Abstract
Pool water must meet certain chemical and microbiological conditions to ensure high water quality and a safe environment for users. A recreational swimming pool treated with a combined disinfection method (chlorination and UV radiation) was monitored for 18 months. Selected chemical and microbiological parameters of the indoor freshwater pool were analyzed, and the in vivo presence of Pseudomonas aeruginosa biofilm was assessed and further correlated to the type of disinfection. P. aeruginosa isolated from biofilm was further examined to determine the effects of combined disinfection methods on the formation and destruction of mature biofilm in vitro. The in vitro application of the combined disinfection methods led to the inhibition of planktonic P. aeruginosa biofilm formation (68.9% compared to the control group) and were more effective in the partial destruction of mature biofilm than individual disinfection methods (from 25.4 to 26.3%). The obtained results indicate the better microbiological and chemical quality of pool water when combined disinfection was applied. Our results contribute to developing the optimization of pool water disinfection methods and biofilm control.
1. Introduction
Swimming pool water is a complex environment where different types of pathogenic microorganisms can be transmitted. Even though pools are mostly intended for recreational activities, to protect the health of users, they must be maintained regularly. Disinfection plays an important role in maintaining pool water quality and is a basic method for reducing the risk of potential microbiological hazards [1,2]. Nevertheless, opportunistic premise plumbing pathogens (OPPPs) often remain in water supply systems or in water for recreation and rehabilitation [3]. Standard doses of chlorine-based disinfectants do not destroy OPPPs, and their presence in aquatic environments increases over time. They either exist as planktonic cells or enclosed within structures known as biofilms [4]. Biofilm is a complex community of microorganisms imbedded in extracellular polymeric substances (EPSs), which are resistant to changes in external living conditions. Pseudomonas aeruginosa is one of the most studied opportunistic pathogens; it can cause minor or major health problems, with its presence sometimes resulting in infection outbreaks. It is a predominant microorganism that causes infections in aquatic environments [5,6,7]. This gram-negative bacterium can grow in adverse living conditions such as low nutrient concentrations, and it can survive high temperature differences. P. aeruginosa has a strong ability to form biofilm and can survive in distilled water, in treated water with residual chlorine levels < 1 mg/L, and in disinfectant solutions, and it shows high resistance to mechanical cleaning processes. Its persistence in swimming pool areas most often depends on its ability to form biofilms on almost any pool surface area [8]. The damp parts of pools contribute greatly to this [9]. Extensive research on forming P. aeruginosa biofilms and biofilm control conditions in swimming pools is scarce. It is known that many procedures and different disinfection methods are available to manage and reduce the risk of infection transmissions in swimming pool settings. In addition to chemical methods, combined methods (chlorination with UV radiation) of water disinfection are increasingly utilized [10,11]. Evidence from various toxicological and epidemiological studies on the adverse health effects of disinfection by-products (DBPs) has raised concerns about the chemical safety of swimming pools. Frequently studied and mentioned harmful by-products are trihalomethanes (THMs) [12,13,14,15,16]. Mainly, the THM concentration depends on the method of pool water disinfection. It has been proven that combined disinfection is an effective method of reducing concentrations of these harmful volatile halogenated hydrocarbons [17]. As has previously been mentioned, biofilms are extremely resistant to chemicals in water environments, including disinfectants [18,19]. Routine examinations of swimming pool biota in microbiological laboratories often do not include bacterial biofilms. Therefore, in this study, in order to follow real conditions that are present in swimming pools, an additional set of removable ceramic tiles was placed (in situ) in the swimming pool shell. The intention was to detect what chemical conditions of swimming pool water lead to suitable conditions for the development of microbiological flora. The presence of P. aeruginosa was analyzed in relation to the selected chemical parameters. The aim of this study was to isolate P. aeruginosa from a mixed biofilm and to examine the further influence of different disinfection methods (chlorination and UV radiation) in in vitro conditions. Additionally, the aim was to determine its influence on the formation of biofilm and the behavior of mature biofilm.
2. Materials and Methods
2.1. Study Area and Sampling
For the analysis of selected parameters (free and total chlorine, and trihalomethanes), an indoor freshwater swimming pool with ceramic tiles was used. The pool dimensions were 14 m in length, 7.5 m in width, depth of 1.45 m with total volume of freshwater 152.25 m3. The pool shell was covered with small 2.5 × 2.5 cm ceramic tiles. An automatic chemical dosing system, at a properly built engine room, ensured constant chemical monitoring. Chlorination (Cl) was carried out with sodium hypochlorite, and a combined disinfection method with a low-pressure, high-efficiency mercury UV lamp (Ultraaqua a/s, Aalborg, Denmark) was utilized in the system and used along with the sodium hypochlorite chlorination (UV + Cl). For the first nine months of the study, the combined method of disinfection (UV + Cl) was continuously applied, whereas in the second nine months of the study, the single disinfection method (only Cl) was used. A schematic view of the indoor swimming pool, sampling points, and locations of the additionally placed ceramic tiles is presented in Scheme 1. The sampling points were selected according to the following criteria: the sampling point 0 represents the disinfected water just before returning to the pool; sampling point 1 represents the entrance to the pool, around the stairs that are more difficult to clean; sampling point 2 represents the center of the pool and is a common point for sampling procedure; and sampling point 3 represents the most distant point to the entrance and is a place where bathers usually gather held by the edge. Water samples were taken 0.5 to 1.0 meters below the water surface using a telescopic rod and put into clean containers with a volume of 0.25, 0.5, or 1 L, depending on the parameter being analyzed. Samples were transported to the laboratory in the controlled temperature conditions and immediately processed or stored at 4 °C until analysis.
