILLA FIORITA | PO | 0.769 | CASA DI CURA PRIVATA ‘DOTT. SPATOCCO’ | CH | 0.995 |
| HABILITA IST.CLINICO-OSP.LE DI SARNICO | BG | 0.992 | KOS CARE S.R.L. VILLA DEI PINI | FI | 0.993 | CASA DI CURA S. FRANCESCO | CH | 1.000 |
| CASA DI CURA S. CAMILLO—BRESCIA | BS | 0.758 | IFCA SPA CASA DI CURA ULIVELLA E GLICINI | FI | 0.702 | CASA DI CURA PIERANGELI | PE | 0.891 |
| ISTITUTO CLINICO S. ANNA—BRESCIA | BS | 0.923 | VILLA MARIA TERESA HOSPITAL SRL | FI | 0.999 | CASA DI CURA VILLA SERENA | PE | 0.865 |
| CASA DI CURA VILLA GEMMA-GARDONE RIV | BS | 0.889 | VILLA DELLE TERME SPA | FI | 0.999 | CASA DI CURA VILLA MARIA SRL | CB | 0.964 |
| IST.CLIN. CITTA’ DI BRESCIA—BRESCIA | BS | 0.891 | CASA DI CURA VAL DI SIEVE SRL | FI | 0.993 | CASA DI CURA PRIVATA VILLA ESTHER S.R.L. | CB | 0.974 |
| ISTITUTO CLINICO S. ROCCO S.P.A.—OME | BS | 0.926 | CASA DI CURA “LEONARDO” | FI | 0.983 | GEA MEDICA IST. EUROPEO DI RIABILITAZIONE | IS | 0.998 |
| FONDAZIONE POLIAMBULANZA—BRESCIA | BS | 0.980 | CASA DI CURA FRATE SOLE SRL | FI | 0.994 | PINETA GRANDE SPA—CDIC VILLA ESTHER | AV | 0.976 |
| CASA DI CURA VILLA BARBARANO—SALO’ | BS | 0.736 | CASA CURA S. CAMILLO FORTE DEI MARMI SRL | LU | 0.505 | CASA DI CURA VILLA MARIA | AV | 0.947 |
| RESIDENZA ANNI AZZURRI—REZZATO | BS | 1.000 | CASA DI CURA MD BARBANTINI | LU | 0.998 | CASA DI CURA S.RITA | AV | 0.972 |
| C.TRO RIAB.E.SPALENZA-FOND.DON GNOCCHI | BS | 0.750 | C. DI CURA M.D. BARBANTINI SANTA CHIARA | LU | 0.757 | CASA DI CURA VILLA MARIA | AV | 0.744 |
| CENTRO MEDICO RICHIEDEI-PALAZZOLO S/O | BS | 0.671 | CASA DI CURA S.ZITA | LU | 0.962 | CASA DI CURA MONTEVERGINE | AV | 0.988 |
| CENTRO CLINICO NEMO | BS | 0.984 | CASA DI CURA VILLE DI NOZZANO | LU | 0.983 | CASA DI CURA VILLA DEI PLATANI | AV | 0.986 |
| CASA DI CURA ANCELLE DELLA CARITA’-CR | CR | 0.998 | CASA DI CURA VILLA TIRRENA | LI | 0.561 | CASA DI CURA PRIVATA VILLA DEI PINI SPA | AV | 0.999 |
| CASA DI CURA S. CAMILLO—CREMONA | CR | 0.837 | CEN.S. MARIA ALLA PINETA F. DON GNOCCHI | MS | 0.999 | CASA DI CURA GE.P.O.S. SRL | BN | 0.998 |
| CASA DI CURA FIGLIE DI S. CAMILLO-CR | CR | 0.887 | CENTRO CHIRURGICO TOSCANO | AR | 0.919 | CASA DI CURA NUOVA CLINICA S.RITA | BN | 0.961 |
| CASA DI CURA S. CLEMENTE—MANTOVA | MN | 0.987 | CASA DI CURA S. GIUSEPPE | AR | 0.792 | CASA DI CURA SAN FRANCESCO | BN | 0.981 |
| OSPEDALE CIVILE DI VOLTA MANTOVANA | MN | 0.799 | RUGANI HOSPITAL SRL | SI | 0.980 | C.M.R. S.P.A.- CENTRO MED. DIAGN. E RIAB. | BN | 0.999 |
| OSPEDALE DI SUZZARA S.P.A. | MN | 0.912 | CASA DI CURA VILLA FIORITA | PG | 0.979 | CASA DI CURA VILLA MARGHERITA SRL | BN | 0.999 |
| OSP. SAN PELLEGRINO—CASTIGLIONE D/S | MN | 0.990 | ISTITUTO CLINICO PORTA SOLE CASA DI CURA | PG | 0.978 | CLINICA SANT`ANNA | CE | 0.998 |
| CASA DI CURA VILLA ESPERIA | PV | 0.991 | CLINICA LAMI | PG | 0.981 | VILLA DEL SOLE | CE | 0.963 |
| CASA DI CURA VILLA FIORITA | CE | 0.972 | CASA DI CURA—VILLA LUCIA HOSPITAL | BA | 0.895 | CASA DI CURA RIABILITATIVA VILLA SOFIA | CT | 0.991 |
| CASA DI CURA SAN PAOLO | CE | 0.659 | CASA DI CURA ‘ MONTE IMPERATORE’ | BA | 0.998 | IST. ONCOLOGICO DEL MEDITERRANEO SPA | CT | 0.830 |
| CASA DI CURA ‘VILLA FIORITA’ SPA | CE | 0.986 | CASA DI CURA ANTHEA | BA | 1.000 | CASA DI CURA MONS. G. CALACIURA CENACOLO | CT | 1.000 |
| CLINICA SAN MICHELE | CE | 0.991 | CASA DI CURA C.B.H. MATER DEI HOSPITAL | BA | 0.966 | CASA DI CURA PROF. E. FALCIDIA SRL | CT | 0.801 |
| CASA DI CURA PINETA GRANDE | CE | 0.924 | CASA DI CURA PROF. BRODETTI | FG | 1.000 | CASA DI CURA MUSUMECI GESCAS | CT | 0.882 |
| MINERVA S.P.A. SANTA MARIA DELLA SALUTE | CE | 0.998 | CASA DI CURA LEONARDO DE LUCA | FG | 0.998 | CASA DI CURA VALSALVA SRL | CT | 0.704 |
| VILLA DEI PINI | CE | 0.794 | CASA DI CURA ‘S. MICHELE’ GEST. BRODETTI | FG | 1.000 | PRIVATE HOSPITAL ARGENTO SRL | CT | 0.853 |
| CASA DI CURA VILLA ORTENSIA | CE | 0.999 | CASA DI CURA UNIVERSO SALUTE—DON UVA | FG | 0.999 | ISTITUTO CLINICO VIDIMURA SRL | CT | 0.887 |
| VILLA DEGLI ULIVI | CE | 0.999 | CASE CURA RIUNITE VILLA SERENA-S. FRANCE | FG | 1.000 | CLINICA SANT’AGATA TIGANO SRL | CT | 0.999 |
| VILLA DELLE MAGNOLIE | CE | 0.960 | CASA DI CURA ‘PROF. PETRUCCIANI’ S.R.L. | LE | 0.991 | I.O.M.I. F. SCALABRINO GANZIRRI | ME | 0.867 |
| CLINICA PADRE PIO S.R.L. | CE | 0.657 | CASA DI CURA VILLA BIANCA | LE | 0.999 | CASA DI CURA S. CAMILLO | ME | 0.899 |
| ALMA MATER S.P.A. ‘VILLA CAMALDOLI’ SPA | NA | 0.997 | CASA DI CURA VILLA VERDE | LE | 1.000 | CASA DI CURA CRISTO RE | ME | 0.877 |
| CASA DI CURA VILLA ANGELA SRL | NA | 1.000 | CASA DI CURA SAN FRANCESCO | LE | 0.998 | CASA DI CURA CARMONA SRL | ME | 0.819 |
| CASA DI CURA CLINIC CENTER SPA | NA | 0.983 | CASA DI CURA CITTA’ DI LECCE | LE | 0.998 | CASA DI CURA VILLA SALUS S.A.S. | ME | 0.914 |
| HERMITAGE CAPODIMONTE SPA | NA | 0.998 | CASA DI CURA RIABILITATIVA EUROITALIA | LE | 0.999 | C.O.T. S.P.A. (CURE ORTOPED. TRAUM.) | ME | 0.897 |
| CASA DI CURA VILLA DELLE QUERCE SPA | NA | 0.985 | UNIVERSO SALUTE SRL POTENZA | PZ | 0.998 | CASA DI CURA VILLA IGEA SRL | ME | 0.999 |
| CLINICA VESUVIO SRL | NA | 0.998 | IGRECO OSPEDALI RIUNITI EX LA MADONNINA | CS | 0.977 | CASA DI CURA CAPPELLANI GIOMI S.P.A. | ME | 0.814 |
| CLINICA MEDITERRANEA SPA | NA | 0.772 | IGRECO OSP. RIUNITI MADONNA DELLA CATENA | CS | 0.980 | CASA DI CURA IGEA S.N.C. | PA | 0.792 |
| CLINICA SANTA PATRIZIA | NA | 0.999 | IGRECO OSPEDALI RIUNITI EX SACRO CUORE | CS | 0.986 | CASA DI CURA CANDELA SPA | PA | 0.916 |
