Longitudinal Analysis of Sucralose at a Water Treatment Wetland
Abstract
:1. Introduction
2. Materials and Methods
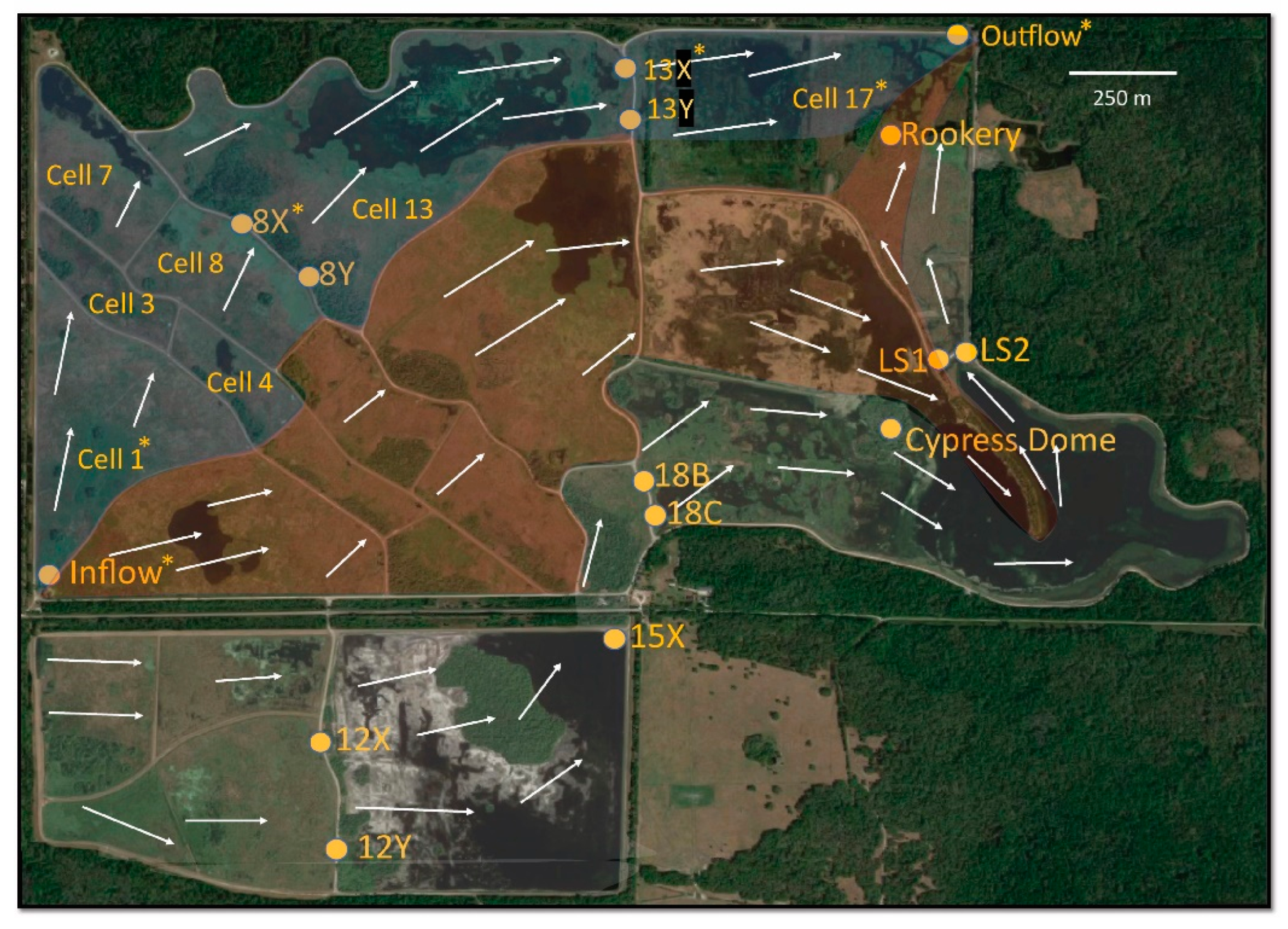
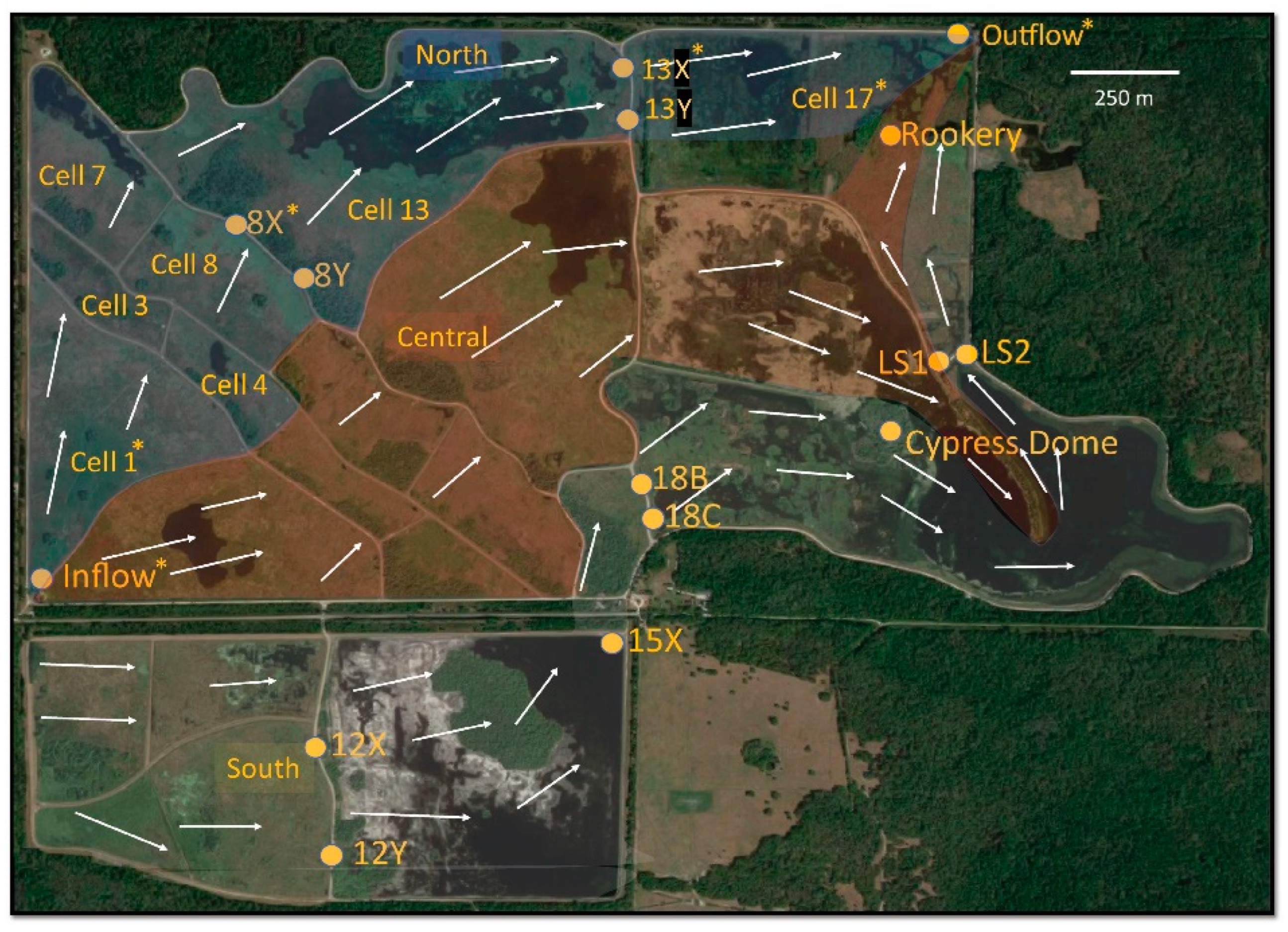