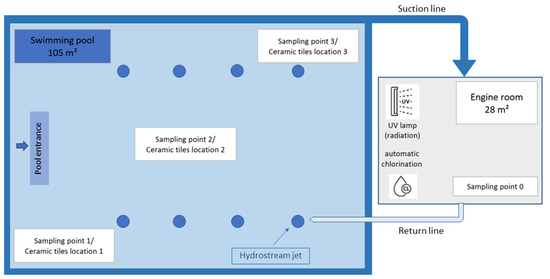
Scheme 1.
Sampling points 0, 1, 2, and 3, and the locations of the additionally placed ceramic tiles 1, 2, and 3 in the indoor swimming pool. A combined disinfection method (UV + Cl) was applied in the first part of the experiment, and the single disinfection method (only Cl) was applied in the second part of the investigation.
2.2. Selected Chemical and Microbiological Parameter Measurements in Pool Water
Selected chemical parameters were tested in accordance with the relevant ISO methods: the free chlorine concentration and total chlorine concentration (EN ISO 7393-2:2018) [20], pH (EN ISO 10523:2012) [21], trihalomethanes (EN ISO 10301:1997) [22], and P. aeruginosa (EN ISO 16266:2008) [23].
2.3. Bacterial Strains and Inoculum Preparation
P. aeruginosa used in the in vitro experiment was isolated in situ from ceramic tiles of the swimming pool placed at location 3. It was identified using biochemical test API-NE (Biomerieux, Paris, France). Furthermore, bacterial strains were cultured on Mueller–Hinton agar (MH, Biolife, Milano Italy) under aerobic conditions at 35 °C for 24 h. Pure bacterial cultures were suspended in MH broth (Biolife, Milano, Italy) of appropriate concentrations of 105 CFU/mL, and the optical density was measured at 600 nm (OD600) (Eppendorf, Bio photometer, model #6131, Hamburg, Germany).
2.4. Treatment of Planktonic Bacteria before Biofilm Formation
The influence of UV light and sodium hypochlorite, and their combination on the bacterial suspension and planktonic form was tested. The ability of bacteria to grow and form biofilm was investigated. Bacterial suspensions of 105 CFU/mL prepared as previously described were transferred to a plastic Petri dish and exposed to UV light at 254 nm (UV lamp—dual wavelength, Muttenz, Switzerland) for 20 s, sodium hypochlorite solution (Cl—chlorination) 0.4 mg/L for 1 min, and a combination of UV light for 20 s and sodium hypochlorite solution 0.4 mg/L for 1 min. A neutralizer was not used. Treated bacterial suspension was poured over the prepared ceramic tiles in agar, as described here, followed by incubation at 35 °C for 5 days to mature biofilm formation. After washing planktonic bacteria and ultrasound treatment to release bacteria in the biofilm, CFUs were determined by planting tenfold dilutions on MH agar. All experiments were performed in triplicate and repeated three times (N = 9).
2.5. Formation of Mature Biofilm
The method of biofilm formation was described according to the procedure developed by Ivanković et al. and modified by the conditions of our laboratory [24]. The individual tiles were mechanically brushed, washed, and then sterilized at 180 °C for 1 h. An agar bacteriological solution (Oxoid, Basingstoke, UK) was prepared according to the manufacturer’s instructions and autoclaved at 121 °C for 15 min. Three sterile tiles were placed in a Petri dish, with the ceramic surface facing up (to simulate a pool). Subsequently, still warm agar solution was poured, making sure that the upper ceramic surface of the tiles remained uncovered. A total of 10 mL of test P. aeruginosa bacteria suspension was poured on the upper side of the tiles that were placed in agar, ensuring that they completely covered their surface, as described (Figure 1). Petri dishes were incubated at 35 °C for 5 days using an orbital shaker (30 rpm), and mature biofilm was formed.
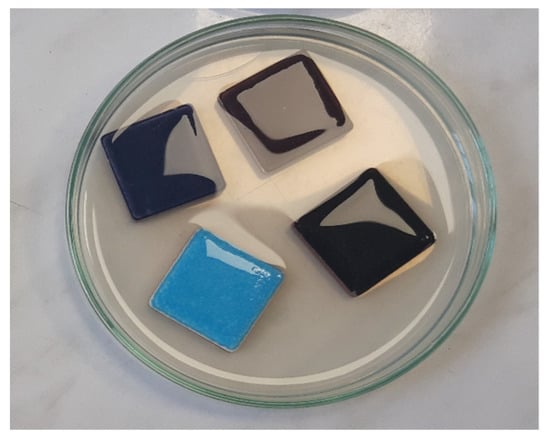
Figure 1.
Sterile ceramic tiles placed in a Petri dish, immersed in agar, and covered with suspension of test P. aeruginosa bacteria.