| CASA DI CURA VILLA CINZIA SRL | NA | 0.974 | CASA DI CURA VILLA DEL SOLE | CS | 0.973 | CASA DI CURE ORESTANO S.R.L. | PA | 0.795 |
| CLINICA SANATRIX SPA | NA | 0.998 | CASA DI CURA TRICARICO ROSANO SRL | CS | 0.967 | CASA DI CURA ‘TRIOLO ZANCLA’ S.P.A. | PA | 0.781 |
| CASA DI CURA VILLA DEI FIORI SRL | NA | 0.891 | CASA DI CURA CASCINI SRL | CS | 0.777 | CASA DI CURA SERENA S.P.A. | PA | 0.845 |
| CASA DI CURA VILLA MAIONE | NA | 0.696 | CASA DI CURA M. MISASI GR.S. BARTOLO | CS | 0.979 | CASA DI CURA NOTO PASQUALINO S.R.L. | PA | 0.892 |
| CASA DI CURA ‘CLINICA S. ANTIMO’ | NA | 0.999 | MEDICAL HOTEL CLIMAT. SPES PIETR.’ARENA’ | CS | 0.977 | NUOVA CASA DI CURA D`ANNA PIA ASS. SRL | PA | 0.999 |
| CASA DI CURA VILLA DEI FIORI | NA | 0.977 | CASA DI CURA SAN FRANCESCO | CS | 0.984 | NUOVA CASA DI CURA DEMMA SRL | PA | 0.627 |
| CASA DI CURA ‘ LA MADONNINA ‘ SRL | NA | 0.976 | ISTITUTO SANT’ANNA—VIA SIRIS 11 | KR | 0.982 | CASA DI CURA MACCHIARELLA S.P.A. | PA | 0.825 |
| CASA DI CURA S. MARIA LA BRUNA SRL | NA | 0.976 | ISTITUTO SANT’ANNA—SS 106, KM 243 | KR | 1.000 | CASA DI CURA TORINA | PA | 0.879 |
| CASA DI CURA VILLA STABIA | NA | 0.966 | CASA DI CURA S.RITA DOTT. CAPARRA | KR | 0.989 | CASA DI CURA VILLA MARGHERITA | PA | 1.000 |
| CARDIOMED S.P.A. | NA | 0.984 | ROMOLO HOSPITAL (EX VILLA EVA) | KR | 0.977 | CASA DI CURE COSENTINO DI KAROL S.R.L. | PA | 0.628 |
| CASA DI CURA MARIA ROSARIA SPA | NA | 0.942 | SADEL DI SALVATORE BAFFA SPA (EX OLIVET) | KR | 0.970 | CASA DI CURA LA MADDALENA S.P.A. | PA | 0.892 |
| STAZIONE CLIMATICA BIANCHI SRL | NA | 0.976 | CASA DI CURA MADONNA DELLO SCOGLIO SRL | KR | 0.982 | CASA DI CURA LATTERI VALSAVA S.R.L. | PA | 0.693 |
| CASA DI SALUTE S. LUCIA SRL | NA | 0.983 | MARRELLI HOSPITAL | KR | 0.952 | CASA DI CURA MARIA ELEONORA HOSPITAL SRL | PA | 0.898 |
| CASA DI CURA A. GRIMALDI | NA | 1.000 | CASA DI CURA VILLA DEL SOLE | CZ | 0.961 | CASA DI CURA CLINICA DEL MEDITERRANEO | RG | 0.719 |
| IOS—CASA DI CURA ‘ MELUCCIO’ SRL | NA | 0.936 | CASA DI CURA VILLA SERENA | CZ | 0.973 | CASA DI CURA SANTA LUCIA GLEF | SR | 0.766 |
| IOS—EX CLINICA ‘ S. FELICE’ SRL | NA | 0.836 | CASA DI CURA VILLA MICHELINO SRL | CZ | 0.978 | CASA DI CURA VILLA MAURITIUS ARC | SR | 0.999 |
| CASA DI CURA S. MARIA DEL POZZO | NA | 0.985 | CASA DI CURA SANT`ANNA HOSPITAL | CZ | 0.973 | IST.ORT. VILLA SALUS I. GALATIOTO SRL | SR | 0.792 |
| HYPPOCRATICA SPA CDC VILLA DEL SOLE | SA | 0.960 | VILLA RACHELE S.R.L. | CZ | 0.999 | C. DI CURA ‘VILLA AZZURRA’—GESIN SRL | SR | 0.896 |
| ICM-ISTITUTO CLINICO MEDITERRANEO S.P.A. | SA | 0.958 | VILLA DEI GERANI | VV | 0.636 | CLINICA VILLA RIZZO | SR | 0.736 |
| LA QUIETE—S.R.L. | SA | 0.998 | ISTITUTO ORTOPEDICO MEZZOGIORNO D`ITALIA | RC | 0.973 | CASA DI CURA VILLA DEI GERANI | TP | 0.801 |
| CASA DI CURA PRIVATA SALUS S.P.A. | SA | 0.965 | CASA DI CURA ‘VILLA AURORA’ | RC | 0.981 | CASA DI CURA SANT`ANNA SRL. | TP | 0.809 |
| CAMPOLONGO HOSPITAL S.P.A. | SA | 0.979 | POLICLINICO ‘MADONNA DELLA CONSOLAZIONE’ | RC | 0.979 | CASA DI CURA MORANA SRL | TP | 1.000 |
| ‘CASA DI CURA PROF.DOTT. LUIGI COBELLIS’ | SA | 0.998 | CASA DI CURA ‘VILLA CAMINITI’ | RC | 0.972 | CASA DI CURA RIABIL. VITTORIA S.R.L | TP | 1.000 |
| CASA DI CURA TORTORELLA SPA | SA | 0.968 | CASA DI CURA ‘VILLA S.ANNA’ | RC | 0.970 | CLINICA TOMMASINI SPA | NU | 0.947 |
| VILLA CHIARUGI SRL | SA | 0.998 | CASA DI CURA ‘VILLA ELISA’ S.P.A. | RC | 0.989 | CASA DI CURA S.ANNA S.R.L. | CA | 0.854 |
| VILLA SILVIA ‘G.F. MONTESANO’ | SA | 0.999 | CASA DI SALUTE IGNAZIO ATTARDI S.P.A. | AG | 0.891 | CASA DI CURA S. ANTONIO S.P.A. | CA | 0.936 |
| CASA DI CURA ‘SALUS’—BRINDISI | BR | 0.998 | SIA CASA DI CURA S. ANNA S.P.A. | AG | 0.889 | CASA DI CURA VILLA ELENA | CA | 0.827 |
| CASA DI CURA BERNARDINI | TA | 0.927 | CASA DI CURA ‘REGINA PACIS’ | CL | 0.548 | NUOVA CASA DI CURA S.R.L. | CA | 0.724 |
| CASA DI CURA D’AMORE S.R.L. | TA | 0.999 | SO.GE.SA. SPA C.DI.C.S. BARBARA | CL | 0.820 | C.C. ‘MADONNA DEL RIMEDIO’—ORISTANO | OR | 0.905 |
| CASA DI CURA SAN CAMILLO | TA | 0.997 | CASA DI CURA MADONNA DEL ROSARIO | CT | 0.999 | KINETIKA SARDEGNA | CA | 0.914 |
| CASA DI CURA SANTA RITA S.R.L. | TA | 0.576 | CASA DI CURA G.B. MORGAGNI S.R.L. | CT | 0.931 | MATER OLBIA HOSPITAL | SS | 0.994 |
| CASA DI CURA VILLA VERDE S.R.L. | TA | 0.999 | MATER DEI DI G.NESI & C. S.P.A | CT | 0.862 | |||
| CASA DI CURA VILLA BIANCA S.R.L. | TA | 0.999 | CASA DI CURA VILLA DEI GERANI SRL | CT | 0.977 | |||
| CENTRO MEDICO RIABILITAZIONE ICS MAUGERI | TA | 1.000 | HUMANITAS ISTITUTO CLINICO CATANESE | CT | 0.888 | mean | 0.923 | |
| FONDAZIONE CITTADELLA DELLA CARITA` | TA | 0.999 | CASA CURA CENTRO CATANESE MED. E CH. | CT | 0.916 | st.dev. | 0.111 | |
| UNIVERSO SALUTE OSP. DON UVA—BISCEGLIE | BT | 0.718 | CASA DI CURA CARMIDE | CT | 0.976 | max | 1.000 | |
| CASA DI CURA SANTA MARIA | BA | 0.999 | CASA DI CURA GIBIINO SRL | CT | 0.808 | min | 0.290 |
Appendix C

References
- Adler, N., Friedman, L., & Sinuany-Stern, Z. (2002). Review of ranking methods in the data envelopment analysis context. European Journal of Operational Research, 140, 249–265. [Google Scholar] [CrossRef]
- AGENAS. (2024). Monitoraggio spesa sanitaria: Andamento finanziamento SSN 2021–2024. Available online: https://www.agenas.gov.it/images/agenas/monitoraggio/spesa_sanitaria/dati_economici/Andamento_finanziamento_SSN_2001-2024.pdf (accessed on 16 August 2025).
