Abstract
During 2006–2008, a pipeline was buried in Vallona lagoon in the Northern Adriatic Sea (Italy). A Before-During-After environmental monitoring programme was scheduled to monitor possible alterations. Bioaccumulation of metal(loid)s, BTs (butyltins) and HMW-PAHs (High Molecular Weight Polycyclic Aromatic Hydrocarbons), and biological responses (Condition index, air Survival—LT50, Acetylcholinesterase, Micronuclei—MN, acyl-CoA oxidase, catalase, malondialdehyde—MDA, and the total oxyradical scavenging capacity—TOSCA) were investigated in Manila clams (Ruditapes philippinarum) from November 2005 to June 2015. In opera (IO) results showed higher levels of HMW-PAHs (73 ± 13 ng/g), BTs (90 ± 38 ng Sn/g) and increasing levels of Pb (6.7 ± 0.7 mg/kg) and Zn (73.6 ± 6.08 mg/kg) probably linked to works. Other contaminant alterations, especially metal(loid)s, before (AO) and after (PO) the burial, were attributed to a general condition of the area and mostly unrelated to works. In addition, LT50, MN and TOSCA showed alterations, probably due to hotspots occurring in IO. TOSCA and MDA increases, right after the burial, were considered delayed responses of IO, whilst other biological responses detected later were connected to the general condition of the area. Comparisons between results of Principal Component Analyses (PCAs) highlighted partial overlapping of AO and IO, whilst PO differed only for contaminants. Visual correlations between PCAs highlighted the biomarkers’ latter response.
1. Introduction
Monitoring of biological indicators is very important and useful for detecting impacts from anthropogenic pressures on aquatic ecosystems [1]. Indeed, the use of bioindicators provide better information than physico-chemical variables on ecosystem conditions, as organisms used in biomonitoring assessment can either integrate the biogeochemical changes within the ecosystem, or respond to several stresses over a long period of time [2]. To assess the potential toxic effects arising from environmental exposure to chemicals, information on their bioaccumulation in biota is essential, since they can provide information on their bioavailability. On the other hand, the measurement of sublethal biological effects also provides information at the molecular, cellular and functional levels, which may be considered as an early-warning tool of adverse effects of environmental stressors, such as contaminants. Indeed, biomarkers allow the assessment of fast responses to external drivers, even under less intense stress levels, and may detect integrated effects from exposure to complex mixtures [3,4,5,6,7,8]. In this context, bivalves, such as clams, represent the most suitable bioindicators in aquatic environments. They are widely distributed, sessile and filter feeding, and easy to sample. Bivalves are the most representative taxa among the benthic biomass in marine and transitional ecosystems, and they are responsible for fundamental ecological processes and functions. Finally, yet importantly, several bivalve species are often of economic value for their human consumption [9,10,11,12,13]. Bivalves are tolerant of, but sensitive to, several chemicals. They reflect environmental exposure levels, due to their limited capability to metabolize contaminants, such as heavy metals, metalloids, polycyclic aromatic hydrocarbons (PAHs) and butyltins (BTs) (see e.g., [4,14,15,16,17,18,19,20]).
Heavy metals and metalloids are naturally present in the Earth’s crust and can be introduced in the environment through geological, biological and anthropogenic processes [21]. In aquatic environments, metal(loid)s tend to partition between aqueous and solid phases, which may consist of sediments, particulates or biota. Hydrodynamism, biochemical processes and environmental conditions, such as pH, salinity and temperature, regulate their fate in marine and coastal waters [22,23,24]. Heavy metals and metalloids can be divided into being essential or non-essential to organisms. The former, such as copper, iron, manganese, and zinc, are indispensable at low concentrations for multiple metabolic activities, but at higher concentrations, they can become toxic; the latter, such as arsenic, barium, cadmium, chrome, mercury, nickel and lead, are generally not required for particular metabolic functions and are toxic even at low concentrations [25].
PAHs include a series of compounds of increasing environmental impact with fused aromatic rings [26,27,28]. Low molecular weight (LMW) PAHs, containing two or three benzene rings show acute toxicity but not chronic toxicity so they are not broad-spectrum carcinogens for marine organisms. High molecular weight (HMW) PAHs present four to six aromatic rings and show lesser acute toxicity, but are potentially more carcinogenic [29,30]. As a result, LMW-PAHs are sometimes classified separately from HMW-PAHs. PAHs released into the environment are due to both natural and, to a much greater extent, anthropogenic sources. Among the factors arising from human activities, pyrolytic and petrogenic sources are the most important. The former includes processes such as burning fossil fuels and emissions from incinerators. On the other hand, petrogenic inputs are considered as contaminations involving the introduction in the environment of petroleum products, such as oil and fuel spills, or coming from the run-off of the road network, industrial discharges from refineries and offshore wells [31]. In petroleum products, HMW PAHs generally show very low concentrations or are undetectable while LMW PAHs and especially their branched homologues are predominant. In combustion processes, HMW PAHs generally predominate over LMW ones [29,30]. So LMW PAHS may be considered petrogenic PAHs and the HMW ones may be considered pyrogenic PAHs.
Tributyltin (TBT) is a synthetic compound and its presence in the marine environment is mainly due to its use as an active component in antifouling paints [32]. TBT was defined as “probably the most toxic chemical compound ever deliberately introduced by societies into natural waters” [33] and it has been totally banned almost all around the world by the AFS Convention since 2008 [34]. Once released into the water, due to its hydrophobicity characteristics, TBT tends to bind to suspended particulate matter. In water, it would be easily degraded by debuthylation into dibutyltin (DBT) and monobutyltin (MBT) [35], but, at the same time, TBT is easily absorbed by organisms due to its liposolubility and accumulates in sediments by binding to organic matter, thus constituting a real reservoir of contamination for several years [36].
Metal(loid)s, PAHs and BTs are problematic issues for marine ecosystems, and some of them and their derived compounds are listed as priority and/or hazardous priority substances in the European Framework Directive (WFD 2000/60/EC) [37].
Biomarkers can respond to toxic stressors with several levels of specificity. However, most of them respond to environmental stress in general [14]. Various physiological responses, including basic functions, such as changing in growth rate, feeding, and reproduction, have been measured and used as biomarkers. Condition index and air survival are thus used to provide measures of bivalves’ well-being status assessments, which are relevant for ecological purposes [14,38,39,40]. Acetylcholinesterase (AChE) is a neurotoxicity biomarker whose inhibition may lead to behavioural impairment and thus it may affect survival, growth or reproduction of organisms [8]. AChE activity is usually inhibited by organophosphate or carbamate pesticides, but other chemicals (i.e., PAH, PCB, heavy metals, metalloids) have also been shown to modulate its activity [8,15,41,42,43,44]. The micronucleus assay is commonly used for evaluating DNA damage. The increase of micronuclei frequency (MN) is indeed a marker of genotoxic pollutants (see [15] and references therein). Peroxisomal enzymes, such as acyl-CoA oxidase (AOX), are involved in lipid metabolism through oxidative reactions and can be considered a specific biomarker of organic xenobiotic in molluscs [16]. Concerning the oxidative stress, the total oxyradical scavenging capacity toward hydroxyl and peroxyl radicals (TOSC HO· and ROO·) is a useful biomarker to quantify the whole capability of tissues to neutralize different forms of oxyradicals [8,45]; while catalase (CAT) is an enzyme involved in the antioxidant system, acting on the important precursor of hydroxyl radicals H2O2 by reducing the reactive oxygen species (ROS) levels induced by stressors [46]. Damage related to oxidative stress could be quantified by the level of the lipidic peroxidation product malondialdehyde (MDA) [8,45].