2.1. Site Description
2.2. Sample Collection
2.3. Sediment Cores
2.4. Solid Phase Extraction
2.5. Sucralose Quantification
2.5.1. Water Column Samples
2.5.2. Sediment Core Samples
2.5.3. Statistical Analysis
2.6. Total Organic Carbon Analysis
3. Results
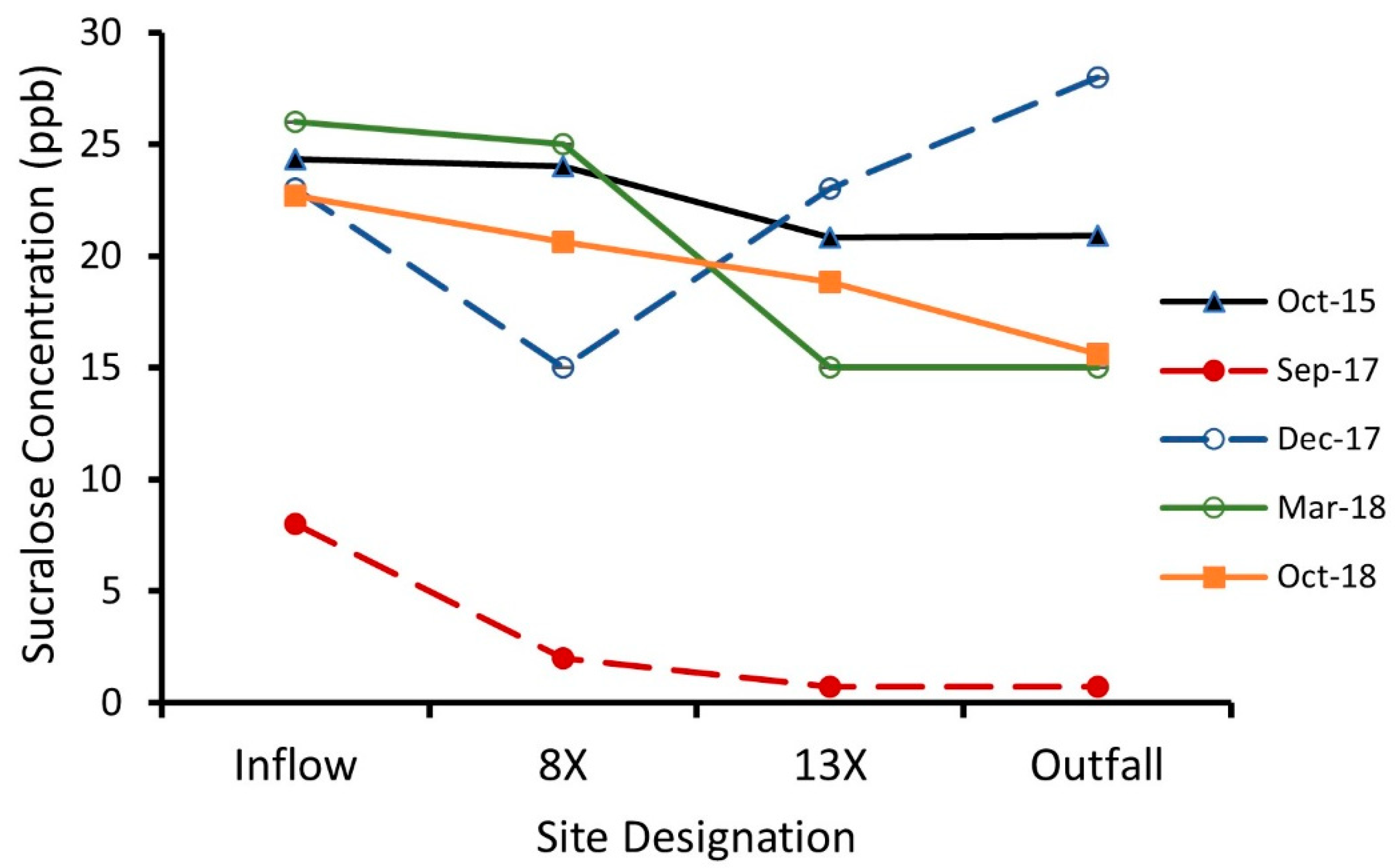
3.1. Sucralose Water Concentration
3.2. Mass Balance
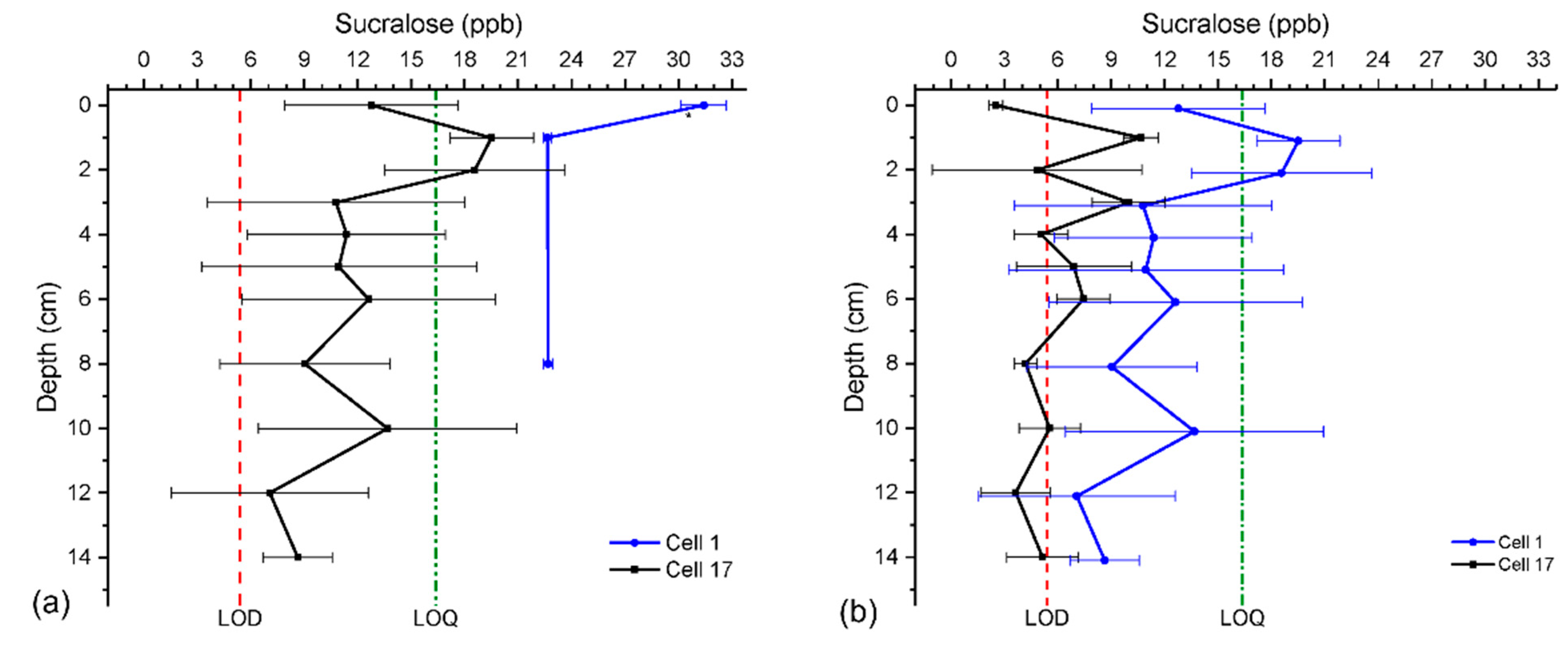
3.3. Sucralose Sediment Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, A. How Sweet It Isn’t. The Wall Street Journal. 6 April 2007. Available online: https://www.wsj.com/articles/SB117582405851161797 (accessed on 27 August 2022).