- Andersen, P., & Petersen, N. C. (1993). A procedure for ranking efficient units in data envelopment analysis. Management Science, 39, 1261–1264. [Google Scholar] [CrossRef]
- Anderson, J. E., Ross, A. J., Macrae, C., & Wiig, S. (2020). Defining adaptive capacity in healthcare: A new framework for researching resilient performance. Applied Ergonomics, 87, 103111. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A., & Emvalomatis, G. (2024). Efficiency measurement in healthcare: The foundations, variables, and models—A narrative literature review. Economics, 18, 20220062. [Google Scholar] [CrossRef]
- Angulo-Meza, L. A., & Lins, M. P. E. (2002). Review of methods for increasing discrimination in data envelopment analysis. Annals of Operations Research, 116, 225–242. [Google Scholar] [CrossRef]
- Antunes, B. B. P., Bastos, L. S. L., Hamacher, S., & Bozza, F. A. (2021). Using data envelopment analysis to perform benchmarking in intensive care units. PLoS ONE, 16(11), e0260025. [Google Scholar] [CrossRef]
- Armeni, P., Borsoi, L., Notarnicola, E., & Rota, S. (2022). La spesa sanitaria: Composizione ed evoluzione nella prospettiva nazionale, regionale ed aziendale. In CERGAS (Ed.), Rapporto OASI 2022: Osservatorio sulle aziende e sul sistema sanitario italiano (pp. 93–150). EGEA. Available online: https://cergas.unibocconi.eu/observatories/oasi_/oasi-report-2022 (accessed on 16 August 2025).
- Asmild, M., Hollingsworth, B., & Birch, S. (2013). The scale of hospital production in different settings: One size does not fit all. Journal of Productivity Analysis, 40, 197–206. [Google Scholar] [CrossRef]
- Auteri, M., Guccio, C., Pammolli, F., Pignataro, G., & Vidoli, F. (2019). Spatial heterogeneity in non-parametric efficiency: An application to Italian hospitals. Social Science and Medicine, 239, 112544. [Google Scholar] [CrossRef]
- Baker, L. C., Phibbs, C. S., Guarino, C., Supina, D., & Reynolds, J. L. (2004). Within-year variation in hospital utilization and its implications for hospital costs. Journal of Health Economics, 23(1), 191–211. [Google Scholar] [CrossRef]
- Banker, R. D., & Chang, H. (2006). The super-efficiency procedure for outlier identification, not for ranking efficient units. European Journal of Operational Research, 175(2), 1311–1320. [Google Scholar] [CrossRef]
- Banker, R. D., Charnes, R. F., & Cooper, W. W. (1984). Some models for estimating technical and scale inefficiencies in data envelopment analysis. Management Science, 30, 1078–1092. [Google Scholar] [CrossRef]
- Barbetta, G. P., Turati, G., & Zago, M. A. (2007). Behavioral differences between public and private not-for-profit hospitals in the Italian national health service. Health Economics, 16, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Barra, C., Lagravinese, R., & Zotti, R. (2022). Exploring hospital efficiency within and between Italian regions: New empirical evidence. Journal of Productivity Analysis, 57, 269–284. [Google Scholar] [CrossRef]
- Battese, G. E., Rao, D. S. P., & O’Donnell, C. J. A. (2004). Metafrontier production function for estimation of technical efficiencies and technology gaps for firms operating under different technologies. Journal of Productivity Analysis, 21, 91–103. [Google Scholar] [CrossRef]
- Bayley, T., Begen, M. A., Rodrigues, F. F., & Barrett, D. (2022). Relative efficiency of radiation treatment centers: An application of data envelopment analysis. Healthcare, 10(6), 1033. [Google Scholar] [CrossRef]
- Bianchi, L., Caravella, S., & Petraglia, C. (2024). Un Paese due cure. I divari Nord-Sud nel diritto alla salute. Informazioni Svimez. Available online: https://www.astrid-online.it/static/upload/sani/sanitai_informazioni_web_ok.pdf (accessed on 22 March 2025).