2.6. Treatments of Mature Biofilm
After incubation for 5 days, the tiles with mature biofilm were transferred to a plastic Petri dish and washed three times in sterile saline solution. Then, the mature biofilm was exposed to various treatments: UV light (UV) for 5 and 20 s, sodium hypochlorite solution (T.T.T., Sveta Nedjelja, Croatia), 0.4 mg/L for 1 min, and a combination of UV light (254 nm) for 5 or 20 s and sodium hypochlorite solution (Cl) 0.4 mg/L for 1 min. After sodium hypochlorite exposure, 10% sodium thiosulphate solution (Kemika, Zagreb, Croatia) was added to remove residual chlorine. Subsequently, tiles were placed in sterile polypropylene tubes with sterile saline and treated in an ultrasonic bath (BactoSonik - Bandelin, Berlin, Germany) for 1 min at 40 kHz. Tenfold serial dilutions were performed, and samples were inoculated on MH agar. After incubation for 24 h at 35 °C, CFU/mL was determined. During the experiment, a control group was maintained. Mature biofilm was grown on the plate under the same conditions and was not exposed to UV light and sodium hypochlorite. Each experiment was performed in triplicate and repeated three times (N = 9).
2.7. Statistical Analyses
Analyses of the selected chemical parameters of pool waters at different applied disinfection methods (Cl and UV + Cl) and sampling points were performed in triplicate, whereas in vitro bacteria assays were performed in three replicates. All experimental data are expressed as the median with minimal and maximal values. All statistical analyses were performed using the software Statistica® v. 14.0 (StatSoft Inc., Tulsa, OK, USA) at the significance level of p < 0.05. Differences between disinfection methods (Cl and UV + Cl) for each chemical parameter at sampling locations and differences between control and experimental groups in in vitro bacterial assays were assessed with the nonparametric Mann-Whitney U test. For correlations between chemical parameters, the number of bathers per day, and P. aeruginosa bacteria in analyzed pool waters with different disinfection methods, nonparametric Kendall-Tau correlation test and principal component analysis (PCA) were used.
3. Results and Discussion
3.1. Swimming Pool Water (In Vivo)
3.1.1. Monitored Parameters in Swimming Pool Water
In this study, an indoor freshwater swimming pool was monitored for a period of 18 months and with periods of applying different disinfection methods. Samples were taken frequently from three locations around the swimming pool and one location from the engine room after applying disinfection methods. For the whole investigation period, pool water temperature was monitored and ranged from 20.8–23.9 °C. The single chlorination method (Cl) was applied in the first half of the experiment, and the combined method UV radiation + chlorination (UV + Cl) was applied in the second half of the investigation. Monitored parameter results are presented in Table 1 as the median with minimum and maximum values.

Table 1.
Monitored parameters and the number of bathers per day for the analyzed swimming pool water at different applied disinfection methods (Cl and UV + Cl) and sampling points (0–3). The data are presented as the median with minimum and maximum values (N = 90 and N = 126 for Cl and UV + Cl disinfection methods, respectively, at each sampling point).
Comparing previously mentioned disinfection methods, significantly higher pH values (p < 0.0098) were obtained by the UV + Cl method than by Cl, ranging from 3.6% to 4.9% higher values depending on the sampling location. Different behavior was observed for free and total chlorine, where significantly lower values of both parameters were found using the UV + Cl disinfection method (p < 0.0001). The percentage decreased with an average value of about 54.1%, with the highest percentage decrease found at sampling point 3 (60.3%; p = 0.0001). The amount of total chlorine followed the behavior of free chlorine; thus, its evident reduction was also observed using the UV + Cl disinfection method (p < 0.0001). The reduction was somewhat lower than with free chlorine and amounted to an average of 40.6%, with the highest reduction found at the sampling point 3 (46.8%; p = 0.0001). The trihalomethane concentrations with the Cl and UV + Cl disinfection methods were in wide ranges of values from 3.20 to 352.0 µg/L and 2.77 to 140.6 µg/L, respectively, depending on the sampling location. Although much lower concentrations of trihalomethanes were detected using the UV + Cl disinfection method (40.7% on average) compared with the Cl method, the reductions were not significantly different compared with the concentrations obtained through the Cl method. The lowest reduction in trihalomethane concentration was observed at sampling point 2 and was counted as only 19.6%, whereas the highest reduction was achieved at location point 0 (53.0%). It is already known that chemical parameters in swimming pool water, such as pH, free and total chlorine, and the possible presence of harmful DBPs (trihalomethanes) provide useful information on water quality [25]. Poor maintenance of these parameters can lead to the potential risk of bacterial growth in the water [26]. A very important parameter is pH, where its misbalance can quickly increase the possibility of reducing disinfection efficiency. The Croatian Regulation on Health Safety of Swimming Pool Water (OG 59/2020), among other parameters, prescribes that the pH levels in pools should be maintained between 6.5 and 7.3 for fresh and sea water, free chlorine concentration should be no more than 1.2 mg/L Cl2, and the highest acceptable level of trihalomethanes is 100 µg/L [27]. Therefore, these physicochemical parameters should be continuously measured and adjusted. It has been documented that the use of combined disinfection methods may reduce exposure to harmful DBPs [28,29,30], which was also observed in this study. Even though the reductions in THMs were not significantly different when comparing the combined and single chlorination method, the reduction at sampling point 0 was expected because that was the sample collected immediately after applying disinfection methods and before any impact of bathers at the swimming pool. Higher concentrations of THMs were also determined by Chu et al. in their survey on swimming pools in London, where they reported a mean value of 132.4 μg/L, a minimum value of 57 μg/L, and a maximum value of 223 μg/L [13].
