Abstract
Electroactive bacteria have a wide range of applications, including electricity production, bioremediation, and the sensing of toxic compounds. Bacterial biofilm formation is often mediated by the second messenger cyclic guanosine monophosphate (c-di-GMP) synthesized by a diguanylate cyclase (DGC). The role of c-di-GMP in the expression of c-type cytochromes has been previously reported. The aim of this study was to determine the bioelectrogenic activity of Cupriavidus metallidurans strain CH34 pJBpleD*, which possesses a constitutively active DGC that increases c-di-GMP levels. Notably, the heterologous expression of the constitutively active DGC in C. metallidurans strain CH34 pJBpleD* showed a higher biofilm formation and increased the electrical current production up to 560%. In addition, C. metallidurans CH34 pJBpleD* showed increased levels of c-type cytochrome-associated transcripts compared with the wild-type strain CH34. Scanning electron microscopies revealed a denser extracellular matrix with an increased exopolymeric substance content in the CH34 pJBpleD* biofilm on the electrode surface. The results of this study suggest that higher levels of c-di-GMP synthesized by a constitutively active diguanylate cyclase in C. metallidurans strain CH34 pJBpleD* activated the formation of an electroactive biofilm on the electrode, enhancing its exoelectrogenic activity.
1. Introduction
Microbial electrochemical technologies (METs) have been applied to the generation of electrical currents, bioremediation of polluted matrices, synthesis of valuable compounds, and sensing of toxic agents [1,2,3,4,5,6]. Diverse strains with an exoelectrogenic activity have been studied to detect environmental signals and translate them into an electrical current [3]. MET-based biosensors have been developed mainly for microbial communities, but also with pure strains for the detection of organic matter in water [7,8]. Few studies have investigated the use of single strains for sensing pollutants in water (e.g., in MET-based biosensors) due to their low electrogenic activity. One strategy to overcome this limitation is to improve the electrogenic activity of pollutant-degrading strains.
An electroactive biofilm is an important factor in the electrogenic output of bioelectrochemical systems (BESs) [5,9,10,11], in which exopolysaccharides act as attachment sites for peripheral redox proteins, allowing the transfer of electrons to distant acceptors [12]. Therefore, improved biofilm formation capabilities increase the power densities in MFCs [13]. Often, the bacterial biofilm formation is mediated by the second messenger bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP). The general consensus is that the cytoplasmic c-di-GMP concentration is the result of the dynamic equilibrium of two antagonist activities: diguanylate cyclase (DGC), which is involved in its synthesis, and phosphodiesterase (PDE), implied in its degradation [14]. C-di-GMP interacts with a broad spectrum of intracellular effectors as riboswitches and a diverse range of proteins, reducing cell motility and promoting extracellular polymeric substance (EPS) synthesis [15,16]. A role of c-di-GMP in the electroactivity of biofilms has been reported, demonstrating an influence on the content of proteins that enable electron transfers from the inner membrane through the outer membrane and beyond as c-type cytochromes [17]. C-di-GMP has been reported to have a critical role the stability of c-type cytochromes into the extracellular matrix of Geobacter sulfurreducens, promoting the biosynthesis of anchoring exopolysaccharides [18]. C-di-GMP has also been shown to promote the expression of genes that encode c-type cytochromes in Shewanella oneideinsis MR-1 [19]. On the other hand, a specific DGC (DgcS) involved in the biofilm formation of S. oneideinsis MR-1 on electrode surfaces has been reported [20].
Cupriavidus metallidurans CH34 is a facultative anaerobe and metal-resistant bacterium [21,22,23,24] whose exoelectrogenic activity during the anaerobic degradation of toluene was recently reported [25]. The heterologous synthesis of the constitutively active DGC PleD* from Caulobacter crescentus in C. metallidurans strain CH34 pJBpleD* was shown to increase c-di-GMP levels and the biofilm formation [23]. The aim of this study was to determine the exoelectrogenic activity of C. metallidurans strain CH34 pJBpleD*. The results indicated that increased c-di-GMP levels in C. metallidurans strain CH34 pJBpleD* led to an overproduction of the electric current from the strain to the electrode in a bioelectrochemical system in concomitance with an increase in the abundance of c-type cytochrome gene transcripts as well as changes to the biofilm topology, including a more compact extracellular matrix.