Coastal lagoons are priority habitats as declared by the European Union in the Habitats Directive, and their environments are very vulnerable to several anthropogenic pressures [47]. Vallona lagoon is one of the lagoons composing the Delta of the Po River, the most important river in Italy (961 km long), which passes from West to East through different regions and main cities of Northern Italy and flows into the Northern Adriatic Sea. The Po River Delta is a wetland of about 400 km2; it includes several lagoon areas and it is extremely important for its natural environmental variability and complexity, as well as for its economic values [48]. All around the Delta, the use of soil is mostly dedicated to agriculture, while typical activities in the lagoons are the extensive breeding of fish species and intense shellfish production, especially of Manila clams (Ruditapes philippinarum) [48,49].
R. philippinarum is often used as a bioindicator in transitional waters as this species can accumulate several pollutants, but can also provide biological responses that are easy to quantify (see e.g., [17,50,51,52]).
During 2006–2008, the burial of a pipeline took place in the middle of the Vallona lagoon to connect the structures of the first offshore LNG (Liquefied Natural Gas) terminal in Italy, in the Northern Adriatic Sea, to facilities on land [53]. In view of this, an environmental monitoring programme was scheduled from 2005 to 2015, which consisted of three phases: (i) before; (ii) during; and (iii) after the construction of the structures [54]. The monitoring programme for the Vallona lagoon was specifically scheduled to assess the possible impacts of the burial of the pipeline on the environment.
Previous results showed that a contamination probably related to the presence of machinery and working boats during the burying activities of the pipeline occurred [55]. In this context, heavy metal, metalloid and BT bioaccumulation were assessed considering HMW-PAH concentrations in clams as a marker of impacts of the construction activities. Moreover, bioaccumulation values were related to biological responses to monitor possible alterations in R. philippinarum populations in the Vallona lagoon by integrating chemical and biological status of the clams.
2. Materials and Methods
2.1. Sampling Strategy
The sampling was carried out in the Vallona lagoon, a transitional water area located in the northern part of the Po River Delta, NE Italy (Figure 1). Manila clams (R. philippinarum) were collected by manual rake at four sampling sites located at different distances from the pipeline: L022 and L023 are the closest stations from the pipeline, while L016 and L017 are the furthest.
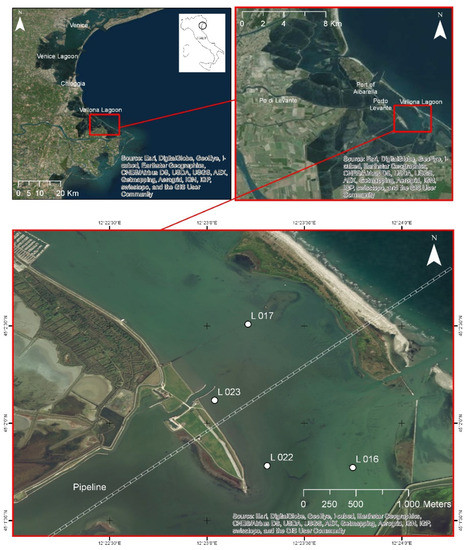
Figure 1.
Maps of the Vallona lagoon (NE, Italy) with localizations of the four sampling sites of Manila clams (R. philippinarum).
Manila clams were sampled before, during and after the activities for the burial of the pipeline that were carried out from November 2006 to May 2008 in alternating phases. Dredging activities occurred in November 2006, pipeline-laying operations were in February 2007 and covering was in May 2008. In this context, November 2005, February, April, July 2006 were considered ante operam (AO), November 2006, February 2007, June and November 2008 as in opera (IO), November 2010, June 2011 and November 2011, June and November 2012, May 2013 and November 2013, and June 2014 as post operam (PO).
After the sample collections, the individuals of R. philippinarum were quickly transported to the laboratory in a refrigerated and dark container.
For chemical analyses, bivalve specimens were dissected in the laboratory and divided into pools, each consisting of the set of soft parts of several individuals in order to ensure at least three replicates for each sampling station.
The dissected organisms were then homogenized with a dispersing tool (Ultra Turrax T 25, IKA® Werke, Germany), lyophilized and then stored at −20 °C in the dark until the analysis. For each sample, all three replicates were subjected to the analytical procedure.
For biochemical analyses, haemolymph and digestive glands were rapidly removed from 200 specimens. For each sampling site, digestive glands were pooled in 10 samples (each consisting of tissues from twenty specimens), frozen in liquid nitrogen and maintained at −80 °C; and an aliquot of haemolymph from 10 specimens was fixed with Carnoy’s solution (3:1 methanol, acetic acid) and stored at +4 °C for the microscopic evaluation of micronuclei frequency.
2.2. Analysis of Polycyclic Aromatics Hydrocarbons (PAHs)
The detailed analytical procedures to analyse the High Molecular Weight (HMW) PAHs (pyrene, fluoranthene, chrysene, benzo(a)anthracene, benzo(a)pyrene, dibenzo(a,h)anthracene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(g,h,i)perylene, indeno(1,2,3-cd)pyrene) in Manila clams of the present study are reported in [56].
2.3. Analysis of Heavy Metals and Metalloids
Metal(loid)s analysed for this study were arsenic, barium, cadmium, chromium, iron, manganese, mercury, nickel, lead, copper, and zinc.
For all metal(loid)s, the analytical procedure is the same as that reported in [56]. In addition, quantification limits were reported in [56] with the exception of Ba (2 µg/g d.w.), Mn and Zn (1.5 µg/g d.w.), and Cu (1 µg/g d.w). The recoveries were greater than 90%, except for Zn (80%) and Cr (82%).
2.4. Analysis of Butyltins (BTs)
The determination of BTs was carried out based on modified methods adopted by [57,58,59].
Briefly, tetrabutyltin (TTBT) was added to 0.5–1 g of each sample as an internal standard. After acidification with hydrochloric acid, BTs were extracted twice with 0.05% w/v tropolone solution in methanol, in an ultrasonic bath. The supernatants were separated by centrifugation (10 min at 1000× g) and saline solution in Milli-Q water (20%) was added. Methanol–water fraction was extracted with 10 mL of dichloromethane for three times. Dichloromethane extracts were dried through anhydrous Na2SO4 and concentrated to about 2 mL under a gentle stream of N2. During evaporation, the solvent was changed into n-hexane. The extract was derivatized with 0.5 mL of CH3MgCl or CH3MgBr (3M). After elimination of the excess reagent with NH4Cl (20% in MilliQ water), the organic phase was concentrated and cleaned-up on SPE columns filled with anhydrous Na2SO4 and Florisil. The eluate (n-hexane) was concentrated to 0.5 mL for injection into GC-MS/MS. All solvents used were of grade for residual pesticide analysis.
The analytical determination was carried out using a gas chromatograph (Trace GC, Thermo-Finnigan, Rodano-Milan, Italy) equipped with TriPlusAS autosampler (Thermo Fisher Scientific, Rodano-Milan, Italy) and coupled to an ion trap mass spectrometer (PolarisQ, Thermo Finnigan, Austin, TX, USA). GC was fitted with a capillary column Rxi®-5ms (length 25 m, i.d. 0.20 mm, film thickness 0.33 µm, Restek, Bellefonte, PA, USA). A splitless mode injection of sample (1.5 µL) was performed at 250 °C, with helium flow of 1.5 mL/min. The GC oven temperature was programmed as follows: 50 °C held for 2 min, from 50 °C to 164 °C at 9 °C/min and held for 5.2 min, from 164 °C to 300 °C at 50 °C/min and held for 9.5 min. The transfer line was kept at 240 °C.