- Bjorvatn, A. (2018). Private or public hospital ownership: Does it really matter? Social Science & Medicine, 196, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Blank, J. L., & Valdmanis, V. G. (2010). Environmental factors and productivity on Dutch hospitals: A semi-parametric approach. Health Care Management Science, 13(1), 27–34. [Google Scholar] [CrossRef]
- Bobini, M., Longo, F., & Ricci, A. (2020). Gli erogatori privati accreditati: Inquadramento e ruolo nella risposta del SSN al COVID-19. In CERGAS-Bocconi (Ed.), Rapporto OASI 2020. EGEA. [Google Scholar]
- Boffardi, R. (2022). How efficient is the Italian health system? Evidence on the role of political-institutional dynamics. Socio-Economic Planning Sciences, 84, 101388. [Google Scholar] [CrossRef]
- Bosque-Mercader, L., & Siciliani, L. (2023). The association between bed occupancy rates and hospital quality in the English national health service. European Journal of Health Economics, 24, 209–236. [Google Scholar] [CrossRef]
- Breiman, L., Friedman, J. H., Olshen, R. A., & Stone, C. J. (1984). Classification and regression trees. Chapman and Hall, Wadsworth Inc. [Google Scholar]
- Bruzzi, S., Ivaldi, E., & Santagata, M. (2022). Measuring regional performance in the Italian NHS: Are disparities decreasing? Social Indicators Research, 159, 1057–1084. [Google Scholar] [CrossRef]
- Burnett, S., Mendel, P., Nunes, F., Wiig, S., van den Bovenkamp, H., Karltun, A., Robert, G., Anderson, J., Vincent, C., & Fulop, N. (2016). Using institutional theory to analyse hospital responses to external demands for finance and quality in five European countries. Journal of Health Services Research and Policy, 21(2), 109–117. [Google Scholar] [CrossRef]
- Buzelli, M. L., & Boyce, T. (2021). The privatization of the Italian national health system and its impact on health emergency preparedness and response: The COVID-19 case. International Journal of Health Services, 51(4), 501–508. [Google Scholar] [CrossRef]
- Campanella, P., Azzolini, E., Izzi, A., Pelone, F., De Meo, C., La Milia, D., Specchia, M. L., & Ricciardi, W. (2017). Hospital efficiency: How to spend less maintaining quality? Annali dell’Istituto Superiore di Sanità, 53(1), 46–53. [Google Scholar]
- Campos, M. S., Fernández-Montes, A., Gavilan, J. M., & Velasco, F. (2016). Public resource usage in health systems: A data envelopment analysis of the efficiency of health systems of autonomous communities in Spain. Public Health, 138, 33–40. [Google Scholar] [CrossRef]
- Cantor, V. J. M., & Poh, K. L. (2018). Integrated analysis of healthcare efficiency: A systematic review. Journal of Medical Systems, 42(1), 8. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, M., Guccio, C., Lisi, D., & Pignataro, G. (2018). Does the extent of per case payment system affect hospital efficiency?: Evidence from the Italian NHS. Public Finance Review, 46(1), 117–149. [Google Scholar] [CrossRef]
- Chan, Y., & Walmsley, R. P. (1997). Learning and understanding the Kruskal-Wallis one-way analysis-of-variance-by-ranks test for differences among three or more independent groups. Physical Therapy & Rehabilitation Journal, 77(12), 1755–1762. [Google Scholar]
- Chang, H., Cheng, M.-A., & Das, S. (2004). Hospital ownership and operating efficiency: Evidence from Taiwan. European Journal of Operational Research, 159, 513–527. [Google Scholar] [CrossRef]
- Chen, K. C., Chen, H. M., Chien, L. N., & Yu, M. M. (2019). Productivity growth and quality changes of hospitals in Taiwan: Does ownership matter? Health Care Management Science, 22, 451–461. [Google Scholar] [CrossRef]
- Cheng, Z., Cai, M., Tao, H., He, Z., Lin, X., Lin, H., & Zuo, Y. (2016). Efficiency and productivity measurement of rural township hospitals in China: A bootstrapping data envelopment analysis. BMJ Open, 6(11), e011911. [Google Scholar] [CrossRef]
- Chern, J. Y., & Wan, T. T. H. (2000). The impact of the prospective payment system on the technical efficiency of hospitals. Journal of Medical Systems, 24, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, J. H., Park, I., Jung, I., & Dey, A. (2017). Complementary effect of patient volume and quality of care on hospital cost efficiency. Health Care Management Science, 20, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Conover, W. J. (1999). Practical nonparametric statistics (3rd ed.). John Wiley & Sons. [Google Scholar]
- Cook, W. D., & Seiford, L. M. (2009). Data envelopment analysis (DEA)—Thirty years on. European Journal of Operational Research, 192(1), 1–17. [Google Scholar] [CrossRef]
- Cooper, W. W., Seiford, L. M., & Tone, K. (2007). Data envelopment analysis: A comprehensive text with models, applications, references and DEA-Solver software. Springer-Verlag US. [Google Scholar]
- Cox, J. C., Green, E. P., & Hennig-Schmidt, H. (2016). Experimental and behavioral economics of healthcare. Journal of Economic Behavior & Organization, 131 Part B, A1–A4. [Google Scholar] [CrossRef]
- Czypionka, T., Kraus, M., Mayer, S., & Röhrling, G. (2014). Efficiency, ownership, and financing of hospitals: The case of Austria. Health Care Management Science, 17(4), 331–347. [Google Scholar] [CrossRef]
- Daidone, S., & D’Amico, F. (2009). Technical efficiency, specialization and ownership form: Evidences from a pooling of Italian hospitals. Journal of Productivity Analysis, 32, 203–216. [Google Scholar] [CrossRef]
- de Belvis, A. G., Meregaglia, M., Morsella, A., Adduci, A., Perilli, A., Cascini, F., Solipaca, A., Fattore, G., Ricciardi, W., Maresso, A., & Scarpetti, G. (2022). Italy: Health system review. Health Systems in Transition, 24(4), 1–236. [Google Scholar]
- Delai, N. (Ed.). (2023). Health & hospitals in Italy, 20th annual report 2022. Franco Angeli. [Google Scholar]
- De Nicola, A., Gitto, S., Mancuso, P., & Valdmanis, V. (2014). Healthcare reform in Italy: An analysis of efficiency based on nonparametric methods. International Journal of Health Planning Management, 29(1), e48–e63. [Google Scholar] [CrossRef]
- Dimas, G., Goula, A., & Soulis, S. (2012). Productive performance and its components in Greek public hospitals. Operational Research, 12(1), 15–27. [Google Scholar] [CrossRef]
- Di Stefano, R. (2023). L’impatto dell’emergenza sanitaria da COVID-19 sulla spesa sanitaria regionale negli anni 2019–2021. Economia e Politica, 1–6. Available online: https://www.economiaepolitica.it/_pdfs/pdf-14556.pdf (accessed on 17 August 2025).
- Domenighetti, G., Vineis, P., De Pietro, C., & Tomada, A. (2010). Ability to pay and equity in access to Italian and British national health services. European Journal of Public Health, 20, 500–503. [Google Scholar] [CrossRef]
- Doyle, J. R., & Green, R. H. (1994). Efficiency and cross-efficiency in DEA: Derivations, meanings and uses. Journal of the Operational Research Society, 45(5), 567–578. [Google Scholar] [CrossRef]
- Dyson, R. G., & Shale, E. (2010). Data envelopment analysis, operational research and uncertainty. Journal of the Operational Research Society, 61(1), 25–34. [Google Scholar] [CrossRef]
- Elliott, D. J., Young, R. S., Brice, J., Aguiar, R., & Kolm, P. (2014). Effect of hospitalist workload on the quality and efficiency of care. JAMA Internal Medicine, 174(5), 786–793. [Google Scholar] [CrossRef] [PubMed]
- Felisini, D., & Salsano, F. (2024). Between public and private: The business history of hospitals in Italy (1968–2018). In P. Fernández Pérez (Ed.), Business history of hospitals in the 20th century (Frontiers in Economic History). Springer Nature Switzerland. [Google Scholar]
- Ferreira, D. C., Figueira, J. R., Greco, S., & Marques, R. C. (2023). Data envelopment analysis models with imperfect knowledge of input and output values: An application to Portuguese public hospitals. Expert Systems with Applications, 231, 120543. [Google Scholar] [CrossRef]
- Ferreira, D. C., Nunes, A. M., & Marques, R. C. (2018). Doctors, nurses, and the optimal scale size in the Portuguese public hospitals. Health Policy, 122, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Flokou, A., Kontodimopoulos, N., & Niakas, D. (2011). Employing post-DEA cross-evaluation and cluster analysis in a sample of Greek NHS hospitals. Journal of Medical Systems, 35(5), 1001–1014. [Google Scholar] [CrossRef]
- Fragkiadakis, G., Doumpos, M., Zopounidis, C., & Germain, C. (2016). Operational and economic efficiency analysis of public hospitals in Greece. Annals of Operational Research, 247(2), 787–806. [Google Scholar] [CrossRef]
- Furukawa, M. F., Raghu, T. S., & Shao, B. B. M. (2010). Electronic medical records and cost efficiency in hospital medical-surgical units. INQUIRY: The Journal of Health Care Organization, Provision, and Financing, 47(2), 110–123. [Google Scholar] [CrossRef]
- Gallaher, J. R., Chise, Y., Reiss, R., Purcell, L. N., Manjolo, M., & Charles, A. (2021). Underutilization of operative capacity at the district hospital level in a resource-limited setting. Journal of Surgical Research, 259, 130–136. [Google Scholar] [CrossRef]
- Garavaglia, G., Lettieri, E., Agasisti, T., & Lopez, S. (2011). Efficiency and quality of care in nursing homes: An Italian case study. Health Care Management Science, 14(1), 22–35. [Google Scholar] [CrossRef]
- Gearhart, R., & Michieka, N. (2018). A comparison of the robust conditional order-m estimation and two stage DEA in measuring healthcare efficiency among California counties. Economic Modelling, 73, 395–406. [Google Scholar] [CrossRef]
- Giancotti, M., Guglielmo, A., & Mauro, M. (2017). Efficiency and optimal size of hospitals: Results of a systematic search. PLoS ONE, 12(3), e0174533. [Google Scholar] [CrossRef]
- Giancotti, M., Rotundo, G., Pipitone, V., & Mauro, M. (2018). Efficiency and optimal size of Italian public hospitals: Results from data envelopment analysis. Epidemiology, Biostatistics and Public Health, 15(4), e12929-1. [Google Scholar] [CrossRef]
- Giudice, L., Preti, L. M., & Ricci, A. (2022). Gli erogatori privati accreditati: Inquadramento e ruolo potenziale nell’implementazione del Piano Nazionale di Ripresa e Resilienza. In CERGAS (Ed.), Rapporto OASI 2022: Osservatorio sulle aziende e sul sistema sanitario italiano (pp. 151–198). EGEA. Available online: https://cergas.unibocconi.eu/observatories/oasi_/oasi-report-2022 (accessed on 15 January 2025).