2. Materials and Methods
2.1. Bacterial Strains and Growth
C. metallidurans strains CH34 and CH34 pJBpleD* were cultured in a low phosphate Tris-buffered mineral salt (LPTMS) broth at 30 °C [23]. C. metallidurans CH34 pJBpleD∗, which expresses the constitutively active DGC PleD* from C. crescentus, was cultured in the presence of 10 µg mL−1 tetracycline. Both strains were also grown in an LB medium (yeast extract 5 g L−1, peptone 10 g L−1, and NaCl 5 g L−1) under aerobic conditions. The cultures (20 mL) grown in the LB medium for 12 h were washed three times with a volume of LPTMS broth and suspended in 2 mL of this medium.
2.2. Biofilm Formation Assays in a Calgary Biofilm Device
The cellular suspensions were obtained directly from colonies grown on LPTMS agar plates at a turbidity equivalent to a McFarland 1.0 standard solution (Thermo R20421). Each suspension was diluted 15 times to inoculate them into a Calgary Biofilm Device (CBD) (Innovotech; product 19111) at 30 °C and 150 rpm over 24 h [26,27]. The biofilms grown on polyethylene glycol surfaces (pegs) were separated and six pegs were broken from the lid using a needle-nose plier. The pegs were then rinsed two times in NaCl 0.85% and sonicated for 10 min at a low frequency in 200 µL of NaCl 0.85% and 1% Tween solution, resulting in a cell suspension that was subsequently serial diluted. Serial dilutions were spotted on the TSA agar plate to quantify the colony forming units (CFU). The remaining pegs on the lid were rinsed two times in NaCl 0.85% and stained with Cristal Violet (0.1%) for 15 min. The plate was air-dried for 16 h. Thereafter, the stained biomass was suspended in 200 mL of 30% acetic acid; the absorbance of this suspension at 595 nm was then measured. This value was normalized by the turbidity at 600 nm of the planktonic fraction of each culture.
2.3. Dual-Chamber Bioelectrochemical Reactor Set-Up and Operation
The reactors were set as a dual-chamber BES separated by a cation exchange membrane (Ultrex CMI-7000S, Membranes International Inc., Ringwood, NJ, USA). For each BES, the cathodic chamber was filled with 290 mL of the LPTMS medium and maintained in an abiotic state whilst 290 mL of the LPTMS medium supplemented with succinate (18 mM) was used in the anodic chamber. Ag/AgCl (RE-1S; Bio-Logic, Grenoble, France) was used as the reference electrode. Rectangular graphite plates with a 65 cm2 surface area were used as working (anode) and counter (cathode) electrodes and were electrically connected with titanium wires covered with a heat-shrinkable tube. The potential of the anode of each system was held constant at +500 mV vs. Ag/AgCl with a potentiostat (Autolab 204, Metrohm, Herisau, Switzerland). The electrogenic performances of each strain were investigated by chronoamperometry. Two experimental runs were performed. The first preliminary run was maintained for 6 days to determine the current density performances of the systems. A second system was operated with the same conditions for 15 days. Two BESs were operated for each run and the anodic chamber of each one was inoculated with C. metallidurans CH34 or C. metallidurans CH34 pJBpleD*. The current outputs were monitored on both BESs for 15 days. One control without cells was set up and operated in parallel.
2.4. Isolation of Total RNA, Reverse Transcription, and qPCR Amplification
The anode-associated biofilms were harvested by scraping one face of the surface of the two electrodes. The total RNA from the biofilms was extracted using a Fast RNA Pro Soil-Direct kit (MP Biomedicals, Illkrich, France) and the protocol for RNA extraction was optimized for the bacterial cells. The biofilm samples were processed immediately after sampling, kept on ice during the RNA extraction, and stored at −80 °C. To completely remove the contaminating DNA, the extracted nucleic acids were treated using RQ1 RNase-Free DNase (Promega Corporation, Madison, WI, USA) with a modification to the reagent quantities as suggested by the manufacturer. The genomic DNA contamination was tested by PCR using primers specific to the 16S rRNA gene. The RNA was quantified using a Qubit Fluorometric Quantification kit (Invitrogen, Life Technologies, Carlsbad, CA, USA) in order to proceed with the same concentrations for each sample using the proportional quantities in the following step. The total cDNA was obtained in quadruplicate from 1 µg of RNA by reverse transcription PCR (RT-PCR) with random primers using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). To ensure the success of the protocol, the resulting cDNA samples were analyzed by PCR using primers specific to the housekeeping 16S rRNA and rpoB genes as a positive control. The cDNA was then diluted 1/10 with nuclease-free water and used as a template for the qPCR experiments. Specific primers were designed to analyze the transcriptional levels of the citC1 and citC2 genes (Table S1). The rpoZ and gyrB genes were used as the housekeeping genes for data normalization. The qPCR reactions were carried out using an initial denaturation step of 3 min at 95 °C, 35 cycles of a denaturation step of 30 s at 95 °C, a primer annealing step of 45 s at 69 °C, and an elongation step of 45 s at 72 °C. The cycling was completed by a final elongation step of 10 min at 72 °C.