The quantification was performed in MS/MS mode, by employing 137 m/z for TBT, 135 m/z for DBT and 135 m/z for MBT (precursor ions: 193 m/z, 151 m/z and 165 m/z, respectively). For the purpose of quality control of the analytical data, analyses of procedure blanks and certified reference material (ERM®-CE 477, EC–DG JRC Institute for Reference Materials and Measurements) were carried out, with recoveries of 92 ± 12%, 86 ± 6% and 88 ± 4% (n = 8) for TBT, DBT and MBT, respectively. For each compound, the reported concentration data are expressed both as cation per gram of dry weight (respectively (C4H9)3Sn+ for TBT, (C4H9)2Sn2+ for DBT and (C4H9)Sn3+ for MBT), and as tin (Sn) per gram of dry weight. The quantification limit of the method is 4 ng TBT/g d.w., 8 ng DBT/g d.w. and 6 ng MBT/g d.w., corresponding to 2 ng Sn/g d.w., 4 ng Sn/g d.w. and 4 ng Sn/g d.w., respectively.
2.5. Biological Responses
Condition index (CI) and Survival test (LT50) were applied following the methods described by [60]. In order to calculate the two indices, forty specimens per sample per index were used as reported by [55].
Biomarkers in clam tissues were measured following protocols described in [16,61], which included spectrophotometric determination of acetylcholinesterase (AChE) in haemolymph and acyl-CoA oxidase (AOX), catalase activity (CAT) and malondialdehyde levels (MDA) in digestive glands; gas chromatographic assay of total antioxidant capacity (TOSCA) towards peroxyl radicals (ROO·) and hydroxyl radicals (HO·) in digestive glands. Genotoxic damage, as micronuclei (MN) frequency in the haemocytes, was performed as described in [15], scoring 2000 cells with preserved cytoplasm for each specimen.
2.6. Statistical Methods
Kruskal–Wallis one way analysis of variance on ranks was applied to detect significant differences in metal(loid)s and BT bioaccumulation, and in biomarkers, comparing monitoring phases, surveys and sites. If significant, pairwise multiple comparisons were also conducted. Significant differences were highlighted with rejection of the null hypothesis at the 5% probability level (p < 0.05).
The Spearman’s correlation test was performed between TBT and DBT + MBT, and all bioaccumulation and biological response data.
Principal component analyses (PCA) were applied to data of contaminants (HMW-PAHs, Heavy Metals, Metalloids and BTs), and of biological responses (CI, LT50, AChE, AOX, CAT, MN, MDA, TOSC_HO·, TOSC_ROO·), respectively, to elucidate on bioaccumulation pattern, probable input sources, and biological responses. Finally, biplots of PCA results of bioaccumulations and biological responses were compared.
Software R studio [62] and STATISTICA Software, release 10 (StatSoft Inc., Tulsa, OK, USA) were used for statistical processing.
All values less than the limit of quantification (LOQ) were replaced with zeros to calculate the sum of HMW-PAHs and BTs, and were considered as ½ LOQ in all the other calculations and analyses.
3. Results
3.1. Contaminant Body Burdens
Values of metal(loid)s and BT bioaccumulations for all surveys are reported in Tables S1–S3, respectively.
Comparing the three monitoring phases (AO vs. IO vs. PO), concentrations of all metal(loid)s and BTs were significantly different (p < 0.05), except for Hg (p > 0.05). Results of Ba, Cr, Fe, Mn, Ni, TBT and DBT showed significantly (p < 0.05) lower values (mean ± s.d.) in PO (3.58 ± 0.46 mg/kg; 3.55 ± 0.53 mg/kg; 680.91 ± 86.6 mg/kg, 19.60 ± 2.58 mg/kg, 7.97 ± 0.87 mg/kg, 33 ± 2.73 ngTBT+/g, 8.13 ± 1 ngDBT+/g, respectively), whilst AO (6.17± 0.85 mg/kg; 5.23 ± 0.83 mg/kg; 902.15 ± 142.83 mg/kg; 29.59 ± 4.57 mg/kg; 8.46 ± 1.01 mg/kg; 164 ± 17.86 ngTBT+/g; 28 ± 3.48 ngDBT+/g, respectively) and IO (4.58 ± 0.5 mg/kg; 4.43 ± 0.46 mg/kg; 1103.32 ± 119.61 mg/kg; 32.27 ± 3.08 mg/kg; 9.71 ± 0.83 mg/kg; 185 ± 11.29 ngTBT+/g; 29 ± 2.44 ngDBT+/g, respectively) had similar results (p > 0.05). The values of As were significantly (p < 0.0001) higher in PO (22.11 ± 2.46 mg/kg) than in AO (12.95 ± 1.67 mg/kg) and IO (15.76 ± 1.93 mg/kg). Levels of Cd were significantly (p < 0.01) lower in IO (0.49 ± 0.05 mg/kg) than in AO (0.73 ± 0.1 mg/kg) and PO (0.59 ± 0.07 mg/kg). Concentrations of Pb resulted significantly (p < 0.0001) higher in IO (6.69 ± 0.66 mg/kg) rather than in the other two phases (AO: 0.99 ± 0.13 mg/kg; PO: 1.62 ± 0.18 mg/kg), Zn showed significantly (p < 0.05) lower values in AO (73.56 ± 6.02 mg/kg) respect to the following phases (IO: 88.31 ± 6.63 ng/g; PO: 91.18 ± 6.61 mg/kg), and Cu had significantly (p < 0.05) higher values in PO (9.55 ± 0.8 mg/kg) than in the previous phases (AO: 8.67 ± 0.78 mg/kg; IO: 9.44 ± 0.53 mg/kg). MBT values were significantly (p < 0.0001) higher in AO (26 ± 3.02 ngMBT+/g) than in the following phases (IO: 7 ± 0.84 ngMBT+/g; PO: <6 ngMBT+/g).
No clear and significant differences were observed between spring–summer surveys (April–July) with respect to autumn–winter (October–February) campaigns (p > 0.05), and no significant differences in concentrations were observed between sampling sites (p > 0.05), except in site L023V, where TBT was significantly higher than L016V (p < 0.05), considering all the surveys.
TBT results were significantly correlated with the sum of DBT and MBT (Spearman’s R = 0.78; p < 0.05), also when considering each single monitoring phase, except for IO values (AO: R = 0.78; p < 0.05; IO: R = 0–0.17, p >0.05; PO: R = 0.38, p < 0.05).
PAH results in Manila clams were presented in [55].
3.2. Biological Responses
Biomarkers results are reported in Table S4.
Comparing all data of each phase, some significant differences (p < 0.01) were evidenced in biomarker results, except for AOX (p > 0.05). Values of MN, as well as TOSCA HO·, were significantly (p < 0.0001) higher in IO (0.54 ± 0.60 ‰, and 485.66 ± 55.73 UTOSC/mg prot., respectively) and PO (0.39 ± 0.54 ‰, and 545.31 ± 85.19 UTOSC/mg prot.) than in AO (0.20 ± 0.36 ‰, and 354.74 ± 60.57 UTOSC/mg prot.). Levels of AChE and CAT were significantly (p < 0.001) lower in PO (10.97 ± 3.63 nmol/min/mg prot., and 34.25 ± 7.24 µmol/min/mg prot.) than in the previous phases (AO: 13.62 ± 4.53 nmol/min/mg prot., and 33.93 ± 7.1 µmol/min/mg prot.; IO: 14.59 ± 2.25 nmol/min/mg prot., and 39.27 ± 6.69 µmol/min/mg prot., respectively). Values of TOSC_ROO· and MDA in PO (542.63 ± 86.75 UTOSC/mg prot., and 38.34 ± 9.37 nmol/min/g tissue) were significantly different from those of the other phases (p < 0.01), the first being higher than IO (421.02 ± 66.48 UTOSC/mg prot.) and the latter higher than AO (28.26 ± 7.88 nmol/min/g tissue).
There were no significant differences (p > 0.05) between sampling sites. Although a light seasonality was evidenced in the AO phase [63], no clear and significant (p > 0.05) differences were highlighted between seasons sampled in the other phases (IO and PO). Results of PO surveys showed higher variability, especially for CAT, AOX, TOSC_HO·. and TOSC_ROO· values.