3.1.2. Microbiological Presence in Swimming Pool Water
In addition to the chemical parameters of the pool waters, the presence of bathers in the pools was also studied, expressed as the number of bathers per day (Table 2).

Table 2.
Coefficients of correlation between chemical parameters, the number of bathers per day, and Pseudomonas aeruginosa bacteria levels of analyzed pool waters treated with chlorination (Cl) disinfection methods and represented by the nonparametric Kendall–Tau correlation test (N = 360; 90 samples × four sampling points). Statistically significant correlations (p < 0.05) are presented in bold-type numbers.
A somewhat lower number of bathers was observed in the pools when the combined (UV + Cl) disinfection method was used (28%). Usually, the highest influence on the formation of toxic compounds in pool water is the amount of organic matter introduced by people using the pools (bathers) [31,32].
Furthermore, to explain the correlations between the chemical parameters of pool waters, the number of bathers, and P. aeruginosa bacteria, which were isolated during the Cl disinfection method, Kendall–Tau correlation analysis was used. The correlation results of pooled data for the combined disinfection method (UV + Cl) are presented in Table 3.

Table 3.
Coefficients of correlation between chemical parameters, the number of bathers per day, and Pseudomonas aeruginosa bacteria levels of analyzed pool waters with the disinfection method of UV radiation with chlorination (UV + Cl) represented by nonparametric Kendall-Tau correlation tests (N = 504; 126 samples × four sampling points). Statistically significant correlations (p < 0.05) are presented in bold-type numbers.
With the Cl disinfection method, strong positive correlations (τ > 0.30) were observed between trihalomethanes and free chlorine and total chlorine (Table 2), indicating that increased concentrations of trihalomethanes in pool water followed stronger water chlorination with higher concentrations of free and total chlorine. The correlations between trihalomethanes and the number of bathers per day and P. aeruginosa were moderate (τ = 0.20 and 0.12), showing that a greater number of bathers has a significant influence on the trihalomethanes’ production and occurrence of P. aeruginosa. This is mainly because of the presence of organic matter from bathers, which is a precursor for forming THMs [33,34]. Examining the sensitivity of Pseudomonas aeruginosa on the amount of chlorine in pools, it is evident that the bacteria were susceptible to the free and total chlorine (τ = −0.16 and −0.16). Furthermore, the Kendall-Tau correlation with the disinfection UV + Cl method indicated a positive moderate influence of pH on the occurrence of trihalomethanes and the number of bathers per day (τ = 0.21 for both cases, Table 3.), whereas the effects of free chlorine on the number of bathers per day and trihalomethanes were strong (τ = −0.38) and moderate (τ = −0.16) but negative. With the combined UV + Cl disinfection method, the correlation of bathers per day with the concentration of trihalomethanes was strong and positive (τ = 0.49). The P. aeruginosa bacteria were not isolated during the disinfection of pool water with the UV + Cl method; therefore, correlation with the chemical parameters and the number of bathers per day was not even assessed. This was the breaking point at this investigation, where the occurrence of P. aeruginosa was significantly correlated with the analyzed application period of the single (Cl) disinfection method.
Furthermore, to determine the effectiveness of the applied disinfection methods of chlorination (Cl) and chlorination with UV radiation (UV + Cl) depending on the chemical parameters of the pool waters, the presence of bathers, and the isolated P. aeruginosa bacteria at different sampling points, statistical principal component analysis (PCA) was used (Figure 2).
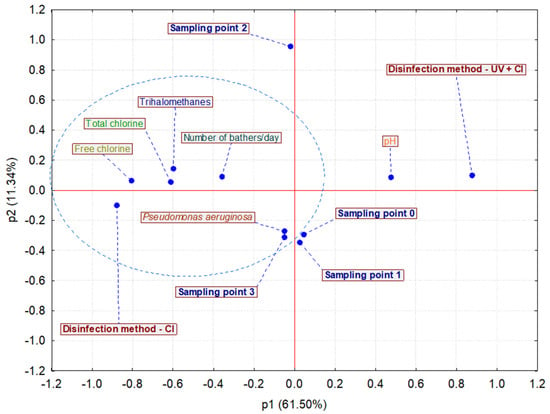
Figure 2.
Selected chemical parameters, the number of bathers per day, and Pseudomonas aeruginosa bacteria levels of analyzed pool waters with the disinfection methods of chlorination (Cl) and chlorination with UV radiation (UV + Cl), represented by principal component analysis (PCA) (N = 864; 216 samples × four sampling points).
A total of 864 pooled data points were included in the analysis (72 samples × 3 replications × 4 sampling points). The Cattell scree test was used to determine the number of main components retained in the analysis. According to this test, three main components (p1, p2, and p3) were retained in the analysis, which explained 83.99% of the total variance. The first main component explained 61.50% of the total variance, and the second and third explained 11.34% and 11.15%, respectively. Figure 2 presents the variable distribution (chemical parameters of pool waters, number of bathers per day, and isolated P. aeruginosa bacteria) depending on the applied disinfection method (Cl and UV + Cl) of pool water and on the sampling points. Most of the analyzed variables (free chlorine, total chlorine, trihalomethanes, number of bathers per day, and P. aeruginosa) were located in the third and fourth quadrants and define the negative side of the main component p1 (left side of the p1). Only the pH parameter was distributed in the second quadrant, on the right side of the main component p1, defining its positive side.