Abundance fold changes in the CH34 pJBpleD∗ strain were assessed by normalizing the transcript levels of the WT strain sample using the copy numbers of the housekeeping rpoZ and gyrB genes as the reference transcript. The calibration curves were obtained in triplicate. The fold changes were assessed by a one-sample t-test. Values outside a [+1, −1] range with p-values below 0.05 were reported as significant.
2.5. Electron Microscopy of the Biofilms
The C. metallidurans biofilms that had formed on the electrode surfaces were analyzed by scanning electron microscopy. The samples were fixed with a 2% glutaraldehyde solution in a phosphate buffer (0.1 M, pH 7.4) and then washed with the same buffer. After dehydration with a series of ethanol, the samples were infiltrated with hexamethyldisilazane, dried overnight, and finally sputter-coated with a thin layer (~10 nm) of gold in order to produce a conductive surface. The SEM-SE imaging was performed using a Zeiss Gemini 500 electron microscope operating with an acceleration voltage of 5 kV.
3. Results and Discussion
3.1. Cell Densities and EPS Production in C. metallidurans Biofilms
The assays were performed in a Calgary Biofilm Device (CBD) in order to compare the attachment skills as well as to quantify the number of adhered cells and the EPS production of CH34 and CH34 pJBpleD* biofilms. Similar cell densities by surface units on each biofilm on pegs were observed after 24 h under the conditions tested in the CBD (Figure 1A). However, a similar CH34 pJBpleD* cell density produced a six-fold higher biofilm formation compared with the CH34 cells (Figure 1B), suggesting a significantly higher production of EPSs by the recombinant cells. This A595/OD600 signal increase in the CBD was three times higher than that previously reported in 96-well microtiter plates [23], probably due to a longer cultivation time and more favorable conditions for the production of EPSs. It has been reported that an improved biofilm formation in engineered S. oneidensis strains caused an increase in the current production [13,28]. The bioelectrogenic activity in bacteria has been related to electroactive components such as outer membrane c-type cytochromes [13], exopolysaccharide attachment sites for peripheral redox proteins [12], extracellular DNA [29], and exopolysaccharide contents [28,30].
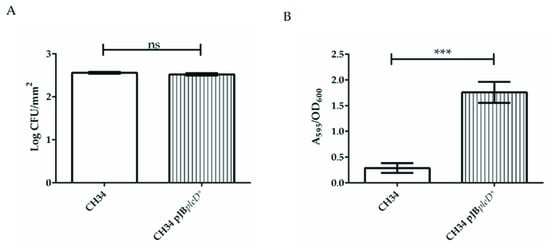
Figure 1.
C. metallidurans CH34 pJBpleD* increased biofilm formation compared with wild-type strain CH34. Cell densities and biomass adherence in the biofilms of C. metallidurans strains CH34 and CH34 pJBpleD*. (A) Cell densities on biofilm surfaces. (B) Biomass adherence on surfaces. Data collected after 24 h incubation at 30 °C. Values correspond with averages ± SD from three independent assays. Significant differences assessed by t-test: ns, non-significant; ***, p < 0.001.