- Dunford, E.K.; Miles, D.R.; Ng, S.W.; Popkin, B. Types and Amounts of Nonnutritive Sweeteners Purchased by US Households: A Comparison of 2002 and 2018 Nielsen Homescan Purchases. J. Acad. Nutr. Diet. 2020, 120, 1662–1671. [Google Scholar] [CrossRef]
- Piernas, C.; Ng, S.W.; Popkin, B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr. Obes. 2013, 8, 294–306. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Welsh, J.A.; Brown, R.J. Low-calorie sweetener consumption is increasing in the United States. Am. J. Clin. Nutr. 2012, 96, 640–646. [Google Scholar] [CrossRef]
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Tian, T.Y.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef]
- Roberts, A.; Renwick, A.G.; Sims, J.; Snodin, D.J. Sucralose Metabolism and Pharmacokinetics in Man. Food Chem. Toxicol. 2000, 38, S31–S41. [Google Scholar] [CrossRef]
- Rother, K.I.; Sylvetsky, A.C.; Walter, P.J.; Garraffo, H.M.; Fields, D.A. Pharmacokinetics of Sucralose and Acesulfame-Potassium in Breast Milk Following Ingestion of Diet Soda. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 466–470. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Brown, S.J.; Spoelstra, J.; Garda, D.; Robertson, W.D.; Smyth, S.A. Variable persistence of artificial sweeteners during wastewater treatment: Implications for future use as tracers. Water Res. 2020, 184, 116124. [Google Scholar] [CrossRef]
- Torres, C.I.; Ramakrishna, S.; Chiu, C.-A.; Nelson, K.G.; Westerhoff, P.; Krajmalnik-Brown, R. Fate of Sucralose During Wastewater Treatment. Environ. Eng. Sci. 2011, 28, 325–331. [Google Scholar] [CrossRef]
- Soh, L.; Connors, K.A.; Brooks, B.W.; Zimmerman, J. Fate of Sucralose through Environmental and Water Treatment Processes and Impact on Plant Indicator Species. Environ. Sci. Technol. 2011, 45, 1363–1369. [Google Scholar] [CrossRef]
- Li, S.; Geng, J.; Wu, G.; Gao, X.; Fu, Y.; Ren, H. Removal of artificial sweeteners and their effects on microbial communities in sequencing batch reactors. Sci. Rep. 2018, 8, 3399. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ren, Y.; Fu, Y.; Gao, X.; Jiang, C.; Wu, G.; Ren, H.; Geng, J. Fate of artificial sweeteners through wastewater treatment plants and water treatment processes. PLoS ONE 2018, 13, e0189867. [Google Scholar] [CrossRef]
- Scheurer, M.; Storck, F.R.; Graf, C.; Brauch, H.-J.; Ruck, W.; Lev, O.; Lange, F.T. Correlation of six anthropogenic markers in wastewater, surface water, bank filtrate, and soil aquifer treatment. J. Environ. Monitor. 2011, 13, 966–973. [Google Scholar] [CrossRef]
- Subedi, B.; Kannan, K. Fate of Artificial Sweeteners in Wastewater Treatment Plants in New York State, U.S.A. Environ. Sci. Technol. 2014, 48, 13668–13674. [Google Scholar] [CrossRef]
- Tran, N.H.; Gan, J.; Nguyen, V.T.; Chen, H.; You, L.; Duarah, A.; Zhang, L.; Gin, K.Y.-H. Sorption and biodegradation of artificial sweeteners in activated sludge processes. Bioresour. Technol. 2015, 197, 329–338. [Google Scholar] [CrossRef]
- Di Marcantonio, C.; Chiavola, A.; Bains, A.; Singhal, N. Effect of oxic/anoxic conditions on the removal of organic micropollutants in the activated sludge process. Environ. Technol. Innov. 2020, 20, 101161. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Eaton, A.; Badruzzaman, M.; Haghani, A.W.; Jacangelo, J.G. Occurrence and Suitability of Sucralose as an Indicator Compound of Wastewater Loading to Surface Waters in Urbanized Regions. Water Res. 2011, 45, 4019–4027. [Google Scholar] [CrossRef]
- Loos, R.; Gawlik, B.M.; Boettcher, K.; Locoro, G.; Contini, S.; Bidoglio, G. Sucralose Screening in European Surface Waters Using a Solid-Phase Extraction-Liquid Chromatography Quadrupole Mass Spectrometry Method. J. Chromatogr. A 2009, 1216, 1126–1131. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Young, R.B.; Vanderford, B.J.; Borch, T.; Snyder, S.A. Artificial Sweetener Sucralose in U.S. Drinking Water Systems. Environ. Sci. Technol. 2011, 45, 8716–8722. [Google Scholar] [CrossRef]