- Giuliani, G., & Gitto, S. (2025). What is the difference between specialisation and diversity in hospitals? Investigating their relationship with efficiency. Socio-Economic Planning Sciences, 100, 102251. [Google Scholar] [CrossRef]
- Grosskopf, S., & Valdmanis, V. (1993). Evaluating hospital performance with case-mix adjusted outputs. Medical Care, 31(6), 525–532. [Google Scholar] [CrossRef] [PubMed]
- Gruca, T. S., & Nath, D. (2001). The technical efficiency of hospitals under a single payer system: The case of Ontario community hospitals. Health Care Management Science, 4, 91–101. [Google Scholar] [CrossRef]
- Guccio, C., Pignataro, G., Romeo, D., & Vidoli, F. (2024). Is austerity good for efficiency, at least? A counterfactual assessment for the Italian NHS. Socio-Economic Planning Sciences, 92, 101798. [Google Scholar] [CrossRef]
- Guerrini, A., Romano, G., Campedelli, B., Moggi, S., & Leardini, C. (2018). Public vs. private in hospital efficiency: Exploring determinants in a competitive environment. International Journal of Public Administration, 41(3), 181–189. [Google Scholar] [CrossRef]
- Huang, T., Li, S., Liu, F., & Diao, H. (2024). A slacks-based measure model for computing game cross-efficiency. Systems, 12(3), 78. [Google Scholar] [CrossRef]
- Huybrechts, I., Declercq, A., Verté, E., Raeymaeckers, P., & Anthierens, S. (2024). How does the external context affect an implementation processes? A qualitative study investigating the impact of macro-level variables on the implementation of goal-oriented primary care. Implement Science, 19(1), 32. [Google Scholar] [CrossRef]
- Jones, R. (2011). Hospital bed occupancy demystified. British Journal of Healthcare Management, 17(6), 242–248. [Google Scholar] [CrossRef]
- Jordi, E., Pley, C., Jowett, M., Jaoude, G. J. A., & Haghparast-Bidgoli, H. (2020). Assessing the efficiency of countries in making progress towards universal health coverage: A data envelopment analysis of 172 countries. BMJ Global Health, 5, e002992. [Google Scholar] [CrossRef] [PubMed]
- Jung, S., Son, J., Kim, C., & Chung, K. (2023). Efficiency measurement using Data Envelopment Analysis (DEA) in public healthcare: Research trends from 2017 to 2022. Processes, 11(3), 811. [Google Scholar] [CrossRef]
- Kaya, S., & Cafrı, R. (2016). Analysis of the efficiency determinants of health systems in OECD countries by DEA and panel Tobit. Social Indicators Research, 129, 113–132. [Google Scholar] [CrossRef]
- Klumpp, M., Loske, D., & Bicciato, S. (2022). COVID-19 health policy evaluation: Integrating health and economic perspectives with a data envelopment analysis approach. European Journal of Health Economics, 23, 1263–1285. [Google Scholar] [CrossRef]
- Kohl, S., Schoenfelder, J., Fügener, A., & Brunner, J. O. (2019). The use of Data Envelopment Analysis (DEA) in healthcare with a focus on hospitals. Health Care Management Science, 22, 245–286. [Google Scholar] [CrossRef]
- Kruse, F. M., Stadhouders, N. W., Adang, E. M., Groenewoud, S., & Jeurissen, P. P. T. (2018). Do private hospitals outperform public hospitals regarding efficiency, accessibility, and quality of care in the European Union? A literature review. International Journal of Health Planning and Management, 33(2), e434–e453. [Google Scholar] [CrossRef]
- La Rocca, A., & Hoholm, T. (2017). Coordination between primary and secondary care: The role of electronic messages and economic incentives. BMC Health Services Research, 17, 149. [Google Scholar] [CrossRef]
- Lee, S.-Y., Chinnam, R. B., Dalkiran, E., Krupp, S., & Nauss, M. (2021). Proactive coordination of inpatient bed management to reduce emergency department patient boarding. International Journal of Production Economics, 231, 107842. [Google Scholar] [CrossRef]
- Lemon, S. C., Roy, J., Clark, M. A., Friedmann, P. D., & Rakowski, W. (2003). Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Annals of Behavioral Medicine, 26(3), 172–181. [Google Scholar] [CrossRef]
- Li, H., & Dong, S. (2015). Measuring and benchmarking technical efficiency of public hospitals in Tianjin, China: A bootstrap–data envelopment analysis approach. INQUIRY: Journal of Health Care Organization, Provision, and Financing, 52, 0046958015605487. [Google Scholar] [CrossRef]
- Li, H., Tao, H., & Li, G. (2021). Predictors and reasons for inappropriate hospitalization days for surgical patients in a tertiary hospital in Wuhan, China: A retrospective study. BMC Health Services Research, 21, 900. [Google Scholar] [CrossRef]
- Liang, L., Wu, J., Cook, W. D., & Zhu, J. (2008). The DEA game cross-efficiency model and its Nash equilibrium. Operations Research, 56, 1278–1288. [Google Scholar] [CrossRef]
- Madsen, F., Ladelund, S., & Linneberg, A. (2014). High levels of bed occupancy associated with increased inpatient and thirty-day hospital mortality in Denmark. Health Affairs, 33(7), 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Mahate, A., Hamidi, S., & Akinci, F. (2016). Measuring the effect of size on technical efficiency of the United Arab Emirates hospitals. Global Journal of Health Science, 9(3), 116. [Google Scholar] [CrossRef]
- Maietti, E., Sanmarchi, F., Toth, F., de Pietro, C., Fantini, M. P., & Golinelli, D. (2023). Changes in private health service utilisation and access to the Italian National Health Service between 2006 and 2019: A cross-sectional comparative study. BMJ Open, 13(5), e070975. [Google Scholar] [CrossRef]
- Mancuso, P., & Valdmanis, V. G. (2016). Care appropriateness and health productivity evolution: A non-parametric analysis of the Italian regional health systems. Applied Health Economics and Health Policy, 14(5), 595–607. [Google Scholar] [CrossRef]
- Mapelli, V. (1999). Il sistema sanitario italiano. Il Mulino. [Google Scholar]
- Maragos, E. K., Maravelakis, P. E., & Linardis, A. I. (2021). A DEA evaluation of the successful implementation of HEALTH2020 policies. Socio-Economic Planning Sciences, 76, 100968. [Google Scholar] [CrossRef]
- Martini, G., Berta, P., Mullahy, J., & Vittadini, G. (2014). The effectiveness–efficiency trade-off in health care: The case of hospitals in Lombardy, Italy. Regional Science and Urban Economics, 49, 217–231. [Google Scholar] [CrossRef]
- Matranga, D., Bono, F., Casuccio, A., Firenze, A., Marsala, L., Giaimo, R., Sapienza, F., & Vitale, F. (2014). Evaluating the effect of organization and context on technical efficiency: A second-stage DEA analysis of Italian hospitals. Epidemiology, Biostatistics, and Public Health, 11(1), e8785.1–e8785.11. [Google Scholar] [CrossRef] [PubMed]
- Matranga, D., & Sapienza, F. (2015). Congestion analysis to evaluate the efficiency and appropriateness of hospitals in Sicily. Health Policy, 119, 324–332. [Google Scholar] [CrossRef]
- Medarević, A., & Vuković, D. (2021). Efficiency and productivity of public hospitals in serbia using DEA-Malmquist model and Tobit regression model, 2015–2019. International Journal of Environmental Research and Public Health, 18(23), 12475. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. (2015). Annuario statistico del Servizio Sanitario Nazionale: Assetto organizzativo, attività e fattori produttivi del SSN, anno 2012. Available online: https://www.quotidianosanita.it/allegati/allegato3094452.pdf (accessed on 11 December 2024).