3.2. Current Production by C. metallidurans Strains
In this study, we observed an increased bioelectrogenic activity in the recombinant C. metallidurans strain CH34 pJBpleD*. During the first run, after a short phase of current stabilization (a few hours), the current densities stabilized at about 15 mA/m2 for the wild-type (WT) strain whereas for strain CH34 pJBpleD*, the current densities remained stable at about 60 mA/m2 (data not shown). In the second run, after one day of stabilization, the current densities remained stable for 8 days for the wild-type strain at about 18 mA/m2 and about 55 mA/m2 for C. metallidurans CH34 pJBpleD* (Figure 2). On day 8, the current densities raised significantly only in the BESs inoculated with strain CH34 pJBpleD*, reaching a maximum of 100 mA/m2. This increase in the current production may be related to a more mature biofilm and the adaptation of the bacteria to the electrochemical respiration conditions. The lag phase in the current production in the BESs with strain CH34 pJBpleD* has also been observed in previous reports [31,32]. A third run was set using the bacterial culture present in the bioelectrochemical systems of the second run (Figure S1). The current densities after the lag phase increased up to 300 mA/m2 for strain CH34 pJBpleD* and up to 150 mA/m2 for the WT strain.
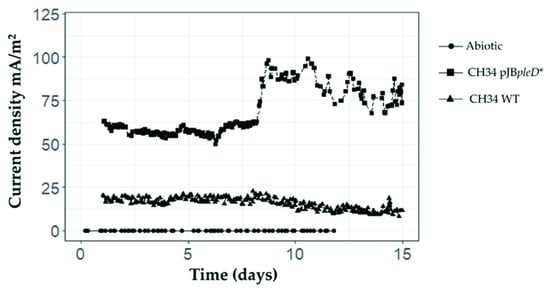
Figure 2.
Increased bioelectrogenic activity of the genetically modified C. metallidurans strain CH34 pJBpleD* in BESs. C. metallidurans CH34 pJBpleD* showed an increase in current densities compared with wild-type strain CH34. In the abiotic control, current densities remained stable between 0–3 mA/m2 during the whole experiment.
The electrogenic properties of C. metallidurans CH34 in BESs using toluene as the sole carbon source have been previously described [25]. However, the low current outputs of strain CH34 are not suitable for biosensing toluene or other toxic compounds. Notably, C. metallidurans CH34 pJBpleD* produced electrical current outputs up to 5.6-fold higher than the WT strain. An increased bioelectrogenic activity (3.4-fold) through the heterologous expression of DGC in the marine bacterium Shewanella oneidensis has been also reported [13]. The highly enhanced current production by C. metallidurans CH34 pJBpleD* supports its potential applications in BESs for bioremediation and for sensing pollutants such as toluene in water, for example.
3.3. Effect of PleD* Expression on C. metallidurans Transcripts
Based on previous reports of the critical role of c-type cytochromes in bacterial electrogenesis and biosynthesis regulation by c-di-GMP, the transcript levels of c-type cytochrome CDS RMET_RS10820 (citC1) and RMET_RS30050 (citC2) were compared between the biofilms of CH34 and CH34 pJBpleD* on the surfaces of the electrodes. The abundance of both c-type cytochrome transcripts encoded in the C. metallidurans CH34 genome showed an increment of 1.88 ± 0.09 and 2.45 ± 0.05, respectively, in strain CH34 pJBpleD* (Figure 3). The transcript abundances of genes, which encode putative determinants of electrogenesis, and the biofilm formation were also assessed. No significant differences were detected (Figure S2).
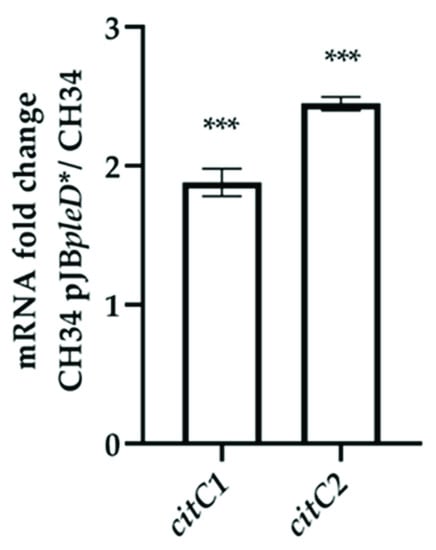
Figure 3.
C. metallidurans CH34 pJBpleD* showed increased levels of c-type cytochrome-associated transcripts compared with the wild-type strain CH34. Each bar represents the mean ratios from one biological replicate analyzed by technical quadruplicates using rpoZ and gyrB as reference genes. Fold change significances were assessed by a one-sample t-test: ***, p < 0.001.