To clarify the involvement or importance of individual variables in the main components’ model definition, the following indicators were used: eigenvector spreadsheet, loading spreadsheet, and variable importance (Table 4).

Table 4.
Eigenvector and loading spreadsheet values for the chemical parameters, number of bathers per day, and Pseudomonas aeruginosa bacterial levels in the analyzed pool waters with the disinfection methods of chlorination (Cl) and chlorination with UV radiation (UV + Cl) represented by principal component analysis (PCA) in the first three main components (N = 864; 216 samples × four sampling points).
Analyzing the eigenvector values, it is evident that the most variables define the main component, p1. Namely, a strong positive effect on the definition of component p1 is shown by the variable pH, whereas the remaining variables, except P. aeruginosa, show a strong but negative effect on p1. The strongest effect on the definition of component p1 is shown by free and total chlorine and the trihalomethane concentration, whereas the effect of pH and bathers is slightly less pronounced. Variable P. aeruginosa defines the main component p2, with a negative correlation, and its effect is also evident. None of the analyzed variables dominated in the definition of the main component p3. Similar behavior to the definition of the main components as in the eigenvector analysis was also achieved by the analysis of the loading spreadsheet values. Finally, by analyzing the importance of variables, it was determined that free chlorine, total chlorine, and trihalomethane concentration played dominant roles in the definition of the model, whereas the contributions of pH, the number of bathers, and the presence of P. aeruginosa were less pronounced to the definition of the model. Analyzing the case distributions (disinfection methods and sampling locations) on the PCA plot, it is apparent that the UV + Cl disinfection method and sampling points 0 and 1 are distributed on the right side of the main component p1, which leads to the assumption that variable pH at these locations defines the right side of component p1. Distributed on the left side of the main component p1 were disinfection method Cl and sampling points 2 and 3. Sampling point 3 is located near the isolated P. aeruginosa bacteria, which leads to the conclusion that the bacteria are most often isolated at this sampling point and that this sampling point represents the biggest problem in swimming pools. Comparing the importance of sampling locations with the model definition, it was determined that locations 0 and 3 had equal importance (0.96 and 0.95, respectively), whereas it was slightly less pronounced at locations 1 and 2 (0.93 and 0.92, respectively).
Thus, all of the components included in the analysis confirmed that it is very important to keep chemical parameters properly maintained in order to ensure the best water quality. Additionally, this ensures a safe and bacteria-free aquatic environment [35,36,37].
There are not many extensive studies on forming P. aeruginosa biofilms and the behavior of mature biofilms under the influence of different disinfection methods; therefore, further investigations in in vitro conditions were performed.
3.2. Pseudomonas Aeruginosa Biofilm Treatment (In Vitro)
Treatment before Biofilm Formation, and Treatment on Mature Biofilm
When P. aeruginosa was isolated in pool water, additional in vitro assays of the bacterium’s sensitivity to various disinfection methods were carried out in laboratory conditions. Assays were performed through treatment of the planktonic form of P. aeruginosa, before biofilm formation, and with the treatment of a 5-day-old biofilm of P. aeruginosa. The results are presented as the total bacterial count expressed as log10 CFU/mL depending on the disinfection method (Figure 3a,b).
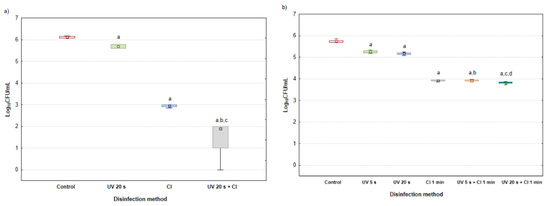
Figure 3.
Total bacterial count expressed as log10 CFU/mL after the treatment of (a) the planktonic form of Pseudomonas aeruginosa, before biofilm formation and after the treatment of (b) the 5-day-old biofilm of Pseudomonas aeruginosa. The results are presented as median with minimum and maximum values (N = 9), performed in triplicate and repeated three times for each experiment. Median values marked with a lowercase letter a represent significant differences between control group and disinfection methods, whereas lowercase letters b, c, and d represent significant differences between disinfection methods (p < 0.05; nonparametric Mann-Whitney U test).
The planktonic form of P. aeruginosa, before biofilm formation, was treated with three disinfection methods, namely, UV radiation for 20 s, chlorination (Cl), and a combination of chlorination and UV radiation for 20 s (UV 20 s + Cl). As can be seen in Figure 3a, the most P. aeruginosa bacterial colonies were found in the control group. The efficiency of each disinfection method was analyzed in comparison with the control group. Each of the used disinfection methods showed a significant reduction in the number of bacteria compared with the control group, with the weakest reduction observed with the UV 20 s disinfection method (6.7%; p = 0.0051); however, UV 20 s + Cl proved to be the strongest disinfection method, with a bacterial reduction of 68.9% (p = 0.0023). Furthermore, the effect of reducing the number of bacteria between the applied disinfection methods was also analyzed. The combination of UV radiation for 20 s with chlorination was more successful in reducing the number of bacteria: by 66.7% (p = 0.0062), compared with the chlorination method, which reduced the number of bacteria by 35.6% (p = 0.0084). Moreover, the obtained in vitro results suggest that the effect of the applied combined method (UV radiation + chlorination) has the potential to control the formation of biofilm in real aquatic environments. The pretreatment on planktonic bacteria with different disinfection methods resulted in slight to significant reductions in planktonic bacteria. Several studies have presented similar results, where the effect on the cell membrane resulted in inhibition of the EPS secretion, and consequently, with damaged DNA [38,39]. UV can significantly contribute to the bacteria-reducing effect of chlorine disinfection.