Condition index and LT50 results were presented in [56].
3.3. Bioaccumulation and Biological Response Patterns
Figure 2 depicts the distribution of Manila clam bioccumulation data according to the first and second components of PCA that accounted for 32.5% and 16% of the total variance, respectively. The first component is mainly related to Ba, Cr, Fe, and Mn, whilst the second component is related to Cu, Zn, DBT, TBT, and HMW-PAHs. In this context, post operam samples differed from those collected during ante operam and in opera. Samples from IO were mostly characterized by HMW-PAHs, TBT and DBT, and slightly also by Cd, Hg, and MBT. Among all samples, those collected in Febraury 2006 and 2007 were separated mostly by HMW-PAHs and BTs. Mn, Fe, Cr, Ni and Ba differed for some AO samples (L022V and L023V of November 2005). Some PO samples from November 2011 are separated from others for Cu and Zn.
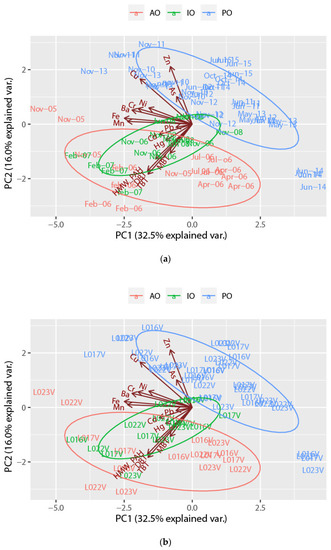
Figure 2.
Biplots of the Manila clam samples, and the loadings of bioaccumulation data, labelled as (a) surveys and (b) sites, on the first two principal components. (AO = ante operam, IO = in opera, PO = post operam).
PCA was also performed on biological response data as presented in Figure 3. First and second principal components explained 30.7% and 17.1% of the total variance, respectively, with the first PC being related to AOX, CAT, TOSC_HO· and TOSC_ROO·, whilst PC2 was related to MDA, TOSC_ROO·, CI and LT50. Results displayed similar overlapping between AO and IO samples, as shown in Figure 2 for bioaccumulation values, while PO samples resulted in more spread. Generally, samples grouped more for surveys rather than sites. Among PO clam samples, those from June 2012 differed from the others for MDA, those from November 2012 for LT50, those from May 2013 for AOX and AChE, whilst those from June 2014 were separated from the others for CI. Finally, samples from November 2013, October 2014 were grouped for TOSC_ROO· and TOSC_HO·.
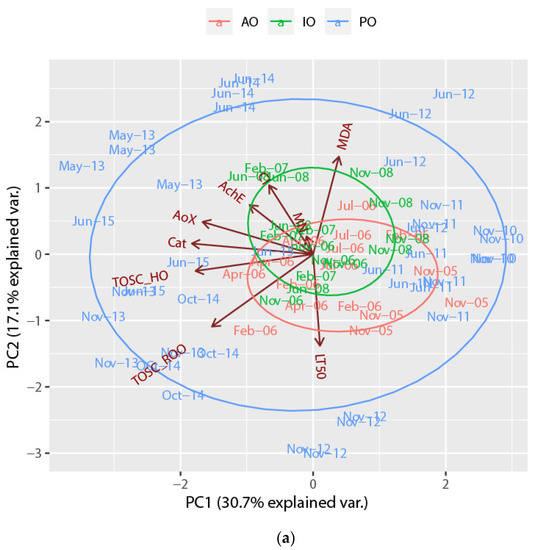
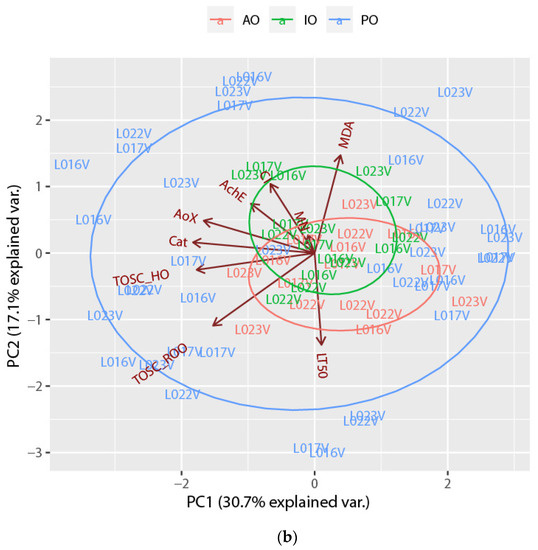
Figure 3.
Biplots of the Manila clam (R. philippinarum) samples and the loadings of biomarkers and biological indices, labelled as (a) surveys and (b) sites, on the first two principal components. (AO = ante operam, IO = in opera, PO = post operam).
Spearman’s correlation results are reported in Table S5. Positive and significant (p < 0.05) correlations resulted between Cu and LT50 and between Cu and all the other metal(loid)s, except for Zn. The results of Ba significantly positive correlated with all the other metal(loid)s, except for As, Cd and Hg, and also correlated with LT50. Significantly positive correlations also resulted between DBT and some heavy metals (Cr, Fe, Mn, Hg, Ni, and Pb), BTs (TBT and MBT), and AChE. Then DBT, along with TBT, were also significantly (p < 0.05) positively correlated with HMW-PAHs. Instead, relationships were significantly (p < 0.05) negative between DBT and biomarkers, such as AOX and TOSC HO·, and between TBT and AOX, MDA, TOSC_HO·, TOSC_ROO·. Then, HMW-PAHs were significantly (p < 0.05) positively correlated with Cr, Fe, Mn, Hg, Ni, Pb, TBT, DBT, and also LT50 and AChE; while significantly negative correlations were found between PAHs and AOX, TOSC_ROO· and TOSC_HO·. TOSC_ROO· and TOSC_HO· values showed negative correlations with Fe, Hg, TBT, in addition to HMW-PAH, and significantly (p < 0.05) positive correlations with CAT and with each other. There were positive and significant correlations between AOX and AChE and CAT, and between MN and As; while it was significantly negative between AChE and As, and between CI and all metal(loid)s (except Cr and Mn) and LT50.
The results of visual correlation between two PCA biplots (bioaccumulations vs. biological responses) are reported in Table 1. Both biplots were partially overlapping between AO and IO samples. In the AO phase samples from November 2005 at sites L022V and L023V were separated for higher levels of Mn, Fe, Cr, Ni and Ba, whilst samples at the same sites (L022V and L023V) from February 2006 were grouped for HMW-PAH, TBT and DBT. Similarly in the IO phase all samples from February 2007 were separated for higher levels of HMW-PAH, TBT, DBT, Cd, Hg, and for CI; in this phase, samples from June 2008 were separated for higher values of CI. In the PO phase samples of the initial surveys were characterized first by higher values of Cu, and Zn in November 2011. Later in June 2012, the values of MDA increased, as well as the values of LT50 in November 2012. Samples from May 2013 were very different for lower concentrations of Cd, Hg, and Pb and higher levels of AOX and AChE. In November 2013, samples were grouped for higher level of Cu, Zn and As as well as for increasing of TOSC_ROO· and TOSC_HO· values; samples from June 2014 were characterized by lower values of Fe, Mn, Cu, Zn, and higher of CI, and lower of LT50. Samples from October 2014 were characterized by lower values of PAH, and higher values of Cu and Zn, TOSC_ROO· and TOSC_HO·. Finally, the last surveys of the study showed that in June 2015 samples were separated by lower HMW-PAH, concentrations and higher values of TOSC_HO·, CAT and AOX.
4. Discussion
Bioaccumulation levels of metal(loid)s, PAHs and BTs, monitored before, during and after the pipeline burying, in Manila clams (R. philippinarum) from Vallona lagoon showed dissimilar patterns when considering the three different phases of the monitoring plan.