The higher abundance of c-type cytochrome transcripts in strain CH34 pJBpleD* was in accordance with previous evidence, supporting the central role of these transmembrane electron carriers in extracellular electron transfers in bacteria as well as biosynthesis regulation by c-di-GMP [17,18,19,33]. In model organisms such as Geobacter sulfurreducens and Shewanella oneidensis, c-type cytochromes play a fundamental role in the electron transfer mechanism from the cell to a solid electron acceptor. In G. sulfurreducens, the periplasmic c-type cytochrome (PpcA) acts as a transmitter in the electron transfer between the inner and the outer cell membranes whereas in S. oneidensis, c-type cytochrome CymA is located in the inner membrane [33]. However, the genomes of G. sulfurreducens and S. oneidensis encode for a broader range of c-type cytochromes [34,35]. Thus, the involvement of this type of protein in the electrogenic capacity of electroactive strains have been assessed, but should be studied further. In C. metallidurans, the involvement of these proteins in the electrogenic capacity of the strain has been determined. Moreover, in S. oneidensis MR-1, the elevated intracellular c-di-GMP concentration increased the expression of c-type cytochromes, which are directly involved in current generation [19].
3.4. Effect of PleD* Expression on Electroactive C. metallidurans Biofilm Topology
Both the WT and recombinant strains established mature biofilms on the electrode surfaces (Figure 4). The CH34 pJBpleD* biofilm showed a more regular and compact extracellular matrix than the CH34 biofilm. The CH34 pJBpleD* extracellular matrix was characterized by the significant presence of EPSs between the cells, showing scarce empty cavities (Figure 4). However, the CH34 pJBpleD* biofilm topology showed fractures and gaps on its matrix. In the SEM preparations, the EPS collapsed down on the cells through the dehydration process and appeared as a stringy, spongy mat; thus, the fractures would be more prevalent if the EPS was denser. A higher proportion of these events in the CH34 pJBpleD* biofilm suggested a more rigid and fragile architecture due to a denser matrix of the EPS compared with the WT biofilm. This was also seen in the EPS dehydration mat covering the CH34 pJBpleD* cells, which were smaller and showed an increase in the surrounding extracellular matrix compared with their WT counterparts. Moreover, the WT biofilm possessed fibrillary structures that were ubiquitously distributed on its surface—as has been described of C. metallidurans biofilms on gold grains [36]—which were not observed on the CH34 pJBpleD* biofilm (Figure 4). The CH34 pJBpleD* biofilm cells resembled the phenotype of starving cells in macrocolony biofilms [37], this could be associated with the fact that CH34 pJBpleD* biofilm cells generate a higher electrical current compared with WT biofilm cells, which may lead to reduced energy resources for cell growth.

Figure 4.
Scanning electron micrographs of the biofilms of C. metallidurans strains CH34 and CH34 pJBpleD* on electrode surfaces after 15 days. Different scales of viewing are shown. CH34 pJBpleD* biofilms showed diminished cell sizes, changes in cell surface, and the increased presence of EPSs in the extracellular matrix.
The biofilm cell densities of both C. metallidurans strains were similar, but the scanning electron microscopic results suggested that the higher EPS content in the biofilm of the recombinant strain could have acted as an extracellular anchoring polysaccharide [18]. In addition, these results suggested a post-transcriptional regulation of the synthesis machinery of the EPS by c-di-GMP in C. metallidurans, as has been reported in the biosynthesis machineries of cellulose and PEL through allosteric c-di-GMP binding sites [38,39].
4. Conclusions
The heterologous expression of constitutively active DGC PleD* in C. metallidurans CH34 favored a biofilm formation, induced an increase of up to 560% in bioelectricity production, and increased the levels of c-type cytochrome transcripts. In addition, important changes in the extracellular topology of the biofilm on the electrode were observed, showing a more regular and compact extracellular matrix and increased EPS content whereas its cell density was maintained in the 24 h biofilm on pegs. These results indicate the close relationship between the biofilm formation, the bioelectrical current generation, the c-type cytochrome content, and the synthesis of the extracellular matrix in relation to the c-di-GMP pathway in bacteria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments9070080/s1, Figure S1: Current density in a third run using as inoculum the bacterial culture present in the bioelectrochemical systems of the second run; Figure S2: Comparison of CH34 pjB:pleD* and CH34 levels of transcripts associated to bacterial electrogenesis and biofilm formation; Table S1: Primers used in this study.