In Figure 3b, the total bacterial count is expressed as log10 CFU/mL after the treatment of 5-day-old biofilms of P. aeruginosa depending on different disinfection methods: UV radiation for 5 and 20 s, chlorination for 1 min, and combinations of UV radiation and chlorination (UV 5 s + Cl 1 min and UV 20 s + Cl 1 min). As in the previous assay, the highest bacterial number was recorded in the control group. Comparing each disinfection method with the control group, a significant reduction in the number of bacteria is observed, ranging from 8.4% (UV 5 s; p = 0.0076) to 33.4% (UV 20 s + Cl 1 min; p = 0.0044). The UV 5 s + Cl 1 min disinfection method reduced the bacterial number by 25.4% (p = 0.0086) compared with the UV 5 s method, whereas with the applied methods of Cl 1 min and UV 5 s + Cl 1 min, no differences were achieved in reducing the number of bacteria. Furthermore, the UV 20 s + Cl 1 min disinfection method induced a significant decrease in the number of bacteria compared with the UV 20 s method (26.3%; p = 0.0051) and compared with the Cl 1 min method (2.6%; p = 0.0131). The effects of all disinfection methods on the mature biofilm were lower as compared with the pretreatment of planktonic bacteria and consequently resulted in a reduction in biofilm formation. Microorganisms in biofilm are more resistant to disinfectants and radiation. While reducing the impact of UV on bacteria in biofilm, EPS plays a key role, either by increasing the length of incident radiation or by the presence of protective factors that absorb UV radiation, such as pigments [40,41]. EPS is also responsible for the resistance to the chlorination of P. aeruginosa in the biofilm. In addition to reducing the availability of reactive sites on the cell, the consumption of residual disinfectants will reduce the effectiveness of disinfection [42]. To destroy the P. aeruginosa biofilm, large, impermissible, concentrations of chlorine disinfectants and a long exposure time would be required [43,44]. On the other hand, the advantage of UV radiation is the absence of harmful by-products, which gives an additional contribution to combined UV + Cl disinfection. The results of this study indicate that this combination of disinfection methods improves the inactivation of bacteria. The absence of bacterial reactivation after 60 s of exposure to UV radiation was determined, as well as the knowledge that the exposure time of UV light also has an impact on the bactericidal effect.
Although studies focusing on swimming pool biofilms are not extensive, they represent a certain investigation challenge. Nevertheless, it is known that biofilms are present in almost every damp part of swimming pools, such as pipe surfaces [8], in filters [45], on shower floors [46], and on inflatables [47], which necessitates more systematic knowledge about their possible control in aquatic systems. The results suggest that combined UV/chlorine disinfection has the potential to reduce the presence of biofilm in aquatic environments. Plankton cultures should be the predominant mechanism in biofilm control and suppression.
4. Conclusions
- ▪
- The obtained results indicate that increased concentrations of trihalomethanes in pool water followed stronger water chlorination with higher concentrations of free and total chlorine. In addition, a greater number of bathers had a significant influence on their presence and on the occurrence of P. aeruginosa in pool water.
- ▪
- The occurrence of P. aeruginosa was significantly correlated with the period of an applied single (Cl) disinfection method.
- ▪
- The combined method, UV radiation/chlorination, showed the best efficiency in the destruction of mature P. aeruginosa biofilm and its ability to form biofilms.
- ▪
- The combined method did have a statistically significant effect on the number of viable bacteria of P. aeruginosa but did not eradicate mature biofilm.
- ▪
- The results of this research can contribute to furthering the understanding of biofilms created in swimming pools as a source of pool water contamination. Therefore, it is necessary to continuously monitor and control the formation of biofilm by improving sanitation and disinfection methods in swimming pools.
Author Contributions
Conceptualization, M.S.Z. and D.T.L.; methodology, G.B.; software, A.M.; validation, M.S.Z., G.B. and I.G.; formal analysis, M.S.Z. and G.B.; investigation, I.G.; data curation, A.M.; writing—original draft preparation, M.S.Z. and D.T.L.; writing—review and editing, I.G.; visualization, D.T.L.; supervision, D.T.L., I.G. and A.M.; funding acquisition, I.G. and D.T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding from commercial or not-for-profit sectors. The in vitro studies were supported by University of Rijeka research grant uniri-biomed-18-171.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article.