- Lang, F.T.; Scheurer, M.; Brauch, H.-J. Artificial sweeteners—A recently recognized class of emerging environmental contaminants: A review. Anal. Bioanal. Chem. 2012, 403, 2503–2518. [Google Scholar] [CrossRef]
- Robertson, W.D.; Van Stempvoort, D.R.; Spoelstra, J.; Brown, S.J.; Schiff, S.L. Degradation of sucralose in groundwater and implications for age dating contaminated groundwater. Water Res. 2016, 88, 653–660. [Google Scholar] [CrossRef]
- Labare, M.P.; Alexander, M. Biodegradation of Sucralose, a Chlorinated Carbohydrate, in Samples of Natural Environments. Environ. Toxicol. Chem. 1993, 12, 797–804. [Google Scholar] [CrossRef]
- Amy-Sagers, C.; Reinhardt, K.; Larson, D.M. Ecotoxicological assessments show sucralose and fluoxetine affect the aquatic plant, Lemna minor. Aquat. Toxicol. 2017, 185, 76–85. [Google Scholar] [CrossRef]
- Tollefsen, K.E.; Nizzetto, L.; Huggett, D.B. Presence, fate and effects of the intense sweetener sucralose in the aquatic environment. Sci. Total Environ. 2012, 438, 510–516. [Google Scholar] [CrossRef]
- Saucedo-Vence, K.; Elizalde-Velázquez, A.; Dublán-García, O.; Galar-Martínez, M.; Islas-Flores, H.; SanJuan-Reyes, N.; García-Medina, S.; Hernandez-Navarro, M.; Gómez-Olivan, L.M. Toxicological hazard induced by sucralose to environmentally relevant concentrations in common carp (Cyprinus carpio). Sci. Total Environ. 2017, 575, 347–357. [Google Scholar] [CrossRef]
- Lillicrap, A.; Langford, K.; Tollefsen, K.E. Bioconcentration of the intense sweetener sucralose in a multitrophic battery of aquatic organisms. Environ. Toxicol. Chem. 2011, 30, 673–681. [Google Scholar] [CrossRef]
- Manasfi, R.; Brienza, M.; Ait-Mouheb, N.; Montemurro, N.; Perez, S.; Chiron, S. Impact of long-term irrigation with municipal reclaimed wastewater on the uptake and degradation of organic contaminants in lettuce and leek. Sci. Total Environ. 2021, 765, 142742. [Google Scholar] [CrossRef]
- Maluly, H.D.B.; Johnston, C.; Giglio, N.D.; Schreiner, L.L.; Robert, A.; Abegaz, E.G. Low-and No-Calorie Sweeteners (LNCS): Critical evaluation of their safety and health risks. Food Sci. Technol. 2021, 40, 1–10. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Wiklund, A.-K.E.; Breitholtz, M.; Bengtsson, B.-E.; Adolsson-Erici, M. Sucralose—An ecotoxicological challenger. Chemosphere 2012, 86, 50–55. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- D’Angelo, E.M.; Reddy, K.R. Regulators of heterotrophic microbial potentials in wetland soils. Soil Biol. Biochem. 1999, 31, 815–830. [Google Scholar] [CrossRef]
- Wang, H.; Jawitz, J.W.; White, J.R. Rejuvenating the largest municipal treatment wetland in Florida. Ecol. Eng. 2006, 26, 132–146. [Google Scholar] [CrossRef]
- Delaune, R.D.; Pezeshki, S.R. The Role of Soil Organic Carbon in Maintaining Surface Elevation in Rapidly Subsiding U.S. Gulf of Mexico Coastal Marshes. Water Air Soil Pollut. 2003, 3, 167–179. [Google Scholar] [CrossRef]
- White, J.R.; Gardner, L.M.; Sees, M.; Corstange, R. The Short-Term Effects of Prescribed Burning on Biomass Removal and the Release of Nitrogen and Phosphorus in a Treatment Wetland. J. Environ. Qual. 2008, 37, 2386–2391. [Google Scholar] [CrossRef]
- Kerr, J.G.; Burford, M.; Olley, J.; Udy, J. Phosphorus Sorption in Soils and Sediments: Implications for Phosphate Supply to a Subtropical River in Southeast Queensland, Autralia. Biogeochemistry 2011, 102, 73–85. [Google Scholar] [CrossRef]
- Bastian, R.K.; Frederick, B.; Haven, R.C.; Lothrop, T.L.; Skene, E.; Oyler, A.R.; Allman, W.; Alexander, A.; deAguilar, C.R.; Ferraro, C.; et al. Wetland Treatment Systems: A Case History; EPA832-R-93_005; United States Environmental Protection Agency: Winter Park, FL, USA, 1993.