PAHs were significantly lower in the post operam phase than in the previous two phases. HMW-PAHs characterized the body burden pattern during the pipeline burial phase, which was mostly connected to the presence of machinery and working boats. On the contrary, in the ante operam phase, an increase in LMW-PAHs resulted as a hotspot, probably due to oil pollution, but not related to the pipeline laying [55]. Similar patterns, with AO and IO being higher than PO, resulted also in body burdens of Ba, Cr, Fe, Mn, Ni, TBT and DBT. On the contrary, MBT was higher before the pipeline burial rather than during and after the laying of it. The only substance with higher concentrations in clams during the burial of the pipeline with respect to the other two monitoring phases was Pb, whilst Zn showed an increase during in opera that did not lower in the following phase (PO). Finally, concentrations of Cu increased just at the end of the monitoring activities in PO.
Sediments, which showed HMW-PAHs always higher than LMW-PAHs, were excluded as a possible source of contamination for clams in Vallona lagoon, mostly due to the different contributions of each compound to the two matrices and low values of Biota-Sediment Accumulation Factors (BSAFs) [55]. The same conclusions were also stated by [56] about sediment contamination and bioavailability of metal(loid)s such as As, Cd, Cr, Hg, Ni and Pb. Indeed, they assumed that in Vallona lagoon, metal(loid)s were linked to mineralogical phases of the sediments, which make them not easily available to clams.
To elucidate on the bioaccumulation pattern of Manila clams, principal component analysis (PCA) was performed, considering HMW-PAHs as a marker of effects connected to the burial of the pipeline. Indeed, in previous results, exclusively focusing on PAHs, HMW-PAHs were related to the presence of machinery and working boats during the activities [55]. Performing PCA to all body burden data, three groups were identified according to the monitoring phase. However, while expecting possible overlapping between ante and post operam, and separation from in opera, data collected after pipeline burial (PO) clearly parted from the other two phases (AO and IO), which were partially overlaid. However, the overlapping seems to be due to a spread of data in the AO monitoring phase. Indeed, before the burial of the pipeline, Mn, Fe, Cr, Ni and Ba levels characterized some samples, whilst during the laying of the pipeline, all data differed for HMW-PAHs, TBT and DBT concentrations. On the contrary, the last phase showed more homogenous data with only some samples characterized by As, Cu and Zn. The positive and significant correlation between TBT, DBT and HMW-PAHs could also confirm their associations with boats involved in the burial of the pipeline, while the other alterations, occurring before and after, could be attributed to a general condition of the area and mostly unrelated to the burying activities. Anyway, in samples collected near the future pipeline in February 2006, TBT showed values comparable to those found during the burial activities at all sites. Therefore, a general presence in the area could also be assumed for this compound. Indeed, TBT application was banned by 2003, and the total and global ban has started only since 2008, just at the end of the burial activities that took place, even if not continuously in time, from the end of 2006 to 2008. However, it would not be surprising to find higher BT levels in clams, as old paint layers on the boat hulls continue to act as sources to the environment [64]. Since the positive and significant correlation between TBT and DBT + MBT, it could also be possible to consider the former as the principal compound introduced in environment and the latter as metabolites of TBT [65].
The environmental quality status in Vallona Lagoon was affected by heavy metals contamination [56]; however, the trend in clams was not so clear even considering the burial of the pipeline as a possible impact. Indeed, metal(loid)s mostly characterized samples before and after in opera activities, rather than during them, with the exception of Pb and Zn. A possible general source of metal(loid)s contamination is the Po di Levante channel, which flows north of the lagoon and its paths laps it, and collects industrial and sewage discharges flow from the Po River, the largest river in Italy. Indeed, it runs across the Pianura Padana with a drainage of 74,000 km2 and more than 16 million people living around the basin. Moreover, a lot of intensive industry and other economic activities, which represent almost half of Italian GDP, surround the Po River basin [66].
According to the Ecological Quality Standards (EQS) set by Italian Regulation (D.lgs. no. 172/2015) for the implementation of Directive 2013/39/UE, only Hg, Fluoranthene and Benzo[a]pyrene are regulated in biota with EQSs of 20 μg/kg w.w., 30 μg/kg w.w. and 5 μg/kg w.w., respectively. Most of the Hg concentrations detected in samples of the present study are above the standard, whilst levels of the two PAH compounds are all far below the EQSs. Manila clams are both bioindicators of environmental quality status and both important as an economical source for human consumptions. Comparing our results with EC Regulation 1881/2006, which set maximum levels for certain contaminants in foodstuffs, concentrations of Cd, Hg, and benzo[a]pyrene were below the limits (1 mg/kg w.w., 0.5 mg/kg w.w., and 10 ng/g w.w., respectively. Only Pb was above the limit (1.5 mg/kg w.w.), but just in samples collected in November 2006, but it quite quickly returned to below the limit after that. Limits for BTs in biota are lacking both considering regulatory levels for human and/or standards for environmental protection. However, a Tolerable Daily Intake (TDI) for TBT and DBT of 0.1 μg/kg b.w. (expressed as Sn) were set, based on the immunotoxic effects of TBT in humans, and was adopted by EFSA (European Food Safe Authority) [67]. Indeed, the main route for human exposure is diet and, among food, fish products, in particular molluscs, are those with the highest levels of concentration to which humans may be exposed (see e.g., [68,69,70]). Considering an average person of 70 kg b.w., no sample would exceed TDI for a daily consumption of about 85 g of Manila clams in Vallona lagoon.
Even if pollution control regulations are only focused on chemical analysis, it is evidently not sufficient to monitor and protect aquatic ecosystem. Indeed, very few substances are regulated for biota, but it must also be considered that sublethal levels of environmental toxicants may cause changes in the physiological condition of molluscs, such as clams R. philippinarum, which could be more hazardous for populations and with dangerous consequences for the environment [8,14]. In the present study, the biomonitoring programme also provided an assessment of physiological indices and biomarkers, to better understand the impacts of the pipeline burial on the Manila clam population.
The Condition index showed the typical seasonal fluctuation, reflecting the reproductive and physiological characteristics of the species in Adriatic lagoons [60,71]. However, significantly lower values resulted in a post operam phase rather than in the previous ones. Environmental conditions, such as water and air temperatures, may explain the lowering, since spring 2013 was colder than usually reported for the same season [55].
The same seasonal fluctuations were also observed for air survival time of clams, which resulted in a low capacity to survive in summer and spring surveys, and better resistance in colder seasons. However, during the burial of the pipeline, air survival showed a decrease, which made this physiological index more sensitive to the stress conditions occurring during the works [55]. Moreover, this stress condition during the works was also evidenced by biomarkers such as MN and TOSC_HO·, which showed an increasing trend probably related to bioaccumulation hotspots occurring during this phase (IO), still detectable after the burial of the pipeline (PO). The increasing of these biochemical responses, together with the increase of TOSC_ROO· and MDA during the post operam phase, indicated the activation of the antioxidant system to counteract the excess of ROS due to the increasing of environmental contaminants, connected with the works [5]; however, this system was not efficient enough to avoid damage at DNA and membrane lipids level. Further damage was evidenced at the nervous level, as suggested by the inhibition of AChE in the post operam phase with respect to the previous ones.
As reported for the bioaccumulation pattern, PCA performed on biological responses showed an overlapping of the two first phases as well as in the bioaccumulation biplot, with a greater spread of data in the PO. Although air survival time should have been the marker of works in our a priori hypothesis, the IO group of data was not clearly characterized by some variables, such as in the bioaccumulation biplot, as also confirmed by no correlations between LT50 and the other biomarker results.