Acknowledgments
The authors are grateful to: Ilirija, shareholding company for providing pool availability for this investigation, and also to the staff of the Institute of Public Health in Zadar County, Department for Health ecology and environmental protection, for their help in sampling and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dilnessa, T.; Demeke, G. Microbiological, physical and chemical quality of swimming water with emphasize bacteriological quality. Glob. J. Med. Res. 2016, 16, 18–27. [Google Scholar]
- Giampaoli, S.; Spica, V.R. Health and safety in recreational waters. Bull. World Health Organ. 2014, 92, 79. [Google Scholar] [CrossRef] [PubMed]
- Vukić Lušić, D.; Maestro, N.; Cenov, A.; Lušić, D.; Smolčić, K.; Tolić, S.; Maestro, D.; Kapetanović, D.; Marinac-Pupavac, S.; Tomić Linšak, D.; et al. Occurrence of P. aeruginosa in water intended for human consumption and in swimming pool water. Environments 2021, 8, 132. [Google Scholar] [CrossRef]
- Harmsen, M.; Yang, L.; Pamp, S.J.; Tolker-Nielsen, T. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Microbiol. Immunol. 2010, 59, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Bjarnsholt, T.; Jensen, P.Ø.; Givskov, M.; Høiby, N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: Current and emerging inhibitors. Future Microbiol. 2013, 8, 901–921. [Google Scholar] [CrossRef]
- Bédard, E.; Prévost, M.; Déziel, E. Pseudomonas aeruginosa in premise plumbing of large buildings. Microbiol. Open 2016, 5, 937–956. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Rice, S.A.; van den Akker, B.; Pomati, F.; Roser, D. A risk assessment of Pseudomonas aeruginosa in swimming pools: A review. J. Water Health 2012, 10, 181–196. [Google Scholar] [CrossRef]
- Guida, M.; Di Onofrio, V.; Gallè, F.; Gesuele, R.; Valeriani, F.; Liguori, R.; Romano Spica, V.; Liguori, G. Pseudomonas aeruginosa in swimming pool water: Evidences and perspectives for a new control strategy. Int. J. Environ. Res. Public Health 2016, 13, 919. [Google Scholar] [CrossRef]
- Ekowati, Y.; Ferrero, G.; Farré, M.J.; Kennedy, M.D.; Buttiglieri, G. Application of UVOX Redox® for swimming pool water treatment: Microbial inactivation, disinfection by-product formation and micro pollutant removal. Chemosphere 2019, 220, 176–184. [Google Scholar] [CrossRef]
- Masschelein, W.J.; Rice, R.G. Ultraviolet Light in Water and Wastewater Sanitation, 1st ed.; Lewis Publishers, CRC Press: Boca Raton, FL, USA, 2002; pp. 9–54. [Google Scholar]
- Kudlek, E.; Lempart, A.; Dudziak, M.; Bujak, M. Impact of the UV lamp power on the formation of swimming pool water treatment By-Products. J. Civ. Eng. Archit. Built Environ. 2018, 11, 131–138. [Google Scholar] [CrossRef]
- Chu, H. Distribution and determinants of trihalomethane concentrations in indoor swimming pools. Occup. Environ. Med. 2002, 59, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, K.T.; Zoh, K.D. Characteristics of trihalomethane (THM) production and associated health risk assessment in swimming pool waters treated with different disinfection methods. Sci. Total Environ. 2009, 407, 1990–1997. [Google Scholar] [CrossRef]
- Villanueva, C.M.; Font-Ribera, L. Health impact of disinfection by-products in swimming pools. Ann. Dell’istituto Super. Sanita 2012, 48, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhoven, K.; Keski-Rahkonen, P.; Barupal, D.K.; Villanueva, C.M.; Font-Ribera, L.; Scalbert, A.; Bodinier, B.; Grimalt, J.O.; Zwiener, C.; Vlaanderen, J.; et al. Effects of exposure to water disinfection by-products in a swimming pool: A metabolome-wide association study. Environ Int. 2018, 111, 60–70. [Google Scholar] [CrossRef]
- Beyer, A.; Worner, H.; van Lierop, R. The Use of UV for Destruction of Combined Chlorine; Version 1.0; Wallace & Tiernan: The Netherlands, 2004; Available online: https://www.pwtag.org.uk/reference/ (accessed on 5 July 2022).
- Høiby, N.; Krogh, J.H.; Moser, C.; Song, Z.; Ciofu, O.; Kharazmi, A. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect/Inst. Pasteur. 2001, 3, 23–35. [Google Scholar] [CrossRef]
- Knezevic, P.; Obreht, D.; Curcin, S.; Petrusic, M.; Aleksic, V.; Kostanjsek, R.; Petrovic, O. Phages of Pseudomonas aeruginosa: Response to environmental factors and in vitro ability to inhibit bacterial growth and biofilm formation. J. Appl. Microbiol. 2011, 111, 245–254. [Google Scholar] [CrossRef]
- ISO 7393-2:2018; Water Quality—Determination of Free Chlorine and Total Chlorine—Part 2: Colorimetric Method Using N, N-Diethyl-1,4-Phenylenediamine, for Routine Control Purposes. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- ISO 10523:2008; Water Quality—Determination of pH. International Organization for Standardization (ISO): Geneva, Switzerland, 2008.
- ISO 10301:1997; Water Quality—Determination of Highly Volatile Halogenated Hydrocarbons—Gas-Chromatographic Methods. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- ISO 16266:2008; Detection and Enumeration of Pseudomonas aeruginosa—Method by Membrane Filtration. International Organization for Standardization (ISO): Geneva, Switzerland, 2008.