- Wang, H.; Jawitz, J.W. Hydralic Analysis of Cell-Network Treatment Wetlands. J. Hydrol. 2006, 330, 721–734. [Google Scholar] [CrossRef]
- Welch, J.; Simmons, V.; Meléndez, E.; Sees, M.; Gold, Y.; Heider, E.C. Assessment of Non-Anthropogenic Addition of Uric Acid to a Water Treatment Wetlands. Environments 2020, 7, 60. [Google Scholar] [CrossRef]
- Heider, E.C.; Valenti, D.; Long, R.L.; Garbou, A.; Rex, M.; Harper, J.K. Quantifying Sucralose in a Water-Treatment Wetlands: Service-Learning in the Analytical Chemistry Laboratory. J. Chem. Educ. 2018, 95, 535–542. [Google Scholar] [CrossRef]
- Altomare, A.J.; Young, N.A.; Beazley, M.J. A preliminary survey of anthropogenic gadolinium in water and sediment of a constructed wetland. J. Environ. Manag. 2020, 255, 109897. [Google Scholar] [CrossRef]
- Griffin, B.A.; Jurinak, J.J. Estimation of Acitivity Coefficients from the Electrical Conductivity of Natural Aquatic Systems and Soil Extracts. Soil Sci. 1973, 116, 26–30. [Google Scholar] [CrossRef]
- Mead, R.N.; Morgan, J.B.; Avery, G.B., Jr.; Kieber, R.J.; Kirk, A.M.; Skrabal, S.A.; Willey, J.D. Occurrence of the Artificial Sweetener Sucralose in Coastal and Marine Waters of the United States. Mar. Chem. 2009, 116, 13–17. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the Consuption of Low-Calorie Sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Z.; Huang, X.; Yuan, W.; Ying, H. Solubility of Sucralose in Different Solvents from (283.15 to 333.15) K. J. Chem. Eng. Data 2010, 55, 2600–2602. [Google Scholar] [CrossRef]
- Ávila, C.; Pelissari, C.; Sezerino, P.H.; Sgroi, M.; Roccaro, P.; García, J. Enhancement of total nitrogen removal through effluent recirculation and fate of PPCPs in a hybrid constructed wetland system treating urban wastewater. Sci. Total Environ. 2017, 584–585, 414–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sampling Year | Cypress Dome (ppb) | Rookery (ppb) |
|---|---|---|
| September 2017 | <5 | <5 |
| December 2017 | 24 ± 5 | 26 ± 5 |
| March 2018 | 27 ± 5 | 31 ± 5 |
| Sampling Year | Influent * mg·day−1 | Outfall mg·day−1 | Comparison of Means t * |
|---|---|---|---|
| October 2015 | 34 ± 9 | 41 ± 12 | 0.98 |
| September 2017 | 14 ± 9 | 2 ± 14 | 1.52 |
| December 2017 | 41 ± 14 | 79 ± 22 | 2.93 |
| March 2018 | 48 ± 13 | 46 ± 17 | 0.23 |
| October 2018 | 42 ± 9 | 48 ± 15 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, N.; Welch, J.; Hill, T.; Sees, M.; Beazley, M.; Heider, E.C. Longitudinal Analysis of Sucralose at a Water Treatment Wetland. Environments 2022, 9, 111. https://doi.org/10.3390/environments9090111
Young N, Welch J, Hill T, Sees M, Beazley M, Heider EC. Longitudinal Analysis of Sucralose at a Water Treatment Wetland. Environments. 2022; 9(9):111. https://doi.org/10.3390/environments9090111
Chicago/Turabian StyleYoung, Nicholas, Joseph Welch, Tyler Hill, Mark Sees, Melanie Beazley, and Emily C. Heider. 2022. "Longitudinal Analysis of Sucralose at a Water Treatment Wetland" Environments 9, no. 9: 111. https://doi.org/10.3390/environments9090111
APA StyleYoung, N., Welch, J., Hill, T., Sees, M., Beazley, M., & Heider, E. C. (2022). Longitudinal Analysis of Sucralose at a Water Treatment Wetland. Environments, 9(9), 111. https://doi.org/10.3390/environments9090111