To compare bioaccumulation data and biomarker results, we first performed correlations matrix and calculated significant relations with Spearman’s R. An interesting negative correlation was evidenced between As and AChE, attesting an inhibiton of acethylcholinesterase activity and neurotoxicity related to the exposure to this metalloid, in agreement with what was found in rats [72]; then a positivite and expected correlation between CAT, TOSC_ROO· and TOSC_HO· was found, all being biomarkers of antioxidative stress.
To integrate the chemical and biological quality status of the clams, a visual comparison between the two PCA biplots, resulting from analyses of bioaccumulation and biomarker data, respectively, was also proposed. Both biplots depicted a partially overlapping between ante operam and in opera phases and a partial separation of the group of bioaccumulation data belonging to post operam surveys. In this context, some samples differed from the groups for bioaccumulation data, as shown during burial activity in February 2006. The trend of biological responses in the following phase seemed to be related to the trend of contaminants and human activities carried out in these phases. In the IO phase, although an apparent good condition was underlined by increasing CI values, the high concentrations of HMW-PAH, probably related to the traffic of boats involved in burial of pipeline activities, induced radical formation in clams, partially counteracted by the activation of the antioxidant system in the PO phase. This stress condition, and lipid membrane damage, appeared at the beginning of the PO phase (2012), as evidenced by the increase of MDA, associated with an increase in some heavy metals such as Cu and Zn in November 2011. It partially persisted in November 2013, with an increasing of Cu, followed by an increasing of antioxidant capability towards oxyradicals [5]. In 2014, a partial revitalization occurred as suggested by the CI increasing, associated with a decrease of heavy metals such as Fe, Mn, Cu, and Zn. In the last two surveys of PO (October 2014 and June 2015), even if a partial decrease of HMW-PAHs was detected, the antioxidant system was still active with an increase of total antioxidant capacity (TOSC_ROO· and HO·) and CAT activity, probably relating to the increase of Cu and Zn [5,72,73].
5. Conclusions
The results of biomonitoring of Manila clams (R. philippinarum) before, during and after the pipeline burial in Vallona lagoon showed an increase of some contaminants in body burdens connected to the works and that sudden biological responses occurred, which could be also considered as a later reaction. However, conditions improved after the in opera phase as reflected by the lowering of bioaccumulation levels and better physiological responses. Indeed, some pollutants also resulted in being at significantly lower levels than before the burial of the pipeline.
Contamination levels were quite comparable to other geographical areas with medium to low pollution impact. As a result, biological responses were related to stress conditions due to working activities, even if a slight effect could be assumed right after the burial. Such a later response was better assessed by visual correlations of PCA biplots that could be adopted as a supporting method to integrate biological responses and bioaccumulation patterns with multiple parameters and compounds to consider.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments9070081/s1, Table S1. Bioaccumulation results (mean ± dev. std.) of metal(loid)s (As, Ba, Cd, Cr, Fe) in R. philippinarum. Table S2. Bioaccumulation results (mean ± dev. std.) of heavy metals (Mn, Hg, Ni, Pb, Cu, Zn) in R. philippinarum. Table S3. Bioaccumulation results (mean ± dev. std.) of BTs in R. philippinarum expressed as ng cation per gram of dry weight and as tin (Sn) per gram of dry weight Table S4. Results of biomarkers (mean ± dev. std.) analysed in Manila clams (R. philippinarum) in Vallona Lagoon. Table S5. Values of R Spearman’s correlations resulted significant (p < 0.05).
Author Contributions
Conceptualization, F.C. and C.V.L.; methodology, C.V.L.; validation, C.V.L., G.M. (Ginevra Moltedo), G.S., F.R., D.B. and C.M.; formal analysis, F.C., M.F., V.B., G.M. (Ginevra Moltedo), B.C., G.M. (Giacomo Martuccio), M.B., M.T.B., G.S. and G.F.; data curation, F.C., B.C. and V.B.; writing—original draft preparation, F.C., G.M. (Ginevra Moltedo) and M.F.; writing—review and editing, all authors; visualization, F.C. and V.B.; supervision, C.V.L.; project managers, C.V.L. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work comes from the environmental monitoring activities of an offshore LNG Terminal located in the northern Adriatic Sea, founded by Adriatic LNG Company.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Morse, J.C.; Bae, Y.J.; Munkhjargal, G.; Sangpradub, N.; Tanida, K.; Vshivkova, T.S.; Wang, B.; Yang, L.; Yule, C.M. Freshwater biomonitoring with macroinvertebrates in East Asia. Front. Ecol. Environ. 2007, 5, 33–42. [Google Scholar] [CrossRef]
- Mezgebu, A. A review on freshwater biomonitoring with benthic invertebrates in Ethiopia. Environ. Sustain. Indic. 2022, 14, 100174. [Google Scholar] [CrossRef]
- Broeg, K.; Lehtonen, K.K. Indices for the assessment of environmental pollution of the Baltic Sea coasts: Integrated assessment of a multi-biomarker approach. Mar. Pollut. Bull. 2006, 53, 508–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, M.; Pauletto, M.; Patarnello, T.; Bargelloni, L.; Marin, M.G.; Matozzo, V. Gene transcription and biomarker responses in the clam Ruditapes philippinarum after exposure to ibuprofen. Aquat. Toxicol. 2013, 126, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Benedetti, M.; Giuliani, M.E.; Regoli, F. Oxidative metabolism of chemical pollutants in marine organisms: Molecular and biochemical biomarkers in environmental toxicology. Ann. N. Y. Acad. Sci. 2015, 1340, 8–19. [Google Scholar] [CrossRef]
- Rato, L.D.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.F.; Leandro, S.M. Homarus gammarus (Crustacea: Decapoda) larvae under an ocean acidification scenario: Responses across different levels of biological organization. Comp. Biochem. Physiol. 2017, 203, 29–38. [Google Scholar] [CrossRef]
- Lemos, M.F.L. Biomarker Studies in Stress Biology: From the Gene to Population, from the Organism to the Application. Biology 2021, 10, 1340. [Google Scholar] [CrossRef]
- Bryan, G.W.; Langston, W.J.; Hummerstone, L.G.; Burt, G.R. A guide to assessment of heavy metal contamination in estuaries using biological indicators. J. Mar. Biol. Assoc. UK 1985, 4, 1–92. [Google Scholar]
- Widdows, J.; Donkin, P. The application of combined tissue residue chemistry and physiological measurements of mussels (Mytilus edulis) for the assessment of environmental pollution. Hydrobiologia 1989, 188/189, 455–461. [Google Scholar] [CrossRef]