- Ministero della Salute. (2023). Annuario statistico del Servizio Sanitario Nazionale: Assetto organizzativo, attività e fattori produttivi del SSN, anno 2022. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3425_allegato.pdf (accessed on 25 February 2025).
- Ministero della Salute. (2024a). Banca dati. Available online: https://www.salute.gov.it/new/it/banche-dati/banca-dati-del-servizio-sanitario-nazionale (accessed on 17 August 2024).
- Ministero della Salute. (2024b). Rapporto annuale sull’attività di ricovero ospedaliero—DATI SDO 2022. Available online: https://www.salute.gov.it/new/it/pubblicazione/rapporto-annuale-sullattivita-di-ricovero-ospedaliero-dati-sdo-2022 (accessed on 18 August 2025).
- Ministero della Salute. (2025). Rapporto annuale sull’attività di ricovero ospedaliero—DATI SDO 2023. Available online: https://www.salute.gov.it/new/it/pubblicazione/rapporto-annuale-sullattivita-di-ricovero-ospedaliero-dati-sdo-2023 (accessed on 18 August 2025).
- Mitropoulos, P. (2022). A metafrontier Global Malmquist framework for hospitals productivity and quality measurement: Evidence from the Greek economic recession. EURO Journal on Decision Processes, 10, 100018. [Google Scholar] [CrossRef]
- Molander, P. (2025). Public versus private healthcare systems in the OECD area—A broad evaluation of performance. European Journal of Health Economics. (in press).
- Nayar, P., & Ozcan, Y. A. (2008). Data envelopment analysis comparison of hospital efficiency and quality. Journal of Medical Systems, 32, 193–199. [Google Scholar] [CrossRef]
- Nayar, P., Ozcan, Y. A., Yu, F., & Nguyen, A. T. (2013). Benchmarking urban acute care hospitals: Efficiency and quality perspectives. Health Care Management Review, 38(2), 137–145. [Google Scholar] [CrossRef]
- Nepomuceno, T. C. C., Piubello Orsini, L., de Carvalho, V. D. H., Poleto, T., & Leardini, C. (2022). The core of healthcare efficiency: A comprehensive bibliometric review on frontier analysis of hospitals. Healthcare, 10, 1316. [Google Scholar] [CrossRef]
- Nuti, S., Daraio, C., Speroni, C., & Vainieri, M. (2011). Relationships between technical efficiency and the quality and costs of health care in Italy. International Journal for Quality in Health Care, 23(3), 324–330. [Google Scholar] [CrossRef][Green Version]
- Oikonomou, N., Tountas, Y., Mariolis, A., Souliotis, K., Athanasakis, K., & Kyriopoulos, J. (2016). Measuring the efficiency of the Greek rural primary health care using a restricted DEA model: The case of southern and western Greece. Health Care Management Science, 19(4), 313–325. [Google Scholar] [CrossRef]
- Ozcan, M., & Peker, S. (2023). A classification and regression tree algorithm for heart disease modeling and prediction. Healthcare Analytics, 3, 100130. [Google Scholar] [CrossRef]
- Ozcan, Y. A. (2009). Quantitative methods in health care management: Techniques and applications (2nd ed.). Jossey-Bass Wiley. [Google Scholar]
- Ozcan, Y. A. (2014). Health care benchmarking and performance evaluation (2nd ed.). Springer. [Google Scholar]
- Paraschi, E. P. (2023). Healthcare efficiency assessment in the southeastern european countries using two-stage DEA analysis. In N. Persiani, I. E. Vannini, A. Romiti, A. Karasavvoglou, & P. Polychronidou (Eds.), Challenges of healthcare systems in the era of COVID-19. Contributions to Management Science. [Google Scholar]
- Perrin, J. M., & Valvona, J. (1986). Does increased physician supply affect quality of care? Health Affairs, 5, 63–72. [Google Scholar] [CrossRef][Green Version]
- Pianori, D., Maietti, E., Lenzi, J., Quargnolo, M., Guicciardi, S., Adja, K. Y. C., Fantini, M. P., & Toth, F. (2020). Sociodemographic and health service organizational factors associated with the choice of the private versus public sector for specialty visits: Evidence from a national survey in Italy. PLoS ONE, 15, e0232827. [Google Scholar] [CrossRef]
- Piubello Orsini, L., Leardini, C., Vernizzi, S., & Campedelli, B. (2021). Inefficiency of public hospitals: A multistage data envelopment analysis in an Italian region. BMC Health Services Research, 21, 1281. [Google Scholar] [CrossRef] [PubMed]
- Puiu, I. A., & Bîlbîie, A. (2025). Measuring productivity in the healthcare sector: A bibliometric and content analysis. Health Economics Review, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Ramanuj, P. P., Scharf, D. M., Ferenchick, E., Spaeth-Rublee, B., & Pincus, H. A. (2018). Measuring efficiency at the interface of behavioral and physical health care. The Journal of Mental Health Policy and Economics, 21(2), 79–86. [Google Scholar] [PubMed]
- Ramírez-Valdivia, M. T., Maturana, S., & Salvo-Garrido, S. (2011). A multiple stage approach for performance improvement of primary healthcare practice. Journal of Medical Systems, 35(5), 1015–1028. [Google Scholar] [CrossRef]
- Rebba, V., & Rizzi, D. (2001). Analisi dell’efficienza relativa delle strutture di ricovero con il metodo DEA: Il caso degli ospedali del Veneto. Politiche Sanitarie, 2(1), 23–43. [Google Scholar]
- Rosko, M. D., & Chilingerian, J. A. (1999). Estimating hospital inefficiency: Does case mix matter? Journal of Medical Systems, 23, 57–71. [Google Scholar] [CrossRef]
- Sala-Garrido, R., Mocholi-Arce, M., Molinos-Senante, M., & Maziotis, A. (2022). Measuring technical, environmental and eco-efficiency in municipal solid waste management in Chile. International Journal of Sustainable Engineering, 15(1), 71–85. [Google Scholar] [CrossRef]
- Salinas-Jiménez, J., & Smith, P. (1996). Data envelopment analysis applied to quality in primary health care. Annals of Operations Research, 67, 141–161. [Google Scholar] [CrossRef]
- Sapienza, F., & Matranga, D. (2018). Evaluating the reform of the healthcare system in Sicily: Variations of efficiency and appropriateness between 2008 and 2010. Epidemiology Biostatistics and Public Health, 15(1), e12593-1–e12593-10. [Google Scholar] [CrossRef]
- Sasso, L., Bagnasco, A., Zanini, M., Catania, G., Aleo, G., Santullo, A., Spandonaro, F., Icardi, G., Watson, R., & Sermeus, W. (2017). The general results of the RN4CAST survey in Italy. Journal of Advanced Nursing, 73, 2028–2030. [Google Scholar] [CrossRef]
- Scott, A., Shiell, A., & Farnworth, M. G. (1993). The value of early discharge: Dispelling some myths. Health Policy, 26(2), 81–91. [Google Scholar] [CrossRef]
- Seiford, L. M., & Zhu, J. (2002). Modeling undesirable factors in efficiency evaluation. European Journal of Operational Research, 142(1), 16–20. [Google Scholar] [CrossRef]