Author Contributions
P.A.-G., A.E.-T., F.F., N.G., R.J.T., A.F. and M.S. conceived and designed the experiments. P.A.-G., A.E.-T. and F.F. performed the experiments. P.A.-G., A.E.-T., F.F., N.G., R.J.T., A.F. and M.S. analyzed the data. M.S., N.G., R.J.T. and A.F. contributed reagents, materials, and analysis tools. P.A.-G., A.E.-T., F.F., A.F. and M.S. prepared the figures and/or tables. P.A.-G., A.E.-T., F.F., N.G., R.J.T., A.F. and M.S. authored or reviewed the drafts of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by ANID Programa de Investigación Asociativa (PIA) Anillo GAMBIO ACT172128 (M.S., P.A.-G.); FONDECYT 1200756 (M.S.) and 1160702 (N.G.) (http://www.fondecyt.cl); USM (M.S.) (http://www.usm.cl); UCH (N.G.) (www.uchile.cl); and NSERC RGPPIN/04811-2015 (R.J.T.) grants.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully thank Tiziano Catelani for the electronic microscopy analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lu, L.; Yazdi, H.; Jin, S.; Zuo, Y.; Fallgren, P.H.; Ren, Z.J. Enhanced bioremediation of hydrocarbon-contaminated soil using pilot-scale bioelectrochemical systems. J. Hazard. Mater. 2014, 274, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Benneton, X.D.; Strik, D.P.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Arends, J.B. The next step towards usable microbial bioelectrochemical sensors? Microb. Biotechnol. 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouler, J.; Di Lorenzo, M. Pesticide detection by a miniature microbial fuel cell under controlled operational disturbances. Water Sci. Technol. 2019, 79, 2231–2241. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Palma, E.; Espinoza-Tofalos, A.; Daghio, M.; Franzetti, A.; Tsiota, P.; Cruz Viggi, C.; Petrangeli Papini, M.; Aulenta, F. Bioelectrochemical treatment of groundwater containing BTEX in a continuous-flow system: Substrate interactions, microbial community analysis, and impact of sulfate as a co-contaminant. New Biotechnol. 2019, 53, 41–48. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Tront, J.M.; Fortner, J.D.; Plötze, M.; Hughes, J.B.; Puzrin, A.M. Microbial fuel cell biosensor for in situ assessment of microbial activity. Biosens. Bioelectron. 2008, 24, 586–590. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2013, 339, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Nancharaiah, Y.V.; Mohan, S.V.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Angelaalincy, M.J.; Krishnaraj, R.N.; Shakambari, G.; Ashokkumar, B.; Kathiresan, S.; Varalakshmi, P. Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Front. Energy Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Y.-Y.; Deng, X.-P.; Ng, C.K.; Cao, B.; Wang, J.-Y.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhanced Shewanella biofilm promotes bioelectricity generation. Biotechnol. Bioeng. 2015, 112, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Duerig, A.; Abel, S.; Folcher, M.; Nicollier, M.; Schwede, T.; Amiot, N.; Giese, B.; Jenal, U. Second messenger-mediated spatiotemporal control of protein degradation regulates bacterial cell cycle progression. Genes Dev. 2009, 23, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Ding, D.W.; Xu, J.; Li, L.; Xie, J.M.; Sun, X. Identifying the potential extracellular electron transfer pathways from a c-type cytochrome network. Mol. Biosyst. 2014, 10, 3138–3146. [Google Scholar] [CrossRef]
- Rollefson, J.B.; Stephen, C.S.; Tien, M.; Bond, D.R. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J. Bacteriol. 2011, 193, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.K.; Jiabao, X.; Cai, Z.; Yang, L.; Thompson, I.P.; Huang, W.E.; Cao, B. Elevated intracellular cyclic-di-GMP level in Shewanella oneidensis increases expression of c-type cytochromes. Microb. Biotechnol. 2020, 13, 1904–1916. [Google Scholar] [CrossRef]
- Matsumoto, A.; Koga, R.; Kanaly, R.A.; Kouzuma, A.; Watanabe, K. Identification of a diguanylate cyclase that facilitates biofilm formation on electrodes by Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2021, 87, e00201–e00221. [Google Scholar] [CrossRef]