- Hopkin, S.P. In situ biological monitoring of pollution in terrestrial and aquatic ecosystem. In Handbook of Ecotoxicology; Blackwell Science Publication: London, UK, 1997; pp. 397–427. [Google Scholar] [CrossRef]
- Deplege, M.H.; Hopkin, S.P. Methods to assess effects on brackish, estuarine and near-coastal water organism. In Scope 53—Methods to Assess the Effects of Chemical on Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 1995; Volume 7, pp. 125–149. [Google Scholar]
- Dolbeth, M.; Crespo, D.; Leston, S.; Solan, M. Realistic scenarios of environmental disturbance lead to functionally importantchanges in benthic species-environment interactions. Mar. Environ. Res. 2019, 150, 104770. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.K. Use of biomarkers in environmental monitoring. Ocean Coast. Manag. 2009, 52, 348–354. [Google Scholar] [CrossRef]
- Gorbi, S.; Virno Lamberti, C.; Notti, A.; Benedetti, M.; Fattorini, D.; Moltedo, G.; Regoli, F. An ecotoxicological protocol with caged mussels, Mytilus galloprovincialis, for monitoring the impact of an offshore platform in the Adriatic Sea. Mar. Environ. Res. 2008, 65, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Bocchetti, R.; Regoli, F. Seasonal variability of oxidative biomarkers, lysosomal parameters, metallothioneins and peroxisomal enzymes in the Mediterranean mussel Mytilus galloprovincialis from Adriatic Sea. Chemosphere 2006, 65, 913–921. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G.; Cassini, A.; Piccinni, E.; Albergoni, V. Metal accumulation and binding protein induction in Mytilus galloprovincialis, Scapharca inaequivalvis, and Tapes philippinarum from the Lagoon of Venice. Arch. Environ. Contam. Toxicol. 2003, 44, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Boscolo Brusà, R.; Cacciatore, F.; Berto, D.; Marin, M.G.; Giani, M. Contamination of natural and cultured mussels (Mytilus galloprovincialis) from the northern Adriatic Sea by tributylin and dibutyltin compounds. Appl. Organomet. Chem. 2004, 18, 614–618. [Google Scholar] [CrossRef]
- Berto, D.; Boscolo Brusà, R.; Cacciatore, F.; Giani, M. Organotins used in antifouling paints: Environmental impact and contamination in a case study (Southern Venice Lagoon). Oceanol. Hydrobiol. Stud. 2006, 35, 270–283. [Google Scholar]
- Byrne, P.A.; O’Halloran, J. The impact of ballast effluent on the Manila clam Tapes semidecussatus. Ecotoxicology 2004, 13, 311–322. [Google Scholar] [CrossRef]
- Summer, K.H.; Halbach, S. I metalli. In Tossicologia; Zanichelli Editore: Bologna, Italy, 2000; Cap. 30; pp. 339–350. [Google Scholar]
- Samiullah, Y. Prediction of the Environmental Fate of Chemicals, 1st ed.; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-1-85166-450-4. [Google Scholar] [CrossRef]
- Saulnier, I.; Mucci, A. Trace metal remobilization following the resuspension of estuarine sediments: Saguenay Fjord, Canada. J. Appl. Geochem. 2000, 15, 191–210. [Google Scholar] [CrossRef]
- Cantwell, M.G.; Burgess, R.M.; Kester, D.R. Release and phase partitioning of metals from anoxic estuarine sediments during periods of simulated resuspension. Environ. Sci. Technol. 2002, 36, 5328–5334. [Google Scholar] [CrossRef]
- Abel, P.D. Water Pollution Biology, 1st ed.; Ellis Horwood: Chichester, UK, 1989; ISBN 0745805124. [Google Scholar]
- Wijayaratne, R.D.; Means, J.C. Sorption of polycyclic aromatic hydrocarbons by natural estuarine colloids. Mar. Environ. Res. 1984, 11, 77–89. [Google Scholar] [CrossRef]
- Poulsen, R.; Gravert, T.K.O.; Tartara, A.; Bensen, H.K.; Gunnarsen, K.C.; Dicová, K.; Nielsen, N.J.; Christensen, J.H. A case study of PAH contamination using blue mussels as a bioindicator in a small Greenlandic fishing harbor. Mar. Pollut. Bull. 2021, 171, 112688. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, A.; Abondio, P.; Frapiccini, E.; Luiselli, D.; Marini, M. Meta-Analysis of a New Georeferenced Database on Polycyclic Aromatic Hydrocarbons in Western and Central Mediterranean Seafood. Appl. Sci. 2022, 12, 2776. [Google Scholar] [CrossRef]
- Stogiannidis, E.; Laane, R.; Whitacre David, M. Source Characterization of Polycyclic Aromatic Hydrocarbons by Using Their Molecular Indices: An Overview of Possibilities. In Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2015; Volume 234, Chapter 2; pp. 49–133. [Google Scholar] [CrossRef]
- Kennish, M.J. Practical Handbook of Estuarine and Marine Pollution, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; ISBN 9780203742488. [Google Scholar] [CrossRef]
- Youngblood, W.W.; Blumer, M. Polycyclic aromatic hydrocarbons in the environment: Homologous series in soils and recent marine sediment. Geochim. Cosmochim. Acta 1975, 39, 1303–1314. [Google Scholar] [CrossRef]
- Alzieu, C. Impact of tributyltin on marine invertebrates. Ecotoxicology 2000, 9, 71–76. [Google Scholar] [CrossRef]
- Goldberg, E.D. TBT: An environmental dilemma. Environment 1986, 28, 17–44. [Google Scholar] [CrossRef]
- Noventa, S.; Berto, D.; Rampazzo, F.; Formalewicz, M.; Gion, C.; Boscolo Brusà, R.; Cacciatore, F.; Berducci, M.T.; Bianchi, J.F.; Onorati, F.; et al. TBT and antifouling strategies: The Italian and European legislation. Energia Ambiente e Innovazione ENEA, January–February 2014; pp. 89–94. [Google Scholar] [CrossRef]
- Blunden, S.J.; Bowen, H.J.M.; Hobbs, L.A.; Smith, P.J. The environmental chemistry of organotin compounds. In Environmental Chemistry; Bowen, H.J.M., Ed.; The Royal Society of Chemistry: London, UK, 1984; Volume 3, pp. 49–77. [Google Scholar] [CrossRef]
- Sousa, A.; Matsudaira, C.; Takahashi, S.; Tanabe, S.; Barroso, C. Integrative assessment of organotin contamination in a southern European estuarine system (Ria de Aveiro NWPortugal): Tracking temporal trends in order to evaluate the effectiveness of the EU ban. Mar. Pollut. Bull. 2007, 54, 1645–1653. [Google Scholar] [CrossRef]
- European Union. Water Framework Directive, Council Directive 2000/60/EC of 23 October 2000 Establishing a framework for Community action in the field of water policy. Off. J. Eur. Union 2000, 327, 1–72. [Google Scholar]
- Smolders, R.; Bervoets, L.; De Coen, W.; Blust, R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004, 129, 99–112. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. First evidence of altered vitellogenin-like protein levels in clam Tapes philippinarum and cockle Cerastoderma glaucum from the Lagoon of Venice. Mar. Pollut. Bull. 2007, 55, 494–504. [Google Scholar] [CrossRef]
- Nesto, N.; Romano, S.; Moschino, V.; Mauri, M.; Da Ros, L. Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the Lagoon of Venice, Italy. Mar. Pollut. Bull. 2007, 55, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Day, K.E.; Scott, I.M. Use of acetylcholinesterase activity to detect sublethal toxicity in stream invertebrates exposed to low concentrations of organophosphate insecticides. Aquat. Toxic. 1990, 18, 101–114. [Google Scholar] [CrossRef]
- Bocquené, G.; Galgani, F. Biological Effects of Contaminants: Cholinesterase Inhibition by Organophosphate and Carbamate Compounds. No. 22; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1998.