- Sexton, T. R., Silkman, R. H., & Hogan, A. J. (1986). Data envelopment analysis: Critique and extensions. In R. H. Silkman (Ed.), Measuring efficiency: An assessment of data envelopment analysis (pp. 73–105). Jossey-Bass. [Google Scholar]
- Sheather, S. J. (2004). Density estimation. Statistical Science, 19(4), 588–597. [Google Scholar] [CrossRef]
- Signorelli, C., Odone, A., Oradini-Alacreu, A., & Pelissero, G. (2020). Universal health coverage in Italy: Lights and shades of the Italian National Health Service which celebrated its 40th anniversary. Health Policy, 124(1), 69–74. [Google Scholar] [CrossRef]
- Silverman, B. W. (1998). Density estimation for statistics and data analysis (1st ed.). Routledge. [Google Scholar]
- Søndergaard, S. F., Rasmussen, B., Kerr, D., Frederiksen, K., Redley, B., Trueman, M., Kolbaek, R., Laursen, H. S., & Bloomer, M. J. (2023). Nurses’ work experiences in hospital wards with single rooms: An integrative review. Journal of Clinical Nursing, 32(19–20), 7036–7049. [Google Scholar] [CrossRef] [PubMed]
- Staat, M. (2006). Efficiency of hospitals in Germany: A DEA–bootstrap approach. Applied Economics, 38(19), 2255–2263. [Google Scholar] [CrossRef]
- Stefko, R., Gavurova, B., & Kocisova, K. (2018). Healthcare efficiency assessment using DEA analysis in the Slovak Republic. Health Economic Review, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Stone, K., Zwiggelaar, R., Jones, P., & Mac Parthaláin, N. (2022). A systematic review of the prediction of hospital length of stay: Towards a unified framework. PLOS Digital Health, 1(4), e0000017. [Google Scholar] [CrossRef]
- Su, W., Hou, Y., Huang, M., Xu, J., Du, Q., & Wang, P. (2023). Evaluating the efficiency of primary health care institutions in China: An improved three-stage data envelopment analysis approach. BMC Health Services Research, 23(1), 995. [Google Scholar] [CrossRef]
- Tiemann, O., & Schreyögg, J. (2012). Changes in hospital efficiency after privatization. Health Care Management Science, 15, 310–326. [Google Scholar] [CrossRef]
- Toth, F. (2020). Reducing waiting times in the Italian NHS: The case of Emilia-Romagna. Social Policy and Administration, 54, 1110–1122. [Google Scholar] [CrossRef]
- Trerise, B., Dodek, P., Leung, A., & Spinelli, J. J. (2001). Underutilization of acute care settings in a tertiary care hospital. International Journal for Quality in Health Care, 13(1), 27–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tynkkynen, L. K., & Vrangbæk, K. (2018). Comparing public and private providers: A scoping review of hospital services in Europe. BMC Health Services Research, 18, 141. [Google Scholar] [CrossRef]
- Valdmanis, V., Rosko, M., Mancuso, P., Tavakoli, M., & Farrar, S. (2017). Measuring performance change in Scottish hospitals: A Malmquist and time-series approach. Health Services and Outcomes Research Methodology, 17, 113–126. [Google Scholar] [CrossRef][Green Version]
- van Doorslaer, E., & Koolman, X. (2004). Explaining the differences in income-related health inequalities across European countries. Health Economics, 13(7), 609–628. [Google Scholar] [CrossRef]
- Varabyova, Y., Blankart, C. R., Torbica, A., & Schreyögg, J. (2017). Comparing the efficiency of hospitals in Italy and Germany: Nonparametric conditional approach based on partial frontier. Health Care Management Science, 20(3), 379–394. [Google Scholar] [CrossRef]
- Vitaliano, D. F. (1987). On the estimation of hospital cost-functions. Journal of Health Economics, 6, 305–318. [Google Scholar] [CrossRef]
- Wang, Y. M., & Chin, K. S. (2010). Some alternative models for DEA cross-efficiency evaluation. International Journal of Production Economics, 128, 332–338. [Google Scholar] [CrossRef]
- Wilson, P. (1995). Detecting influential observations in data envelopment analysis. Journal of Productivity Analysis, 6(1), 27–45. [Google Scholar] [CrossRef]
- Wu, J., Liang, L., & Chen, Y. (2009). DEA game cross-efficiency approach to Olympic rankings. Omega, 37, 909–918. [Google Scholar] [CrossRef]
- Zare, H., Tavana, M., Mardani, A., Masoudian, S., & Saraji, M. K. (2019). A hybrid data envelopment analysis and game theory model for performance measurement in healthcare. Health Care Management Science, 22, 475–488. [Google Scholar] [CrossRef]
- Zarrin, M., Schoenfelder, J., & Brunner, J. O. (2022). Homogeneity and best practice analyses in hospital performance management: An analytical framework. Health Care Management Science, 25, 406–425. [Google Scholar] [CrossRef]
- Zhu, J. (2003). Quantitative models for performance evaluation and benchmarking: Data envelopment analysis with spreadsheets and DEA Excel solver. Kluwer Academic Publishers. [Google Scholar]
- Zimmerman, R. K., Balasubramani, G. K., Nowalk, M. P., Eng, H., Urbanski, L., Jackson, M. L., Jackson, L. A., McLean, H. Q., Belongia, E. A., Monto, A. S., Malosh, R. E., Gaglani, M., Clipper, L., Flannery, B., & Wisniewski, S. R. (2016). Classification and regression tree (CART) analysis to predict influenza in primary care patients. BMC Infectious Disease, 16(1), 503. [Google Scholar] [CrossRef]
- Zinn, J., & Flood, A. B. (2009). Commentary: Slack resources in health care organizations--fat to be trimmed or muscle to be exercised? Health Services Research, 44(3), 812–820. [Google Scholar] [CrossRef]











| Region | Regional Budget Deficit (€ per Capita) | Total SSN Expenditure for Accredited Private Care (€ per Capita) | Regional SSN Funding Allocation (€ per Capita) | Resident Population | Total Number of Healthcare Facilities |
|---|---|---|---|---|---|
| Piedmont | 6.8 | 356 | 2011.58 | 4,256,350 | 33 |
| Aosta Valley | 3.7 | 162 | 1994.16 | 123,360 | 1 |
| Lombardy | −3.0 | 550 | 1964.70 | 9,943,004 | 63 |
| Trentino–South Tyrole | −4.4 | 263 | 1935.59 | 1,073,574 | 10 |
| Veneto | −4.6 | 334 | 1968.34 | 4,847,745 | 13 |
| Friuli Venezia-Giulia | 0.6 | 221 | 2013.15 | 1,194,647 | 4 |
| Liguria | 0.3 | 261 | 2044.09 | 1,509,227 | 6 |
| Emilia-Romagna | −4.3 | 347 | 1986.95 | 4,425,366 | 36 |
| Tuscany | 51.7 | 250 | 1999.62 | 3,663,191 | 19 |
| Umbria | −0.2 | 212 | 2013.25 | 858,812 | 5 |
| Marche | −10.5 | 296 | 2006.52 | 1,487,150 | 7 |
| Latium | −2.9 | 566 | 1952.80 | 5,714,882 | 59 |
| Abruzzo | −1.0 | 289 | 1989.89 | 1,275,950 | 9 |
| Molise | 135.0 | 534 | 2026.36 | 292,150 | 3 |
| Campania | 13.3 | 431 | 1921.80 | 5,624,420 | 59 |
| Apulia | 29.8 | 419 | 1947.77 | 3,922,941 | 26 |
| Basilicata | −14.3 | 241 | 1999.38 | 541,168 | 1 |
| Calabria | −74.8 | 270 | 1967.71 | 1,855,454 | 28 |
| Sicily | −1.9 | 423 | 1937.38 | 4,833,329 | 54 |
| Sardinia | 3.9 | 292 | 1998.22 | 1,587,413 | 8 |