- Ivanković, T.; Goić-Barišić, I.; Hrenović, J. Reduced susceptibility to disinfectants of Acinetobacter baumannii biofilms on glass and ceramic. Arh. Za Hig. Rada I Toksikol. 2017, 68, 99–108. [Google Scholar] [CrossRef]
- Zwiener, C.; Richardson, S.D.; DeMarini, D.M.; Grummt, T.; Glauner, T.; Frimmel, F. Drowning in disinfection by-products? Assessing swimming pool water. Environ. Sci. Technol. 2007, 41, 363–372. [Google Scholar] [CrossRef]
- Mustapha, U.F.; Abobi, S.M.; Quarcoo, G. Physicochemical and bacteriological quality of public swimming pools in the Tamale Metropolis, Ghana. J 2020, 3, 236–249. [Google Scholar] [CrossRef]
- Official Gazette of the Republic of Croatia 59/2020; Regulation on sanitary-technical and hygienic conditions of swimming pools and on the health safety of Pool Waters; Ministry of Health: Zagreb, Croatia, 2020.
- Karimi, B. Formation of disinfection by-products in the swimming pool water treated with different disinfection types. Desalination Water Treat 2020, 175, 174–181. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Jahed, G.R.; Zarei, A. Investigation of low-pressure ultraviolet radiation on inactivation of rhabitidae nematode from water. Iran. J. Public Health 2013, 42, 314–319. [Google Scholar]
- Cassan, D.; Mercier, B.; Castex, F.; Rambaud, A. Effects of medium-pressure UV lamps radiation on water quality in a chlorinated indoor swimming pool. Chemosphere 2006, 62, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Bożym, M.; Kłosok-Bazan, I.; Wzorek, M. Analyzing THM Concentrations in selected indoor swimming pool waters in the Opole Region. Pol. J. Environ. Stud. 2018, 27, 1001–1008. [Google Scholar] [CrossRef]
- Dyck, R.; Sadiq, R.; Rodriguez, M.J.; Simard, S.; Tardif, R. Trihalomethane exposures in indoor swimming pools: A level III fugacity model. Water Res. 2011, 45, 5084–5098. [Google Scholar] [CrossRef]
- Li, J.; Blatchley, E.R., III. Volatile disinfection by-product formation resulting from chlorination of organic nitrogen precursors in swimming pools. Environ. Sci. Technol. 2007, 41, 6732–6739. [Google Scholar] [CrossRef]
- Manasfi, T.; Temime-Roussel, B.; Coulomb, B.; Vassalo, L.; Boudenne, J.L. Occurrence of brominated disinfection by-products in the air and water of chlorinated seawater swimming pools. Int. J. Hyg. Environ. Health 2017, 220, 583–590. [Google Scholar] [CrossRef]
- Chambers, V.K.; Creasy, J.D.; Joy, J.S. Modelling free and total chlorine decay in potable water distribution systems. J. Water Sci. Res. Technol. Aqua. 1995, 44, 60–69. [Google Scholar]
- Cheema, W.A.; Kaarsholm, K.M.S.; Andersen, H.R. Combined UV treatment and ozonation for the removal of by-product precursors in swimming pool water. Water Res. 2017, 110, 141–149. [Google Scholar] [CrossRef]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection by products in swimming pool: Occurrences, implications and future needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef]
- Chevremont, A.C.; Farnet, A.M.; Sergent, M.; Coulomb, B.; Boudenne, J.L. Multivariate optimization of facal bioindicator inactivation by coupling UV-A and UV-C LEDs. Desalination 2012, 285, 219–225. [Google Scholar] [CrossRef]
- Marconnet, C.; Houari, A.; Seyer, D.; Djafer, M.; Coriton, G.; Heim, V.; Di Martino, P. Membrane biofouling control by UV irradiation. Desalination 2011, 276, 75–81. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, B.; Lu, Y.; Mei, Y.; Shen, L. Advances in application of ultraviolet irradiation for biofilm control in water and wastewater infrastructure. J. Hazard. Mater. 2022, 421, 126682. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 9, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Hessler, C.M.; Panmanee, W.; Hassett, D.J.; Seo, Y. Pseudomonas aeruginosa inactivation mechanism is affected by capsular extracellular polymeric substances reactivity with chlorine and monochloramine. FEMS Microbiol. Ecol. 2013, 83, 101–111. [Google Scholar] [CrossRef]
- Chen, C.I.; Griebe, T.; Characklis, W.G. Biocide action of monochloramine on biofilm systems of Pseudomonas aeruginosa. Biofouling 1993, 7, 1–17. [Google Scholar] [CrossRef]
- Wende, E.V.D. Biocide ACTION of chlorine on Pseudomonas aeruginosa Biofilm. Ph.D. Dissertation, Montana State University-Bozeman, College of Engineering, Bozeman, MT, USA, 1991. [Google Scholar]
- Uhl, W.; Hartmann, C. Disinfection by-products and microbial contamination in the treatment of pool water with granular activated carbon. Water Sci. Technol. 2005, 52, 71–76. [Google Scholar] [CrossRef][Green Version]
- Leoni, E.; Legnani, p.; Mucci, M.T.; Pirani, R. Prevalence of mycobacteria in a swimming pool environment. J. Appl. Microbiol. 1999, 87, 683–688. [Google Scholar] [CrossRef]
- Tate, D.; Mawer, S.; Newton, A. Outbreak of Pseudomonas aeruginosa folliculitis associated with a swimming pool inflatable. Epidemiol. Infect. 2003, 130, 187–192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).