- Escartín, E.; Porte, C. The use of cholinesterase and carboxylesterase activities from Mytilus galloprovincialis in pollution monitoring. Environ. Toxicol. Chem. 1997, 16, 2090–2095. [Google Scholar] [CrossRef]
- Solé, M.; Baena, M.; Arnau, S.; Carrasson, M.; Maynou, F.; Cartes, J.E. Muscular cholinesterase activities and lipid peroxidation levels as biomarkers in several Mediterranean marine fish species and their relationship with ecological variables. Environ. Int. 2010, 36, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Gorbi, S.; Regoli, F. Total oxyradical scavenging capacity as an index of susceptibility to oxidative stress in marine organisms. Comments Toxicol. 2003, 9, 303–322. [Google Scholar] [CrossRef]
- Di Giulio, R.T.; Benson, W.H.; Sanders, B.M.; van Veld, P.A. Biochemical Mechanisms: Metabolism, Adaptation, and Toxicity. In Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment, 2nd ed.; Rand, G.M., Ed.; Taylor and Francis: Abingdon, UK, 2003; pp. 523–560. [Google Scholar]
- European Union. Habitats Directive, Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Simeoni, U.; Corbau, C. A review of the Delta Po evolution (Italy) related to climatic changes and human impacts. Geomorphology 2009, 107, 64–71. [Google Scholar] [CrossRef]
- Vincenzi, S.; De Leo, G.A.; Munari, C.; Mistri, M. Rapid estimation of potential yield for data-poor Tapes philippinarum fisheries in North Adriatic coastal lagoons. Hydrobiologia 2014, 724, 267–277. [Google Scholar] [CrossRef]
- Nasci, C.; Da Ros, L.; Nesto, N.; Sperni, L.; Passarini, F.; Pavoni, B. Biochemical and histochemical responses to environmental contaminants in clam, Tapes philippinarum, transplanted to different polluted areas of Venice Lagoon, Italy. Mar. Environ. Res. 2000, 50, 425–430. [Google Scholar] [CrossRef]
- Bortoli, A.; Troncon, A.; Dariol, S.; Pellizzato, F.; Pavoni, B. Butyltins and phenyltins in biota and sediments from the Lagoon of Venice. Oceanologia 2003, 45, 7–23. [Google Scholar]
- Marin, M.G.; Boscolo Brusà, R.; Cella, A.; Degetto, S.; Da Ros, L. Field validation of autometallographical black silver deposit (BSD) extent in three bivalve species from the Lagoon of Venice, Italy (Mytilus galloprovincialis, Tapes philippinarum, Scapharca inaequivalvis) for metal bioavailability assessment. Sci. Total Environ. 2006, 371, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Virno Lamberti, C.; Tomassetti, P.; Ceracchi, S.; Gabellini, M. Water Column Study in the Monitoring Plan of the First Italian Offshore LNG Terminal. Int. J. Environ. Sci. 2020, 26, 556187. [Google Scholar]
- Virno Lamberti, C.; Gabellini, M.; Maggi, C.; Nonnis, O.; Manfra, L.; Ceracchi, S.; Trabucco, B.; Moltedo, G.; Onorati, F.; Franceschini, G.; et al. An Envrionmental Monitoring Plan for the Construction and Operation of a Marine Terminal for Regasifying Liquefied Natural Gas (LNG) in North Adriatic Sea; Hughes, T.B., Ed.; Nova Publisher: New York, NY, USA, 2013; Volume 5, pp. 115–134. [Google Scholar]
- Cacciatore, F.; Bernarello, V.; Boscolo Brusà, R.; Sesta, G.; Franceschini, G.; Maggi, C.; Gabellini, M.; Virno Lamberti, C. PAH (Polycyclic Aromatic Hydrocarbon) bioaccumulation and PAHs/shell weight index in Ruditapes philippinarum (Adams & Reeve, 1850) from the Vallona lagoon (northern Adriatic Sea, NE Italy). Ecotoxicol. Environ. Safe 2018, 148, 787–798. [Google Scholar] [CrossRef]
- Maggi, C.; Berducci, M.T.; Di Lorenzo, B.; Dattolo, M.; Cozzolino, A.; Mariotti, S.; Fabrizi, V.; Spaziani, R.; Virno Lamberti, C. Temporal evolution of the environmental quality of the Vallona Lagoon (Northern Mediterranean, Adriatic Sea). Mar. Pollut. Bull. 2017, 125, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Binato, G.; Biancotto, G.; Piro, R.; Angeletti, R. Atomic absorption spectrometric screening and gas chromatographic-mass spectrometric determination of organotin compounds in marine mussels: An application in samples from the Venetian Lagoon. Fresenius. J. Anal. Chem. 1998, 361, 333–337. [Google Scholar] [CrossRef]
- Morabito, R.; Chiavarini, S.; Cremisini, C. Speciation of organotin compounds in environmental samples by GC-MS. In Quality Assurance for Environmental Analysis; Quevauviller, P., Maier, E.A., Griepink, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 17, pp. 435–464. [Google Scholar] [CrossRef]
- Morabito, R. Metodo per la determinazione di composti organostannici in sedimenti e matrici biologiche tramite GC -MS e GC-FPD. In Metodologie Analitiche di Riferimento del Programma di Monitoraggio per il Controllo Dell’ambiente Marino Costiero (Triennio 2001–2003); Cicero, A.M., Di Girolamo, I., Eds.; Ministero Dell’ambiente e Della Tutela del Territorio, ICRAM: Rome, Italy, 2001; Appendice I. [Google Scholar]
- Boscolo, R.; Cornello, M.; Giovanardi, O. Condition index and air survival time to compare three kind of Manila clam Tapes philippinarum (Adams & Reeve) farming systems. Aquac. Int. 2003, 11, 243–254. [Google Scholar] [CrossRef]
- Costa, S.; Coppola, F.; Pretti, C.; Intorre, L.; Meucci, V.; Soares, A.M.V.M.; Freitas, R.; Solé, M. The influence of climate change related factors on the response of two clam species to diclofenac. Ecotoxicol. Environ. Saf. 2020, 189, 109899. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 13 May 2022).
- Bocchetti, R.; Virno Lamberti, C.; Pisanelli, B.; Razzetti, E.M.; Maggi, C.; Catalano, B.; Sesta, G.; Martuccio, G.; Gabellini, M.; Regoli, F. Seasonal variations of exposure biomarkers, oxidative stress responses and cell damage in the clams, Tapes philippinarum, and mussels, Mytilus galloprovincialis, from Adriatic sea. Mar. Environ. Res. 2008, 66, 24–26. [Google Scholar] [CrossRef]
- Eklund, B.; Eklund, D. Pleasure Boatyard Soils are Often Highly Contaminated. Environ. Manag. 2014, 53, 930–946. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.K.; Takahashi, S.; Min, B.Y.; Tanabe, S. Butyltin residues in blue mussels (Mytilus edulis) and ark shells (Scapharca broughtonii) collected from Korean coastal waters. Environ. Pollut. 2002, 117, 475–486. [Google Scholar] [CrossRef]
- Corsolini, S.; Mazzoni, M.; Polesello, S.; Rusconi, M.; Valsecchi, S. Perfluorinated alkyl acids in bivalves, water, and sediments of the Po river Delata (Adriatic Sea). Organohalogen Compd. 2014, 76, 684–687. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission to assess the health risks to consumers associated with exposure to organotins in foodstuffs (Question N EFSA-Q-2003-110). Adopted on 22 September 2004. EFSA J. 2004, 102, 1–119. Available online: http://www.efsa.eu.int.1/119 (accessed on 23 June 2022).
- Belfroid, A.C.; Purperhart, M.; Ariese, F. Organotin Levels in Seafood. Mar. Pollut. Bull. 2000, 40, 226–232. [Google Scholar] [CrossRef]
- Airaksinen, R.; Rantakokko, P.; Turunen, A.W.; Vartiainen, T.; Vuorinen, P.J.; Lappalainen, A.; Vihervuori, A.; Mannio, J.; Hallikainen, A. Organotin intake through fish consumption in Finland. Environ. Res. 2010, 110, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Moon, H.B.; Choi, H.G. Intake and potential health risk of Butyltin compounds from seafood consumption in Korea. Arch. Environ. Contam. Toxicol. 2012, 62, 333–340. [Google Scholar] [CrossRef]
- Marin, M.G.; Moschino, V.; Deppieri, M.; Lucchetta, L. Variations in gross biochemical composition, energy value and condition index of T. philippinarum from the Lagoon of Venice. Aquaculture 2003, 219, 859–871. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Tchounwou, P.B. Serum Acetyl Cholinesterase as a Biomarker of Arsenic Induced Neurotoxicity in Sprague-Dawley Rats. Int. J. Environ. Res. Public Health 2005, 2, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Schiavo, S.; Xiangli, D.; Rametta, G.; Miglietta, M.L.; Oliviero, M.; Changwen, W.; Manzo, S. Early ecotoxic effects of ZnO nanoparticle chronic exposure in Mytilus galloprovincialis revealed by transcription of apoptosis and antioxidant-related genes. Ecotoxicology 2018, 27, 369–384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