| Variable | Type | Unit of Measurement | Statistics | |||
|---|---|---|---|---|---|---|
| Mean | St. Dev. | Max | Min | |||
| Bed capacity | input | number of units | 92.637 | 70.417 | 664 | 6 |
| Active wards | input | number of units | 4.3671 | 3.4827 | 23 | 1 |
| Doctors | input | number of persons | 57.036 | 56.075 | 391 | 1 |
| Nurses | input | number of persons | 56.601 | 64.114 | 676 | 2 |
| Administrative and technical staff | input | number of persons | 85.241 | 79.387 | 748 | 3 |
| Hospital admissions | output (good) | number of units | 1927.628 | 2175.117 | 23,221 | 17 |
| Hospitalization days | output (good) | number of units | 17,973.330 | 14,963.840 | 134,910 | 73 |
| Bed non-occupancy rate | output (bad) | percentage | 9.13% | 11.71% | 81.29% | 0.00% |
| Inactive ward rate | output (bad) | percentage | 8.03% | 13.31% | 75.00% | 0.00% |
| Hospitalization mismatch rate | output (bad) | percentage | 43.53% | 21.29% | 97.35% | 0.00% |
| Region | Geographic Area | Bed Capacity | Active Wards | Doctors | Nurses | Administrative and Technical Staff |
|---|---|---|---|---|---|---|
| Piedmont | North | 86.576 | 3.242 | 32.424 | 38.000 | 74.212 |
| Aosta Valley | North | 125 | 3 | 26 | 31 | 31 |
| Lombardy | North | 130.381 | 5.905 | 97.302 | 97.143 | 142.984 |
| Trentino–South Tyrole | North | 87.700 | 2.400 | 30.100 | 31.200 | 56.700 |
| Veneto | North | 113.615 | 3.923 | 69.769 | 79.462 | 114.846 |
| Friuli Venezia-Giulia | North | 117.250 | 6.750 | 99.250 | 88.500 | 153.750 |
| Liguria | North | 52.000 | 2.333 | 27.333 | 32.500 | 44.500 |
| Emilia-Romagna | North | 90.750 | 5.306 | 79.861 | 62.694 | 73.472 |
| Tuscany | Center | 72.053 | 3.421 | 54.000 | 46.000 | 69.421 |
| Umbria | Center | 49.800 | 3.200 | 51.200 | 20.600 | 36.600 |
| Marche | Center | 123.571 | 4.143 | 61.714 | 66.000 | 112.714 |
| Latium | Center | 97.898 | 3.305 | 66.763 | 66.898 | 100.627 |
| Abruzzo | South and islands | 115.111 | 5.333 | 56.667 | 72.778 | 92.222 |
| Molise | South and islands | 46.667 | 2.000 | 27.333 | 21.333 | 42.000 |
| Campania | South and islands | 85.085 | 4.441 | 39.932 | 47.458 | 70.458 |
| Apulia | South and islands | 95.923 | 5.000 | 40.538 | 56.615 | 84.077 |
| Basilicata | South and islands | 40 | 2 | 10 | 54 | 198 |
| Calabria | South and islands | 60.714 | 2.500 | 31.357 | 28.643 | 56.750 |
| Sicily | South and islands | 73.722 | 4.722 | 41.574 | 32.981 | 51.778 |
| Sardinia | South and islands | 107.875 | 9.000 | 83.250 | 70.125 | 82.750 |
| Region | Cross-Efficiency Mean | Median | Max | Min | Number of Full Efficient Healthcare Facilities (*) |
|---|---|---|---|---|---|
| Piedmont | 0.931 | 0.998 | 1.000 | 0.617 | 1 |
| Aosta Valley | 0.994 | 0.994 | 0.994 | 0.994 | 0 |
| Lombardy | 0.906 | 0.973 | 1.000 | 0.290 | 4 |
| Trentino–South Tyrol | 0.985 | 0.990 | 1.000 | 0.952 | 2 |
| Veneto | 0.922 | 0.976 | 1.000 | 0.731 | 2 |
| Friuli Venezia-Giulia | 0.832 | 0.845 | 0.950 | 0.688 | 0 |
| Liguria | 0.871 | 0.985 | 1.000 | 0.623 | 1 |
| Emilia-Romagna | 0.900 | 0.978 | 1.000 | 0.624 | 1 |
| Tuscany | 0.887 | 0.980 | 0.999 | 0.505 | 0 |
| Umbria | 0.966 | 0.978 | 0.981 | 0.916 | 0 |
| Marche | 0.964 | 0.999 | 1.000 | 0.851 | 3 |
| Latium | 0.947 | 0.981 | 1.000 | 0.516 | 6 |
| Abruzzo | 0.952 | 0.972 | 1.000 | 0.865 | 2 |
| Molise | 0.979 | 0.974 | 0.998 | 0.964 | 0 |
| Campania | 0.951 | 0.981 | 1.000 | 0.657 | 2 |
| Apulia | 0.963 | 0.999 | 1.000 | 0.576 | 6 |
| Basilicata | 0.998 | 0.998 | 0.998 | 0.998 | 0 |
| Calabria | 0.958 | 0.977 | 1.000 | 0.636 | 1 |
| Sicily | 0.861 | 0.881 | 1.000 | 0.548 | 4 |
| Sardinia | 0.887 | 0.909 | 0.994 | 0.724 | 0 |
| Sample | Node | N | Percent | Mean |
|---|---|---|---|---|
| training | 3 | 139 | 63.5% | 0.989 |
| 4 | 23 | 10.5% | 0.897 | |
| 5 | 33 | 15.1% | 0.834 | |
| 6 | 24 | 11.0% | 0.665 | |
| test | 3 | 147 | 65.3% | 0.987 |
| 4 | 22 | 9.8% | 0.915 | |
| 5 | 36 | 16.0% | 0.835 | |
| 6 | 20 | 8.9% | 0.661 |
| Sample | Estimate | Std. Error |
|---|---|---|
| training | 0.002 | 0.001 |
| test | 0.001 | 0.000 |
| Grouping Criteria | Groups | Cross-Efficiency Statistics | ||||
|---|---|---|---|---|---|---|
| Median | Mean | St. Dev. | Max | Min | ||
| facility size | Q1 | 0.998 | 0.934 | 0.119 | 1.000 | 0.548 |
| Q2 | 0.980 | 0.916 | 0.128 | 1.000 | 0.290 | |
| Q3 | 0.977 | 0.924 | 0.107 | 1.000 | 0.432 | |
| Q4 | 0.939 | 0.919 | 0.087 | 1.000 | 0.505 | |
| macro-region | N | 0.982 | 0.913 | 0.125 | 1.000 | 0.290 |
| C | 0.979 | 0.937 | 0.108 | 1.000 | 0.505 | |
| S | 0.973 | 0.926 | 0.099 | 1.000 | 0.548 | |
| entire sample | 0.978 | 0.923 | 0.111 | 1.000 | 0.290 | |
| Cross-Efficiency (Median Value) | Sum of Ranks | H-Test | Prob. | Dunn’s Test | ||||
|---|---|---|---|---|---|---|---|---|
| N | C | S | N | C | S | |||
| 0.982 | 0.980 | 0.974 | 36,810 | 21,179.5 | 40,800.5 | 1.252 | 0.535 | - |
| Cross-Efficiency (Median Value) | Sum of Ranks | H-Test | Prob. | Dunn’s Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| 0.998 | 0.980 | 0.977 | 0.939 | 30,318 | 24,828 | 23,414.5 | 20,229.5 | 29.213 | 0.000 | Q1–Q2; Q1–Q3; Q1–Q4 |
| [1] | [2] | [3] | [4] | [5] | [6] | |
|---|---|---|---|---|---|---|
| [1] cross-efficiency | - | |||||
| [2] regional budget deficit | 0.055/−0.176 | - | ||||
| [3] total SSN expenditure for accredited private care | −0.022/−0.168 | 0.368/0.101 | - | |||
| [4] regional SSN funding allocation | −0.149/−0.050 | 0.261/0.244 | −0.390/−0.454 | - | ||
| [5] resident population | −0.315/−0.550 | −0.096/−0.074 | 0.684/0.707 (*) | −0.523/−0.556 | - | |
| [6] total number of healthcare facilities | −0.216/−0.402 | −0.158/−0.074 | 0.718/0.716 (*) | −0.660/−0.679 (*) | 0.883/0.925 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
lo Storto, C. Evaluating the Efficiency of the Private Healthcare Facilities in Italy: A Game Cross-Efficiency DEA Modeling Framework. Adm. Sci. 2025, 15, 355. https://doi.org/10.3390/admsci15090355
lo Storto C. Evaluating the Efficiency of the Private Healthcare Facilities in Italy: A Game Cross-Efficiency DEA Modeling Framework. Administrative Sciences. 2025; 15(9):355. https://doi.org/10.3390/admsci15090355
Chicago/Turabian Stylelo Storto, Corrado. 2025. "Evaluating the Efficiency of the Private Healthcare Facilities in Italy: A Game Cross-Efficiency DEA Modeling Framework" Administrative Sciences 15, no. 9: 355. https://doi.org/10.3390/admsci15090355
APA Stylelo Storto, C. (2025). Evaluating the Efficiency of the Private Healthcare Facilities in Italy: A Game Cross-Efficiency DEA Modeling Framework. Administrative Sciences, 15(9), 355. https://doi.org/10.3390/admsci15090355