- Monsieurs, P.; Moors, H.; Van Houdt, R.; Janssen, P.J.; Janssen, A.; Coninx, I.; Mergeay, M.; Leys, N. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. Biometals 2011, 24, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Yáñez, C.; González, M.; Lobos, S.; Smalla, K.; Seeger, M. Characterization of the metabolically modified heavy metal-resistant Cupriavidus metallidurans strain MSR33 generated for mercury bioremediation. PLoS ONE 2011, 6, e17555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alviz-Gazitua, P.; Fuentes-Alburquenque, S.; Rojas, L.A.; Turner, R.J.; Guiliani, N.; Seeger, M. The response of Cupriavidus metallidurans CH34 to cadmium involves inhibition of the initiation of biofilm formation, decrease in intracellular c-di-GMP levels, and a novel metal regulated phosphodiesterase. Front. Microbiol. 2019, 10, 1–17. [Google Scholar]
- Alviz-Gazitua, P.; Durán, R.E.; Millacura, F.A.; Cárdenas, F.; Rojas, L.A.; Seeger, M. Cupriavidus metallidurans CH34 possesses aromatic catabolic versatility and degrades benzene in the presence of mercury and cadmium. Microorganisms 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Espinoza Tofalos, A.; Daghio, M.; González, M.; Papacchini, M.; Franzetti, A.; Seeger, M. Toluene degradation by Cupriavidus metallidurans CH34 in nitrate-reducing conditions and in bioelectrochemical systems. FEMS Microbiol. Lett. 2018, 365, fny119. [Google Scholar]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.J.; Stremick, C.A.; Turner, R.J.; Allan, N.D.; Olson, M.E.; Ceri, H. Microtiter susceptibility testing of microbes growing on peg lids: A miniaturized biofilm model for high-throughput screening. Nat. Protoc. 2010, 5, 1236. [Google Scholar] [CrossRef]
- Kouzuma, A.; Oba, H.; Tajima, N.; Hashimoto, K.; Watanabe, K. Electrochemical selection and characterization of a high current-generating Shewanella oneidensis mutant with altered cell-surface morphology and biofilm-related gene expression. BMC Microbiol. 2014, 14, 190. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef]
- Kitayama, M.; Koga, R.; Kasai, T.; Kouzuma, A.; Watanabe, K. Structures, compositions, and activities of live Shewanella biofilms formed on graphite electrodes in electrochemical flow cells. Appl. Environ. Microbiol. 2017, 83, e00903-17. [Google Scholar] [CrossRef] [Green Version]
- Daghio, M.; Vaiopoulou, E.; Patil, S.A.; Suárez-Suárez, A.; Head, I.M.; Franzetti, A.; Rabaey, K. Anodes stimulate anaerobic toluene degradation via sulfur cycling in marine sediments. Appl. Environ. Microbiol. 2016, 82, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daghio, M.; Espinoza Tofalos, A.; Leoni, B.; Cristiani, P.; Papacchini, M.; Jalilnejad, E.; Bestetti, G.; Franzetti, A. Bioelectrochemical BTEX removal at different voltages: Assessment of the degradation and characterization of the microbial communities. J. Hazard. Mater. 2018, 341, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation–the foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Methe, B.; Nelson, K.E.; Eisen, J.; Paulsen, I.; Nelson, W.; Heidelberg, J.; Wu, D.; Wu, M.; Ward, N.; Beanan, M.J.; et al. Genome of Geobacter sulfurreducens: Metal reduction in subsurface environments. Science 2003, 302, 1967–1969. [Google Scholar] [CrossRef] [Green Version]
- Heidelberg, J.F.; Paulsen, I.T.; Nelson, K.E.; Gaidos, E.J.; Nelson, W.C.; Read, T.D.; Eisen, J.A.; Seshadri, R.; Ward, N.; Methe, B.; et al. Genome sequence of the dissimilatory metal ion–reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 2002, 20, 1118–1123. [Google Scholar] [CrossRef]
- Fairbrother, L.; Etschmann, B.; Brugger, J.; Shapter, J.; Southam, G.; Reith, F. Biomineralization of gold in biofilms of Cupriavidus metallidurans. Environ. Sci. Technol. 2013, 47, 2628–2635. [Google Scholar] [CrossRef]
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—The spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471. [Google Scholar] [CrossRef] [Green Version]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hengge, R. Linking bacterial growth, survival, and multicellularity—Small signaling molecules as triggers and drivers. Curr. Opin. Microbiol. 2020, 55, 57–66. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).